?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Molten salt has been regarded as a versatile and environmental-friendly method for the material preparation and waste destruction. In this work, molten FeCl3 was utilized for the generation of magnetic biochar (MBC) derived from simultaneous activation and magnetization of biomass. The sample characterization indicated that MBC had a rough surface with BET surface area of 404 m2/g and total pore volume of 0.35cm3/g. Highly dispersed Fe3O4 and nitrogen could be deposited on the surface, leading to an excellent magnetization property. The MBC exhibited a great 2,4-Dichlorophenol (2.4-DCP) and atrazine removal performance in solution with the maximum adsorption capacity achieved 298.12 mg/g and 102.17 mg/g. Kinetics results demonstrated that MBC adsorption met the Pseudo-first-order model better. Molten NaOH-Na2CO3 was provided for the re-activation of exhausted MBC. 2,4-DCP was firstly desorbed from the MBC and subsequently destructed by the active species in the melt medium. Chlorine can be captured in the molten alkaline medium from the XRD pattern of residues.The MBC could be easily recovered with a yield of 98.2% and fixed carbon content of 61.0% after the molten salt regeneration process. With no 2,4-DCP detected, 65.5% and 31.69% of initial Cl was found in washing water and residues with the molten NaOH-Na2CO3, respectively. After 4 cycles of regeneration and adsorption, 60.55%-72.22% of initial adsorption capacity can be kept. This preparation and regeneration method can be an effective way to reduce the risk of secondary pollution of chlorinated organic compounds during adsorbent regeneration.
Implications: Molten salt (MS) is a salt or multiple salts with a low melting point, and has been applied in many sectors and is regarded as a crucial role in terms of energy, environmental, and resource sustainability. In our paper, magnetic biochar was prepared by one-step activation and magnetization of fir dust using molten FeCl3∙6H2O. Meanwhile, a regeneration method using molten alkaline salt was provided. Magnetic biochar generated in our study performed well in the 2,4-dichlorophenol and atrazine adsorption. After four cycles of regeneration and adsorption, 72.2% of initial 2,4-DCP adsorption capacity can be kept.
Introduction
Biomass generated from agriculture, such as stalks, straw, leaves, roots, husk, nutshells, and waste wood, has resulted in formidable pollution all over the world (Yin et al. Citation2014). Pyrolysis of biomass can convert the organics to biochar powders for various applications, including supercapacitors (Hao et al. Citation2016; Lu et al. Citation2016a; Citation2016b; Citation2016c), contaminant adsorption (dyes (Kong et al. Citation2016; Shang et al. Citation2015b), heavy metals (Kalinke et al. Citation2016), organic substances (Oh and Seo Citation2016; Peng et al. Citation2016), catalyst support for electro-oxidation (Lobos et al. Citation2016), and soil remediation (Nagodavithane, Singh, and Fang Citation2014), along with fertilizer enhancement (Liang et al. Citation2006; Zhang et al. Citation2013). But frequently, the recycling of biochar from a solution medium is very complicated by means of a centrifugation or filtration process, partially restricting the extensive generalization (Thines et al. Citation2017). This difficulty is overcome by the development of magnetic biochar (MBC), which attached with metallic ions on the surface to enhance its magnetic effect. Many researchers studied the carbonization process of the biomass and exploited multiple preparation methods for the purpose of magnetization recently. Therein co-precipitation to generate magnetic substances prior to heat was first developed with a two-step carbonization process (Saravanan et al. Citation2012; Yu et al. Citation2013). Then the methods of calcination (Baig et al. Citation2014; Reguyal, Sarmah, and Gao Citation2016), ball-milling(Shan et al. Citation2016), impregnation (Yang et al. Citation2016), electromagnetization (Jung et al. Citation2016), and molten salts (Yang et al. Citation2016) were gradually developed for the production of magnetic biochar and removal of contaminants in laboratory scale.
Molten salt (MS) is a salt or eutectic salts with a low melting point, has been applied in many sectors, and is regarded as having a crucial role in terms of energy, environment, and resource sustainability (Frangini and Masi Citation2016; Reddy, Rao, and Chowdari Citation2013). Specifically, MS is capable of sustaining high space–time yields for materials preparation/processing and triggering reactions at appreciable rates without the assistance of costly and sophisticated catalysts (Lu et al. Citation2016c; Reddy, Khai, and Chowdari Citation2015a; Reddy et al. Citation2015b). It has been reported that such MS has considerable solubility and diffusivity required for solid-phase reactions, which can greatly reduce the synthesis temperature (Reddy, Rao, and Chowdari Citation2013; Shang et al. Citation2015b). The most common salts are typically chlorides, nitrate, carbonates, and hydroxides, owing to their availability and low cost (Lu et al. Citation2016b). Many materials including ferrite (Cao et al., Citation2014; Ji et al. Citation2011; Selvamani, Balamurugan, and Sreenija Citation2016), titanates (Liu, Li, and Du Citation2016b), metal oxide nanomaterials (Liu et al. Citation2015, Citation2016a; Reddy et al. Citation2014a, Citation2014b; Shang et al. Citation2015a; Wang et al. Citation2014; Xia et al. Citation2011), and other nanotubes and nanosheets (Zhao et al. Citation2012) have been synthesized with those salts.
Molten salt carbonization of the carbon-rich biomass was recently reported using the molten carbonates and chlorides, such as zinc chloride, lithium chloride, and sodium carbonate (Lu et al. Citation2016c), as chlorides can reduce the carbonization temperature, promote the decomposition of carbonaceous material, and meanwhile restrict the formation of tar (Özhan et al. Citation2014). Compared with the conventional process, MS can complete the carbonization and two-activation process within a single step. In addition, the microstructure of carbon materials can be changed in the molten salts, and physicochemical properties are different because of their function as structure-directing agents/modifiers (Lu et al. Citation2016a). However, so far, most of the research work reported concentrates on the preparation method and sorptive properties from liquid phase. When the MBCs reach their saturation limit, they fail to adsorb the targeted pollutants (Marques et al. Citation2017). Though they can be easily separated from the liquid phase, the exhausted materials, in many cases, lack an sustainable desorption and reactivation method.
Molten carbonates were reported to be an effective reactivation agent for altering the surface chemistry of activated carbon and significantly enhanced the surface area, which is tightly related to the reuse of the treated carbon (Lu et al. Citation2015). In this process, high-temperature (usually 550–750ºC) carbonate mixtures are energy-intensive and costly (Flandinet et al. Citation2012; Yao, Li, and Zhao Citation2011). Thus, researchers have focused a significant amount of attention on lowering the eutectic point of molten salts (Raade and Padowitz Citation2011).
With the advantage of MS, we performed a molten salt system to convert fir dust to porous MBC. First, MBC was prepared and characterization of MBC was investigated. After that, MBC was used to eliminate the 2,4-dichlorophenol (DCP) and atrazine in aqueous medium. The adsorption performance including kinetics and isotherm were studied subsequently. Finally, this study creatively used the molten NaOH–Na2CO3 to regenerate the saturated MBC, which absorbed large amounts of DCP and atrazine. Most of the adsorbate could be destroyed and chlorine retained by the alkaline medium, which demonstrated the molten salt is a scalable and environmentally friendly route to prepare and reactivate MBC.
Materials and methods
Synthesis of the magnetic biochar
Fir dust from a local lumber in Shanghai (China) was chosen as a precursor for the MBC; it was ground into powder (<0.5 mm) and dried at 60ºC for 24 hr. All the stored samples undertook nothing about washing or purification protocols. For preparing MBC, 2 g of the dried fir dust and 5 g of FeCl3∙6H2O and 0.5 g NaNO3 (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) were premixed with the mortar, and then placed into the nickel crucible with a volume of 30 mL. The resulting materials were carbonized in a horizontal tube furnace (Hefei Kejing Materials Technology Co., Ltd). Samples were heated from room temperature to 450ºC with a heating rate of 10ºC/min and kept for 120 min under nitrogen atmosphere. The activated samples were cooled inside the tube furnace in the presence of nitrogen flow. After that, deionized water was used to wash the samples until no chlorine ions (Cl−) could be detected in the filtrate by AgNO3 solution (Shang et al. Citation2015b). Fir dust without molten salt was set for comparison under the same procedures.
Characterization of the samples
The morphologies and microstructures of the samples were characterized by scanning electron microscopy/energy dispersive x-ray spectroscopy and mapping system (SEM-EDS, Inspect F, FEI Co., USA). The crystallinity of MBC was measured using x-ray diffraction (XRD, D8 Advance Sol-X, Bruker Co., USA). Surface area, pore diameter, and the total pore volume of MBC were determined by N2 vapor adsorption studies at 77 K using a microporosity system (Micromeritics ASAP 2460). Magnetic moment of MBC was carried out at room temperature with a vibrating sample magnetometer (VSM, Lake Shore Cryotroni, USA). Raman spectra were recorded with a Renishaw InVia-Reflex Confocal Laser Micro Raman Spectrometer using a 514-nm laser diode as the excitation.
Abatement of DCP and atrazine
Adsorption performance of MBC for the DCP and atrazine was tested. For the atrazine removal experiments, methanol was added to deionized water prior to making the atrazine solution. The kinetics adsorption experiments were conducted in a 250-mL flask; 0.1 g of MBC was immersed in the 200 mL DCP and atrazine solution with a concentration of 50 mg/L. The concentration of DCP remaining in the solution was determined by high-performance liquid chromatography (Agilent 1200, America) equipped with a TC- C18 reverse-phase column (250 × 4.6 mm) (Wang, Jiang, and Liu Citation2008). The mobile phase was methanol with water (80:20, v/v) at a flow rate of 1.0 mL/min and ultraviolet (UV) absorbance detection at 280 nm. The injection volume is 20 mL with a variable-wavelength detector (VWD). To measure the concentration of atrazine, 10 mL of the medium was poured into a separatory funnel and the atrazine in the medium was repartitioned with an identical volume of chloroform after 5 min of shaking. The test methods was reported in the previous study (Yang et al. Citation2017). The properties of the reagents are presented in . Adsorption kinetics and adsorption equilibrium experiment on MBC were investigated with different models, which are shown in the supplemental information.
Table 1. Characteristic of FeCl3, 2,4-DCP and atrazine.
Destruction of DCP and reactivation with alkaline molten salt
There was 4000 mg/L of DCP adsorbed on the 5 g MBC for 24 hr, and the reagent remaining in the water (unabsorbed) was determined using the HPLC method. The saturated MBC was freeze-dried before molten salt destruction.
A 90%NaOH–10%Na2CO3 with a eutectic temperature of 286ºC () was selected as the molten salt for the destruction experiment performed with a self-made reactor (Figure S1), and the procedure included following steps: (1) Premixed NaOH/Na2CO3 (10 g/10 g) was loaded in the reactor vessel and heated to the 400ºC at 10ºC/min. (2) After the reactor temperature reached the experiment condition, air was purged into the reactor and 2 g of saturated MBC was conducted through a pipe and squirted into the molten salt. (3) Subsequently, the organic crack reaction started and kept for about 120 min. (4) After cooling to room temperature naturally, the solid phase was removed from the vessel and lixiviated in distilled water for 24 hr prior to final filtration. (5) The chlorine ion content in washing water and residues was determined by ion chromatography (Dionex, ICS-1000), and the chlorine retention efficiency (CRE) was calculated as follows:
Table 2. Salt composition, melting points, and eutectic temperatures (Yao, Li, and Zhao Citation2011).
where m0 means the initial mass of elemental chlorine of 2,4-DCP, and mt represents the mass of chlorine ion in washing water and residues.
Results and discussion
Harvest of magnetic carbon in molten salt
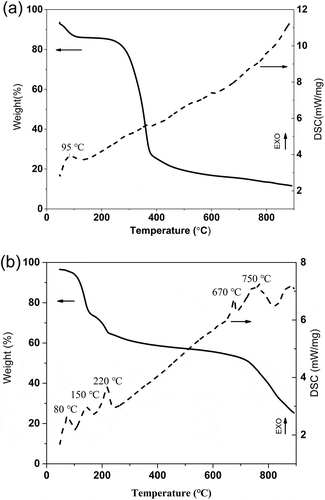
Molten salt carbonization of fir dust with and without FeCl3∙6H2O was studied by thermogravimetry–differential scanning calorimetry (TG-DSC) measurement in N2 atmosphere (). For the fir dust, the initial weight loss was at 95ºC due to the loss of free and bound water present in the fir dust matrix. Major thermal events were occurred approximately between 230 and 400ºC, related to the 59.6% of organic substances decomposition and carbonization process of cellulose and lignin ().
For the fir dust mixed with molten salt, the weight loss curve was more complicated. Decomposition peaks of cellulose and lignin (230–400ºC) still existed (), while the weight loss and exothermic peaks became weakened in comparison with those of the fir dust, which can be attributed to the high content of FeCl3 (Shang et al. Citation2015b). Furthermore, an additional peak at around 220ºC indicated the melt of FeCl3 according to the features of FeCl3 (). When the temperature rose above 500ºC, the carbonization process was almost finished and the production of carbon and other reductants (such as hydrogen) may react with molten FeCl3 (Yang et al. Citation2016), resulting in the formation of FeCl2, for which the melt temperature was 670ºC (eq 8). After that, the weight loss was mainly ascribed to the vaporization of FeCl3 and FeCl2:
The formation mechanism of Fe3O4 has been discussed in many studies, as explained by eqs 2–8 (Kong et al. Citation2016; Lu et al. Citation2016c). FeCl3 can be easily hydrolyzed to Fe(OH)3 and then transformed to FeO(OH) with the help of H2O. which is generated from the decomposition of cellulose and lignin. As is shown in , the initial weight loss was at 95ºC due to the loss of free and bound water present in the fir dust matrix. In addition, FeCl3∙6H2O (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) used in the experiment can supply the H2O, partly generating the FeO(OH). Then, with the formation of some reducing components such as H2, CO, and amorphous carbon, the FeO(OH) would be reduced to Fe3O4 at high temperature (Liu et al. Citation2013).
Characterization of magnetic biochar
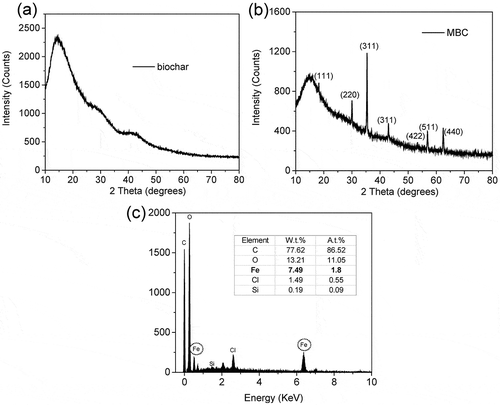
Fir sawdust was converted to magnetic biochar after carbonizing in molten salt for 120 min at 450℃. The product was confirmed by XRD and EDS spectra (). According to the standard diffraction spectrum (JCPDS card 19–0629), the observed peaks at 2θ of 18.269(111), 30.095(220), 35.422(311), 37.052(222), 43.052(400), 53.391(422), 56.942(511), 62.515(440), 65.743(531), and 74.960(622) are identified as cubic Fe3O4 (magnetite) (), while the biochar is in general weakly x-ray ordered, which was proved to be amorphous carbon ().
Figure 1. TG-DSC curves of the fir sawdust (a) and the fir sawdust mixed with FeCl3∙6H2O powder (b).
The stacked sp2 nature of carbon is still obvious from the strongly broadened (002) and (110) humps located near 21–26° and 42°, respectively. These structural features are responsible for the coexistence of strong D and G bands in the Raman spectra. For different biomass-derived biochars, there are common characters associated with structural disorder and the presence of foreign atoms and functional groups trapped (or grafted) between the aromatic sp2 layers (Liu and Antonietti Citation2014).
Microstructures of MBC was investigated with SEM analysis (). Carbon sheet and cubic Fe3O4 were synthesized by carbonization of the fir dust in molten FeCl3 at 450ºC. The carbon sheets have a rough surface and pores within it, while the cubic Fe3O4 was coated on the surface and exhibited different sizes. Such morphological transformations are also supported by obtaining N2 adsorption/desorption isotherms at 77 K. With a porosity, the MBC has a BET surface area of 404 m2/g and a total pore volume of 0.35 cm3/g ( and ). Due to such heterogeneous structure, MBC is expected to exhibit special adsorption capacity of the substrate (Cho et al. Citation2016). The EDS elemental maps of MBC provides further insights on the elemental distribution of C, O, and Fe, which demonstrated the nonuniform distribution of the carbon, oxygen, and iron. From the acquired EDS spectrum, 7.49 wt.% and 1.80 wt.% of determined weight and atomic percentages of iron was detected at the surface of MBC (). Moreover, 77.62 wt.% of carbon was determined for the carbon sheet. The carbon content was verified to be 72.09 wt.% by the elemental analysis of the MBC in .
Table 3. Elemental analysis of MBC.
Figure 3. SEM images at different expansion ratios (a–d) and carbon, oxygen, and iron maps of MBC obtained by molten salt route (e–g). A color version is available online.
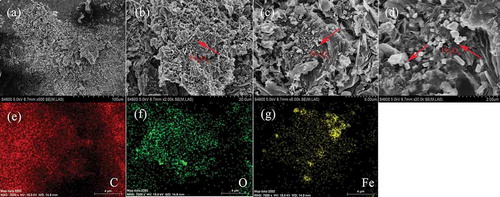
Figure 4. (a) Magnetic hysteresis loops of MBC. Digital image (inset) shows the magnetic separation by an ordinary magnet after 20 sec. (b) Raman spectrum of MBC. (c) N2 adsorption/desorption isotherms. (d) Pore size distribution.
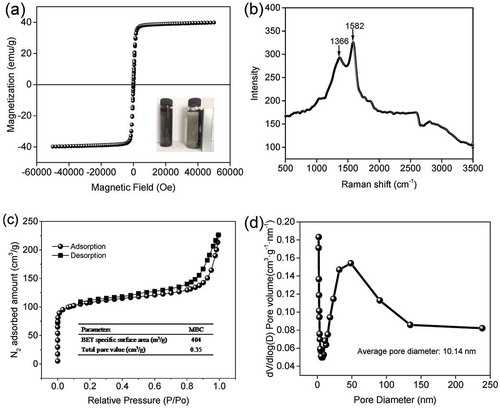
The magnetic properties of the prepared MBC were measured by VSM at room temperature (298 K) in an external magnetic field ranging from −60,000 to 60,000 Oe, and the obtained magnetic curves are presented in . It can be inferred that the presence of magnetic hysteresis loop implies MBC possesses superparamagnetic characteristics with a saturation magnetization value (Ms) of approximately 40.02 emu g−1, suggesting the easy separation properties from the solution system with a magnet.
Adsorption performance for 2,4-DCP and atrazine
Kinetic studies are performed for the 2,4-DCP and atrazine adsorption on MBC samples with initial concentration of 50 mg/L and 25 mg/L, which is presented in and . Approximately 30.488% and 40.66% of the DCP and atrazine, respectively, were removed within 100 min on 0.1 g MBC. With the time prolonged, the concentration of both of them stayed unchanged, which means the adsorption equilibrium had been reached at that time. With the increase of MBC amount from 0.1 g to 0.3 g, equilibrium concentration decreased, and almost 92.86% and 82.41% of DCP and atrazine were removed, respectively, suggesting that the MBC is highly effective for the abatement of these contaminants. The adsorption capacities of MBC were 298.12 mg/g and 102.17 mg/g. The DCP and atrazine can easily sorb on the surface of MBC because of the BET surface area of 404 m2/g and a total pore volume of 0.35 cm3/g. According to the pseudo-first-order and pseudo-second-order model, which were applied for the adsorption kinetics ( and ), pseudo-first-order is more suitable for describing the adsorption process through the comparison of R squared. Moreover, as the k value (adsorption constant) of DCP is comparatively greater thanfor atrazine, the adsorption process is faster, which is of great significance in practical application for rapid removal (Table S1).
Figure 5. Adsorption kinetics for the 2,4-DCP (a) and atrazine (b) on MBC. Results of fitting experimental data to the first-order, the pseudo-second-order for the 2,4-DCP (c) and atrazine (d). Adsorption equilibrium isotherms for the 2,4-DCP (e) and atrazine (f). Initial concentration range, 20–200 mg L−1; temperature range, 10–30ºC; adsorbents dosage, 0.05 g; working volume, 50 mL; contact time, 24 hr.
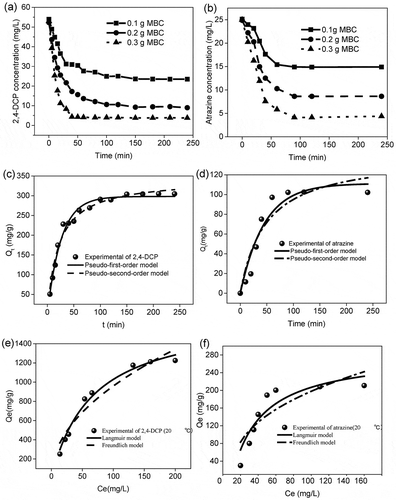
Adsorption isotherms of DCP and atrazine on MBC are shown in and . The data obtained from experiments can be fitted to two-parameter isotherm models (i.e., Langmuir and Freundlich). The relevant constants calculated from these two models are shown in Table S2. For DCP, the Langmuir model and Freundlich model both can fit the data well, which suggests the DCP adsorption is occurring on the carbon surface and in pores at the same time. For atrazine, the Langmuir model is more suitable for describing the adsorption process than the Freundlich model, and the better fitting suggests the atrazine adsorption occurring on the pores of the MBC (Li et al. Citation2017; Wei et al. Citation2016).
Mutual desorption and treatment of DCP and atrazine with alkaline molten salt
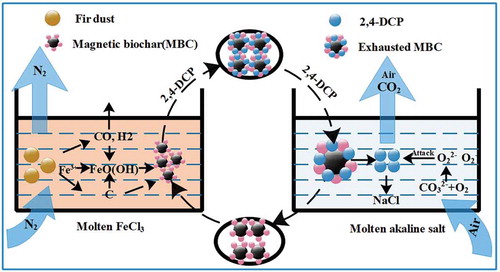
Figure S1 displays the reactor with molten salt regeneration. Typically, premixed NaOH/Na2CO3 were loaded in the reactor vessel and heated to 400ºC at 10ºC/min. After the reactor temperature reached the experiment condition, carrier gas and saturated MBC were purged into the reactor through a pipe and squirted into the molten salt. The DCP was boiled when contacting the molten salt medium, and the boiling DCP reacted with alkaline medium during escape from the system. DCP gases flowed through the molten salt medium and thus were destroyed.
shows the chlorine distribution after the destruction of exhausted MBC. In molten NaOH–Na2CO3, 56.83% and 8.67% of the Cl were detected in the washing water and residues, respectively, which meant at least 65.5% of Cl was removed from the 2,4-DCP. Based on the previous research, molten alkaline salt can be an effective and environmentally friendly medium for the organic waste destruction. Oxidation of the organic compounds is catalyzed by the salt and destruction of the organics is achieved. Hydrocarbons are converted to carbon dioxide and steam (Kovarik, Navratil, and John Citation2015). Meanwhile, acid gases cannot escape from the trap of alkaline molten medium, leading to a harmless effect on the atmosphere (eqs 9–10). However, not all elemental Cl in the adsorbent was converted to chlorine ion. Incomplete oxidation of 2,4-DCP occurred (Upadhye, Pruneda, and Watkins Citation1997). For atrazine, the DRE is much lower than 2,4-DCP, as the dechlorination rate was 31.69%, suggesting the atrazine was hard to decompose in the molten salt medium:
Table 4. The content of Cl− and pesticides in the washing water and residues with molten NaOH–Na2CO3 method.
Control experiment demonstrated that thermal treatment of SMBC can partly remove the absorbed pesticides; 43.82% and 56.38% of the 2,4-DCP and atrazine, respectively, desorbed when the temperature is 400ºC (). Molten salt is a robust and valid route for the destruction of 2,4-DCP, with it being barely detected in the washing water and residues. However, during the process, actually 10–15% (data not shown) of Fe3O4 loss may occur and the magnetic property is be weakened, according to the test. Despite the magnetic property change, this is also a promising process with several advantages: (1) The absorbed substances are desorbed and meanwhile destroyed by the alkaline molten salt. (2) The molten salt process is a versatile way to destroy the organics, especially for the organic chlorine. (3) The destruction process is an environmentally friendly one. (4) There is one step for the organics destruction and biochar reactivation.
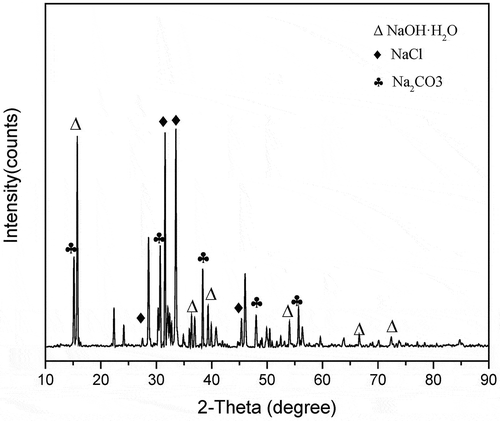
The XRD pattern of salt after reaction is shown in . It can be seen that the major crystalline phases were NaOH and Na2CO3, with NaCl detected. It can be inferred from the preceding results that a typical thermal desorption and destruction performs like this (): (1) Air (oxygen) was dissolved in the molten salt, forming an oxidizing species; (2) DCP desorbed from the MBC by the thermal process; and (3) oxidizing species attack the DCP with dissociation of C–Cl and C–O bonds and the benzene ring (Li et al. Citation2014; Yao, Li, and Zhao Citation2013). Intermediate substances are generated and may also react with oxidizing species. (4) Dechlorination reaction by the free radical chain reaction generates HCl, which promptly is taken in by alkaline melt instead of escaping from the reactor with the air flow. The retained rate of Cl is closely relevant to the retention time in molten salt. Some HCl still exhausts because of the partly nonmelting space and heterogeneous system at 400ºC. As the NaCl is generated, things may be different with a more complicated ternary salt system. Nevertheless, those cannot be determined or tested.
After reaction, the biochar was weighed and tested for the BET and fixed carbon content. The results are shown in . The MBC could be easily recovered with a yield of 98.2% and fixed carbon content of 61.0% after the molten salt regeneration process (). However, the yield decreased to 68.7% and 71.2% for DCP and atrazine, respectively, during the regeneration of saturated MBC. The fixed carbon content was also decreased in this process. Magnetic biochar may be slightly oxidized during the regeneration of saturated MBC due to its amorphous structure, so the fixed carbon content decreased. Owing to the varying adsorption capacity of MBC for DCP and atrazine, the yield, BET and pore volume were different.
Table 5. Fixed carbon content, pore volume, and relative sample yield for the samples derived in molten salt systems at 400ºC.
The regenerated MBC was tested for the adsorption performance; 298.12 mg/g and 102.17 mg/g, respectively, of the 2,4-DCP and atrazine were adsorbed with the reactivation MBC (), indicating the reserved adsorption performance for those materials, despite the magnetic property change. Almost 72.22–77.89% of the initial adsorption capacity can be kept after two to four saturation–reactivation cycles with molten salt medium. This demonstrated that the regeneration method can be an effective way to reduce the risk of secondary pollution of chlorinated organic compounds during adsorbent regeneration.
Table 6. Absorption capacity of regenerated MBC in several cycles.
Conclusion
Carbon sheet and cubic Fe3O4 were synthesized by carbonization of the fir dust in molten FeCl3∙6H2O at 450ºC. SEM showed this kind of MBC has a rough surface. With a poor porosity, the MBC has a BET surface area of 404 m2/g and a total pore volume of 0.35 cm3/g. MBC showed a great capacity for chlorine-containing pesticides adsorption, with the optimum MBC dosage being 2.0 g/L. Kinetics results indicated that MBC adsorption met the pseudo-first-order model, and the maximum adsorption capacity achieved 1609.4 mg/g. In addition, a molten salt method using molten NaOH–KOH and NaOH–Na2CO3 was performed for the regeneration of saturated MBC. The MBC could be easily recovered with a yield of 98.2% and fixed carbon content of 61.0% after the molten salt regeneration process. With contact for 120 min, the attained reactivated MBC can retain 72.22–77.89% of the initial 2,4-DCP capacity after two to four cycles. Simultaneously, 2.4-DCP steam, desorbed from the MBC, was destroyed by the molten salt medium; 65.5% and 31.69% of initial Cl was acquired together in washing water and residues with the molten NaOH–KOH and NaOH–NaCO3, respectively.
Acknowledgment
The authors are grateful to the State Key Laboratory of Pollution Control and Resource Reuse, China, which provided them with sample testing and analyzing.
Supplemental Material
Download PDF (110.2 KB)supplemental data
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Shi-jin Dai
Shi-jin Dai is a Ph.D. student in the School of Environmental Science and Engineering at Tongji University, Shanghai, China.
You-cai Zhao
You-cai Zhao is a professor of environmental engineering at the School of Environmental Science and Engineering, Tongji University, Shanghai, China.
Dong-jie Niu
Dong-jie Niu is an associate professor of environmental engineering at the School of Environmental Science and Engineering, Tongji University, Shanghai, China.
Qiang Li
Qiang Li is a senior engineer of China Everbright Greentech Limited, Shenzhen, China.
Yu Chen
Yu CHEN is a postgraduate student in the School of Environmental Science and Engineering at Tongji University, Shanghai, China.
References
- Baig, S. A., J. Zhu, N. Muhammad, T. Sheng, and X. Xu. 2014. Effect of synthesis methods on magnetic Kans grass biochar for enhanced As(III, V) adsorption from aqueous solutions. Biomass Bioenergy 71:299–310. doi:10.1016/j.biombioe.2014.09.027.
- Cao, Q., Z. Liu, and R. Che. 2014. Ordered mesoporous CoFe2O4 nanoparticles: Molten-salt-assisted rapid nanocasting synthesis and the effects of calcining heating rate. New J. Chem. 38 (7):3193–3198. doi:10.1039/c4nj00235k.
- Cho, D.-W., J. Lee, Y. S. Ok, E. E. Kwon, and H. Song. 2016. Fabrication of a novel magnetic carbon nanocomposite adsorbent via pyrolysis of sugar. Chemosphere 163:305–312. doi:10.1016/j.chemosphere.2016.08.025.
- Flandinet, L., F. Tedjar, V. Ghetta, and J. Fouletier. 2012. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J. Hazard. Mater. 213–214:485–490. doi:10.1016/j.jhazmat.2012.02.037.
- Frangini, S., and A. Masi. 2016. Molten carbonates for advanced and sustainable energy applications: Part I. Revisiting molten carbonate properties from a sustainable viewpoint. Int. J. Hydrogen Energy 41 (41):18739–18746. doi:10.1016/j.ijhydene.2015.12.073.
- Hao, X., J. Wang, B. Ding, L. Shen, Y. Xu, Y. Wang, Z. Chang, H. Dou, X. Lu, and X. Zhang. 2016. Heteroatom Doped Porous Carbon Nanosheets: General Preparation and Enhanced Capacitive Properties. Chem. 22(46):16668–16674. doi:10.1002/chem.201602922.
- Ji, G., X. Lin, Y. Sun, S. A. A. Trimizi, H. Su, and Y. Du. 2011. Molten salt growth and magnetic properties of octahedral CoFe2O4 crystals: Effects of synthesis conditions. Cryst. Eng. Comm. 13 (21):6451–6456. doi:10.1039/c1ce05459g.
- Jung, K.-W., B. H. Choi, T.-U. Jeong, and K.-H. Ahn. 2016. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. Bioresour. Technol. 220:672–676. doi:10.1016/j.biortech.2016.09.035.
- Kalinke, C., A. S. Mangrich, L. H. Marcolino-Junior, and M. F. Bergamini. 2016. Biochar prepared from castor oil cake at different temperatures: A voltammetric study applied for Pb2+, Cd2+ and Cu2+ ions preconcentration. J. Hazard. Mater. 318:526–532. doi:10.1016/j.jhazmat.2016.07.041.
- Kong, W. X., F. Zhao, H. J. Guan, Y. F. Zhao, H. S. Zhang, and B. Zhang. 2016. Highly adsorptive mesoporous carbon from biomass using molten-salt route. J. Mater. Sci. 51 (14):6793–6800. doi:10.1007/s10853-016-9966-8.
- Kovarik, P., J. D. Navratil, and J. John. 2015. Scientific and Engineering Literature Mini Review of Molten Salt Oxidation for Radioactive Waste Treatment and Organic Compound Gasification as well as Spent Salt Treatment. Sci. Technol. Nucl. Installations 2015:1-10. doi:10.1155/2015/407842.
- Li, J.-H., X.-F. Sun, Z.-T. Yao, and X.-Y. Zhao. 2014. Remediation of 1, 2, 3-trichlorobenzene contaminated soil using a combined thermal desorption–Molten salt oxidation reactor system. Chemosphere 97:125–129. doi:10.1016/j.chemosphere.2013.10.047.
- Li, R., J. J. Wang, B. Zhou, Z. Zhang, S. Liu, S. Lei, and R. Xiao. 2017. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 147:96–107. doi:10.1016/j.jclepro.2017.01.069.
- Liang, B., J. Lehmann, D. Solomon, J. Kinyangi, J. Grossman, B. O’Neill, J. O. Skjemstad, J. Thies, F. J. Luizão, and J. Petersen. 2006. Black carbon increases cation exchange capacity in soil. Soil Sci. Soc. America J. 70 (5):1719–1730. doi:10.2136/sssaj2005.0383.
- Liu, G., W.-J. Sun, S.-S. Tang, S.-Q. Liang, and J. Liu. 2015. Synthesis of alpha-Fe2O3@SnO2 core-shell nanoparticles via low-temperature molten salt reaction route. Transactions of Nonferrous Metals Society of China 25 (11):3651–3656. doi:10.1016/S1003-6326(15)64076-6.
- Liu, -T.-T., M.-H. Wang, H.-P. Zhang, and Y.-Z. Zhao. 2016a. Molten salt synthesis of doped nanocrystalline ZnO powders and applications in varistor ceramics. J. Mater. Science-Materials S 27 (4):3704–3709. doi:10.1007/s10854-015-4211-9.
- Liu, W. J., K. Tian, H. Jiang, and H. Q. Yu. 2013. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 3 (33):2419. doi:10.1038/srep02419.
- Liu, X., and M. Antonietti. 2014. Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon N. Y. 69:460–466. doi:10.1016/j.carbon.2013.12.049.
- Liu, X., J. Li, and H. Du. 2016b. Preparation and characterization of Zn 2 TiO 4 micro/nano crystals by molten salt method. J. Mater. Sci. Mater. s 27 (12):1–5. doi:10.1007/s10854-016-5474-5.
- Lobos, M. L. N., J. M. Sieben, V. Comignani, M. Duarte, M. A. Volpe, and E. L. Moyano. 2016. Biochar from pyrolysis of cellulose: An alternative catalyst support for the electro-oxidation of methanol. Int. J. Hydrogen Energy 41 (25):10695–10706. doi:10.1016/j.ijhydene.2016.04.041.
- Lu, B., Z. Xiao, H. Zhu, W. Xiao, W. Wu, and D. Wang. 2015. Enhanced capacitive properties of commercial activated carbon by re-activation in molten carbonates. J. Power. Sources 298:74–82. doi:10.1016/j.jpowsour.2015.08.047.
- Lu, B. H., L. Y. Hu, H. Y. Yin, X. H. Mao, W. Xiao, and D. H. Wang. 2016a. Preparation and application of capacitive carbon from bamboo shells by one step molten carbonates carbonization. Int. J. Hydrogen Energy 41 (41):18713–18720. doi:10.1016/j.ijhydene.2016.05.083.
- Lu, B. H., L. Y. Hu, H. Y. Yin, W. Xiao, and D. H. Wang. 2016b. One-step molten salt carbonization (MSC) of firwood biomass for capacitive carbon. RSC Adv 6 (108):106485–106490. doi:10.1039/C6RA22191B.
- Lu, B. H., J. Zhou, Y. Q. Song, H. L. Wang, W. Xiao, and D. H. Wang. 2016c. Molten-salt treatment of waste biomass for preparation of carbon with enhanced capacitive properties and electrocatalytic activity towards oxygen reduction. Faraday Discuss. 190:147–159. doi:10.1039/C5FD00215J.
- Marques, S. C. R., J. M. Marcuzzo, M. R. Baldan, A. S. Mestre, and A. P. Carvalho. 2017. Pharmaceuticals removal by activated carbons: Role of morphology on cyclic thermal regeneration. Chem. Eng. J. 321:233–244. doi:10.1016/j.cej.2017.03.101.
- Nagodavithane, C. L., B. Singh, and Y. Fang. 2014. Effect of ageing on surface charge characteristics and adsorption behaviour of cadmium and arsenate in two contrasting soils amended with biochar. Soil Res. 52 (2):155–163. doi:10.1071/SR13187.
- Oh, S. Y., and Y. D. Seo. 2016. Sorption of halogenated phenols and pharmaceuticals to biochar: Affecting factors and mechanisms. Environ. Sci. Pollut. Res. 23 (2):1–11. doi:10.1007/s11356-015-5714-x.
- Özhan, A., Ö. Şahin, M. M. Küçük, and C. Saka. 2014. Preparation and characterization of activated carbon from pine cone by microwave-induced ZnCl2 activation and its effects on the adsorption of methylene blue. Cellulose 21 (4):2457–2467. doi:10.1007/s10570-014-0299-y.
- Peng, B., L. Chen, C. Que, K. Yang, F. Deng, X. Deng, G. Shi, G. Xu, and M. Wu. 2016. Adsorption of Antibiotics on Graphene and Biochar in Aqueous Solutions Induced by π-π Interactions. Sci. Rep. 6 (6):31920. doi:10.1038/srep31920.
- Raade, J. W., and D. Padowitz. 2011. Development of molten salt heat transfer fluid with low melting point and high thermal stability. J. Solar Energy Eng. 133 (3):031013. doi:10.1115/1.4004243.
- Reddy, M. V., S. Adams, G. T. J. Liang, I. F. Mingze, T. A. Huynh Van, and B. V. R. Chowdari. 2014a. Low temperature molten salt synthesis of anatase TiO2 and its electrochemical properties. Solid State Ionics 262:120–123. doi:10.1016/j.ssi.2013.11.030.
- Reddy, M. V., C. T. Cherian, K. Ramanathan, K. C. W. Jie, T. Y. W. Daryl, T. Y. Hao, S. Adams, K. P. Loh, and B. V. R. Chowdari. 2014b. Molten synthesis of ZnO.Fe 3 O 4 and Fe 2 O 3 and its electrochemical performance. Electrochim. Acta 118:75–80. doi:10.1016/j.electacta.2013.11.125.
- Reddy, M. V., V. H. Khai, and B. V. R. Chowdari. 2015a. Facile one pot molten salt synthesis of nano (M 1/2 Sb 1/2 Sn)O 4 (M=V, Fe, In). Mater. Lett. 140:115–118. doi:10.1016/j.matlet.2014.10.145.
- Reddy, M. V., G. V. S. Rao, and B. V. R. Chowdari. 2013. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 113 (7):5364–5457. doi:10.1021/cr3001884.
- Reddy, M. V., L. Y. Tse, K. Z. B. Wen, and B. V. R. Chowdari. 2015b. Low temperature molten salt preparation of nano-SnO2 as anode for lithium-ion batteries. Mater. Lett. 138:231–234. doi:10.1016/j.matlet.2014.09.108.
- Reguyal, F., A. K. Sarmah, and W. Gao. 2016. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 321::868–878. doi:10.1016/j.jhazmat.2016.10.006.
- Saravanan, P., V. T. P. Vinod, B. Sreedhar, and R. B. Sashidhar. 2012. Gum kondagogu modified magnetic nano-adsorbent: An efficient protocol for removal of various toxic metal ions. Mater. Sci. Eng. C 32 (3):581–586. doi:10.1016/j.msec.2011.12.015.
- Selvamani, S., S. Balamurugan, and S. V. Sreenija. 2016. Facile Synthesis of Nanocrystalline CuFe2O4 Materials by Molten Salt Flux Method. In Dae Solid State Physics Symposium 2015, eds. R. Chitra, S. Bhattacharya, and N. K. Sahoo, Vol. 1731, 597–603. Melville, NY: AIP Publishing LLC.
- Shan, D., S. Deng, T. Zhao, B. Wang, Y. Wang, J. Huang, G. Yu, J. Winglee, and M. R. Wiesner. 2016. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 305:156–163. doi:10.1016/j.jhazmat.2015.11.047.
- Shang, C., G. Ji, W. Liu, X. Zhang, H. Lv, and Y. Du. 2015a. One-pot in situ molten salt synthesis of octahedral Fe3O4 for efficient microwave absorption application. RSC Adv 5 (98):80450–80456. doi:10.1039/C5RA15949K.
- Shang, H. S., Y. J. Lu, F. Zhao, C. Chao, B. Zhang, and H. S. Zhang. 2015b. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv 5 (92):75728–75734. doi:10.1039/C5RA12406A.
- Thines, K. R., E. C. Abdullah, N. M. Mubarak, and M. Ruthiraan. 2017. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renewable Sustainable Energy Reviews 67:257–276. doi:10.1016/j.rser.2016.09.057.
- Upadhye, R. S., C. O. Pruneda, and B. E. Watkins 1997. Recent advances in the molten salt technology for the destruction of energetic materials. Proceedings of the 1997 Air & Waste Management Association’s 90th Annual Meeting & Exhibition, June 8, 1997 - June 13, 1997, Toronto, Can. Air & Waste Management Assoc.
- Wang, L., X. Jiang, and Y. Liu. 2008. Degradation of bisphenol A and formation of hydrogen peroxide induced by glow discharge plasma in aqueous solutions. J. Hazard. Mater 154 (1–3):1106–1114. doi:10.1016/j.jhazmat.2007.11.016.
- Wang, W., L. Liu, Y. Ma, and W. Wang. 2014. Synthesis and optical property of plate-like ZnO nanocrystals prepared by a NaCl molten salt method. New Chem. Mater. 42 (6):135–137.
- Wei, D., H. H. Ngo, W. Guo, W. Xu, Y. Zhang, B. Du, and Q. Wei. 2016. Biosorption of effluent organic matter onto magnetic biochar composite: Behavior of fluorescent components and their binding properties. Bioresour. Technol. 214:259–265. doi:10.1016/j.biortech.2016.04.109.
- Xia, G., N. Li, D. Li, R. Liu, N. Xiao, and D. Tian. 2011. Molten-salt decomposition synthesis of SnO2 nanoparticles as anode materials for lithium ion batteries. Mater. Lett. 65(23–24):3377–3379. doi:10.1016/j.matlet.2011.07.008.
- Yang, F., L. Sun, W. Zhang, and Y. Zhang. 2017. One-pot synthesis of porous carbon foam derived from corn straw: Atrazine adsorption equilibrium and kinetics. Environ. Sci. Nano 4(3):625–635. doi:10.1039/C6EN00574H.
- Yang, J., Y. Zhao, S. Ma, B. Zhu, J. Y. Zhang, and C. Zheng. 2016. Mercury removal by magnetic biochar derived from simultaneous activation and magnetization of sawdust. Environ. Sci. Technol. 50(21):12040–12047. doi:10.1021/acs.est.6b03743.
- Yao, Z., J. Li, and X. Zhao. 2011. Molten salt oxidation: A versatile and promising technology for the destruction of organic-containing wastes. Chemosphere 84 (9):1167–1174. doi:10.1016/j.chemosphere.2011.05.061.
- Yao, Z.-T., J.-H. Li, and X.-Y. Zhao. 2013. Destruction of decabromodiphenyl ether (BDE-209) in a ternary carbonate molten salt reactor. J. Environ. Manage. 127:244–248. doi:10.1016/j.jenvman.2013.04.040.
- Yin, H., B. Lu, Y. Xu, D. Tang, X. Mao, W. Xiao, D. Wang, and A. N. Alshawabkeh. 2014. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Sci. Technol. 48 (14):8101–8108. doi:10.1021/es501739v.
- Yu, J. X., L. Y. Wang, R. A. Chi, Y. F. Zhang, Z. G. Xu, and J. Guo. 2013. Competitive adsorption of Pb 2+ and Cd 2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl. Surf. Sci. 268 (268):163–170. doi:10.1016/j.apsusc.2012.12.047.
- Zhang, Q. Z., X. H. Wang, Z. L. Du, X. R. Liu, and Y. D. Wang. 2013. Impact of biochar on nitrate accumulation in an alkaline soil. Soil Res. 51 (6):521–528. doi:10.1071/SR13153.
- Zhao, H., S. Yang, H. You, Y. Wu, and B. Ding. 2012. Synthesis of surfactant-free Pt concave nanoparticles in a freshly-made or recycled molten salt. Green Chem. 14 (11):3197–3203. doi:10.1039/c2gc35995b.