 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An ozone abatement strategy for the South Coast Air Basin (SoCAB) has been proposed by the South Coast Air Quality Management District (SCAQMD) and the California Air Resources Board (ARB). The proposed emissions reduction strategy is focused on the reduction of nitrogen oxide (NOx) emissions by the year 2030. Two high PM2.5 concentration episodes with high ammonium nitrate compositions occurring during September and November 2008 were simulated with the Community Multi-scale Air Quality model (CMAQ). All simulations were made with same meteorological files provided by the SCAQMD to allow them to be more directly compared with their previous modeling studies. Although there was an overall under-prediction bias, the CMAQ simulations were within an overall normalized mean error of 50%; a range that is considered acceptable performance for PM modeling. A range of simulations of these episodes were made to evaluate sensitivity to NOx and ammonia emissions inputs for the future year 2030. It was found that the current ozone control strategy will reduce daily average PM2.5 concentrations. However, the targeted NOx reductions for ozone were not found to be optimal for reducing PM2.5 concentrations. Ammonia emission reductions reduced PM2.5 and this might be considered as part of a PM2.5 control strategy.
Implications: The SCAQMD and the ARB have proposed an ozone abatement strategy for the SoCAB that focuses on NOx emission reductions. Their strategy will affect both ozone and PM2.5. Two episodes that occurred during September and November 2008 with high PM2.5 concentrations and high ammonium nitrate composition were selected for simulation with different levels of nitrogen oxide and ammonia emissions for the future year 2030. It was found that the ozone control strategy will reduce maximum daily average PM2.5 concentrations but its effect on PM2.5 concentrations is not optimal.
Introduction
There have been several modeling studies of ozone (O3) and particulate matter (PM) conducted for the South Coast Air Basin (SoCAB; e.g., Chen et al. Citation2013; Kelly et al. Citation2014). In contrast to these studies, the objective of the research presented here is to evaluate the effect of an ozone control strategy on particulate matter (PM) concentrations for the SoCAB.
The South Coast Air Quality Management District (SCAQMD) has set emission reduction goals for volatile organic compounds (VOC) and nitrogen oxides (NOx = NO + NO2) between 2008 and 2030 with the objective of reaching compliance with federal ozone standards in the SoCAB. The SCAQMD Air Quality Management Plan (South Coast Air Quality Management District [SCAQMD] Citation2013; Citation2017) is very focused on NOx emission reductions. Fujita et al. (Citation2016) used the Community Multi-scale Air Quality model (CMAQ; Binkowski Citation1999; Binkowski and Shankar Citation1995; Byun and Ching Citation1999; Byun and Schere Citation2006) to simulate the response of ozone concentrations to the planned emission reductions in the SoCAB between the base year 2008 and the future year 2030 (Fujita et al. Citation2016). In contrast to the SCAQMD Plan (Citation2013), Fujita et al. found that an NOx-focused ozone abatement strategy will cause higher ozone concentrations in the western and central SoCAB over a prolonged time until very large NOx reductions are achieved.
Although the SCAQMD Air Quality Management Plan (SCAQMD Citation2013; Citation2017) focuses on ozone abatement through NOx reductions, this strategy could affect the concentrations of other air pollutants such as PM. Fujita et al. (Citation2016) did not examine the consequences of the emission reductions on PM because this was beyond their scope. This study is an extension of Fujita et al. (Citation2016) with its purpose to examine the effects of the SCAQMD ozone abatement plan on PM concentrations. Given that this study extends Fujita et al. (Citation2016), a resulting limitation is that we focus more deeply on the SCAQMD (Citation2013) plan rather than the updated SCAQMD (Citation2017) plan.
PM may be grouped into two categories, primary PM and secondary PM. Both of these categories include fine particulate matter with an aerodynamic diameter of 2.5 µm or less (PM2.5). Primary PM is directly emitted into the atmosphere by emission sources. Primary PM includes black or elemental carbon, soot, and fine particles formed by combustion processes, most coarse PM (with diameters greater than 10 μm; PM10), windblown crustal material, fugitive dust, and similar substances.
Primary PM concentrations are not very likely to be directly affected by changes in VOC and NOx emissions. The lack of a direct relationship between primary PM concentrations and the emissions of VOC and NOx makes a detailed analysis of primary PM changes beyond the scope of this research project. However, changes in direct PM emissions between 2008 and 2030 are expected (SCAQMD 2013, 2017). These changes to primary PM emissions were implemented in the emissions inventories used for the CMAQ modeling presented here.
Secondary PM is the component of PM that is formed in the atmosphere through chemical reactions. The formation of secondary PM is a highly nonlinear function of VOC and NOx emissions. The chemistry that produces secondary PM is strongly coupled with the gas-phase chemistry of ozone formation because the oxidation products of NOx, sulfur dioxide (SO2), and VOC include nitric acid (HNO3), sulfuric acid (H2SO4), and other products with low equilibrium vapor pressures that can partition into the aerosol phase to yield PM (Calvert et al. Citation2015). The atmospheric acids HNO3 and H2SO4 react with ammonia (NH3) to produce the PM2.5 components ammonium nitrate (NH4NO3), ammonium bisulfate (NH4HSO4), and ammonium sulfate ((NH4)2SO4), if sufficient NH3 is present. These compounds are produced through oxidation reactions involving the hydroxyl radical (HO) that is central also to the chemistry of ozone formation. The composition of VOC is important because different organic compounds have different reactivity for producing ozone. Reductions in VOC and NOx emissions affect the formation of other secondary PM components such as secondary organic aerosol (SOA). The gas-phase chemical production of PM from precursor emissions yields smaller particles that contribute to PM2.5. PM2.5 is of particular concern because fine particles have deleterious effects on health when inhaled (Brunekreef and Holgate Citation2002; Cohen et al. Citation2017; Grahame, Klemm, and Schlesinger Citation2014; Sicard et al. Citation2010; Stewart et al. Citation2017).
A reasonable expectation is that reductions in NOx emissions would reduce the formation of secondary NH4NO3 aerosol. However, reductions in VOC or NOx emissions do not always lead to lower ozone levels in the SoCAB (Fujita et al. Citation2016), and the same could be true for PM. For these reasons the Community Multi-scale Air Quality (CMAQ), a comprehensive air quality model, was used to evaluate the effects of the proposed ozone abatement strategy on PM2.5.
In this paper we present an evaluation of trends in PM2.5 concentrations and the components nitrate, sulfate, organic carbon (OC), and elemental carbon (EC) for the SoCAB. Two high PM2.5 episodes with high NH4NO3 composition were selected for CMAQ modeling based on the examination of the PM measurements for the year 2008. The two 2008 episodes were simulated by CMAQ and a modeling evaluation was performed. For the 2008 episodes the effect of VOC emission inventory increases on secondary PM2.5 were simulated. Then the effect of six NOx and four ammonia (NH3) emission reduction scenarios on PM2.5 were simulated for the future year 2030. Finally, the simulation results and their implications for the effect of the proposed ozone control strategy on PM2.5 are presented.
Measurements and trends in PM2.5 concentrations over the SoCAB
The high level of emissions due to the SoCAB’s population, its location by the Pacific Ocean, and its surrounding mountain topography make it difficult for air quality standards to be attained for ozone and PM. There is a population of 17.8 million people living in an area of 10,743 square miles in the SoCAB (Fujita et al. Citation2016). PM measurements made at ambient air monitoring sites operating in the SoCAB were used to update PM trends, to identify high PM episodes for CMAQ modeling, and to evaluate CMAQ model performance. Six ambient air monitoring sites operating in the SoCAB reported daily mass concentrations of PM2.5 in 2008, with four of these stations reporting chemical speciation data as shown in .
Figure 1. Map of the South Coast Air Basin with approximate locations of the PM2.5 monitoring sites that reported daily mass concentration in 2008 and were used in this study. Filled dots indicate sites that also reported chemical speciation data.
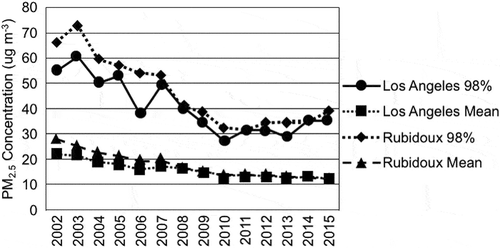
Trends in the annual mean and 98th percentile of daily PM2.5 concentrations at the Central Los Angeles site (CELA) and Rubidoux (RUBI) sites are shown in . The daily PM2.5 concentrations decreased between 2002 and 2010 at Los Angeles and Rubidoux. Following 2010, the PM2.5 concentrations level off and may have increased slightly by 2015 at Los Angeles, while at Rubidoux the PM2.5 concentrations remained almost constant between 2010 and 2015.
Figure 2. Trend in annual mean and 98th percentile of daily PM2.5 concentrations at the Central Los Angeles and Rubidoux sites.
Trends in chemical constituents of PM, organic carbon, elemental carbon, nitrate, and sulfate were examined. Nitrate is the chemical component with the greatest concentrations that is most likely affected by changes in VOC and NOx emissions, as discussed in the Results section of this paper. The annual mean nitrate concentrations at the Rubidoux site were greater than those at the Los Angeles site between 2003 and 2014, as shown in . The annual mean average nitrate concentrations at Rubidoux were near 12 μg m−3, while at Central Los Angeles they were near 6.5 μg m−3 during 2002. The annual mean nitrate concentration decreased at both sites after 2002 and the nitrate concentrations were near 4 μg m−3 at both Rubidoux and Central Los Angeles during 2014.
Figure 3. Trends in nitrate concentrations at the Central Los Angeles and Rubidoux sites are shown. The top plot shows the trend in the annual mean average, while the lower plot shows the nitrate contribution to the NAAQS PM2.5 design value.
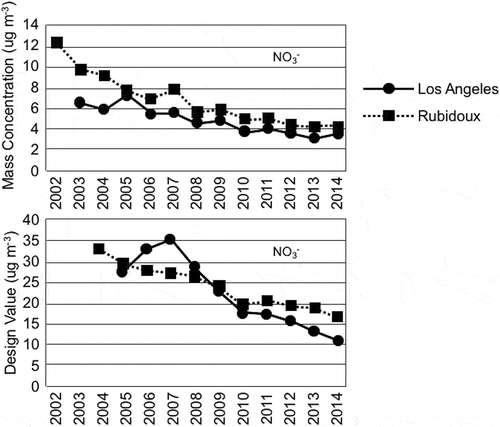
The nitrate “design values” (analogous to the design value for ozone, this is the 3-year running average of the annual 98th percentile of daily mean PM concentrations) at the two sites had similar magnitudes. The nitrate design values were in the range of 30 to near 35 μg m−3 at both sites near the years 2004 to 2006. The design value at the Los Angeles site increased initially and then fell from 2007 to 2014, while at the Rubidoux site the design value fell more steadily during this time. The design value at Los Angeles decreased to 37% of its initial value and the design value at Rubidoux decreased to 50% of its initial value. The nitrate design value was near 17 μg m−3 at Rubidoux and near 10 μg m−3 at Central Los Angeles during 2014.
The annual mean sulfate concentrations are significantly lower than the annual mean nitrate concentrations at Rubidoux and the Central Los Angeles sites, as shown in . The annual mean sulfate concentrations were greater at the Central Los Angeles site than at the Rubidoux site. The mean sulfate concentrations at Central Los Angeles ranged from 3 to 4.5 μg m−3 during the early 2000s, while at Rubidoux they ranged from near 2.5 to 3.5 μg m−3 during this same time period. There was an increase in the annual mean sulfate concentration at Central Los Angeles during 2005, but there was a decrease to 44% of its initial value between 2002 and 2010. At the Rubidoux site there was a decrease in the annual mean sulfate concentration to 40% of its initial value between 2002 and 2010. By 2010 the annual mean sulfate concentration decreased to a range of 1.5 to 2.0 μg m−3 at both sites. There was little change in the sulfate concentrations at both sites between 2010 and 2014.
Figure 4. Trends in sulfate concentrations at the Central Los Angeles and Rubidoux sites are shown. The top plot shows the trend in the annual mean average, while the lower plot shows the sulfate contribution to the NAAQS PM2.5 design value.
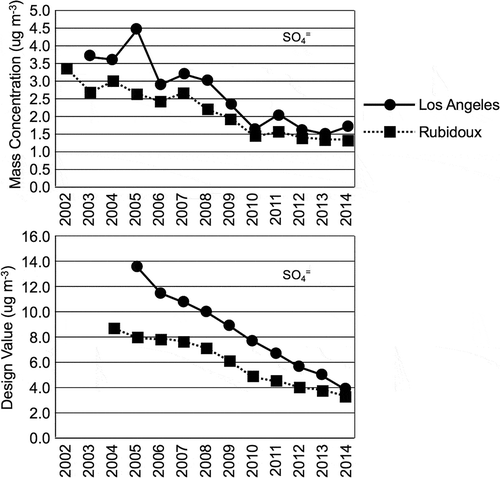
The sulfate design values were 8.5 μg m−3 at Rubidoux during 2004 and 13.5 μg m−3 at Central Los Angeles during 2005. The sulfate design values decreased steadily between 2004 and 2014 at both sites. The decrease was greatest at Los Angeles, decreasing to 27% of its initial value, compared with 39% at Rubidoux. The sulfate design values fell to near 4 μg m−3 at both sites by 2014. These comparisons show that the nitrate component of the aerosol is significantly larger than the sulfate component.
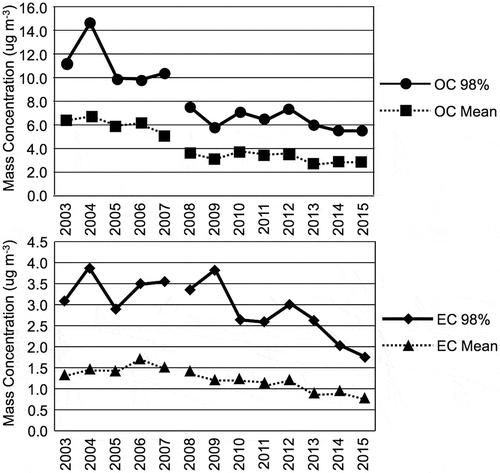
It’s more difficult to assess the relative significance of the OC and EC components of PM2.5 found in the SoCAB relative to nitrate and sulfate. shows that OC at the Central Los Angeles site appears to decrease significantly from over 6 to 3 μg m−3 while the EC drops from a peak near 1.6 to 0.75 μg m−3. However, the apparent drops in OC and EC are more likely due to changes in measurement methods. The figure shows data from the Chemical Speciation Network (CSN). Changes were made to the CSN protocol for the measurement of OC and EC during 2007. The goal was to make CSN measurements more consistent with the U.S. Interagency Monitoring of PROtected Visual Environments (IMPROVE) network. The IMPROVE network implemented a new temperature protocol for its thermal methods used to measure OC and EC (Chow et al. Citation2007). Spada and Hyslop (Citation2018) present a comparison of OC and EC measurements from the two networks using measurements made before and after the protocol changes. Spada and Hyslop found that the CSN protocol changes increased its measured EC and decreased measured OC mass concentrations. Therefore, it’s very likely that there has been little change in EC and OC concentrations at the Central Los Angeles site between 2003 and 2015. This data analysis shows that overall the EC and OC PM2.5 components are not as large as the nitrate components.
Figure 5. Trend in annual mean and 98th percentile of CSN protocol organic and elemental carbon concentrations at the Central Los Angeles site. A change in the analysis method for carbon fractions was implemented in 2007, so data collected before and after 2007 may not be fully comparable.
Selection of episodes for the 2008 base year
The episodes selected for modeling had PM2.5 concentrations with high nitrate particulate fractions during the 2008 base year. It was required that the selected 2008 episodes have chemically speciated data for model evaluation. The episodes chosen for modeling included two of the highest PM concentrations during the 2008 aerosol season. The two selected episodes had high particulate nitrate concentrations as well. This selection procedure is analogous with previously accepted EPA State Implementation Plan (SIP) modeling protocols for ozone and with other PM modeling studies (e.g. Kleeman, Ying, and Kaduwela Citation2005; Nguyen and Dabdub Citation2002). Some PM modeling studies have investigated continental domains with simulations of 1-year duration (Makar et al. Citation2009), and this seems necessary if the effects of very long-range transport of precursors are to be investigated, but this is not relevant for our SoCAB simulations.
Ozone SIP modeling is not usually made for average days; instead, highly polluted episodes are chosen, and this same strategy has been followed in this work. Typically for an ozone SIP, a field study is conducted, and intensive measurements are made during highly polluted events and then these are modeled. Events that are atypical, such as episodes influenced by nearby wildfires, are excluded from the process. The two episodes were modeled and analysis of these simulations showed that both led to similar conclusions. This suggests that our methodology is robust.
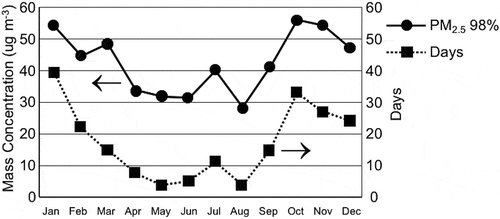
The highest daily PM2.5 concentrations usually occur during the fall and winter seasons in the SoCAB. shows daily PM2.5 concentration data statistics by month for the years between 2002 and 2015 at the Central Los Angeles site. The majority of high daily PM2.5 concentrations occurred between September and February for these years.
Figure 6. Daily PM2.5 concentration data by month for the years between 2002 and 2015 are shown for the Central Los Angeles site. Plotted are the 98th percentiles of the PM2.5 concentrations (upper lines) and the number of days during the time period when the PM2.5 concentrations were greater than 35 μg m−3 (lower line).
The highest two PM levels during 2008 were found to occur during the fall and winter seasons in the SoCAB. The first episode occurred from Friday September 12 to Monday September 15 with the greatest PM concentration on the 13th. The second episode occurred from Tuesday November 11 to Monday November 24. These two episodes are shown in and and, based on the available speciation data, they were driven by high nitrate (NO3−) concentrations. These two episodes were very appropriate for this modeling study because nitrate is a secondary aerosol pollutant.
Figure 7. Top panel presents a time-series plot of daily mean PM2.5 concentrations for September 2008. The dashed-line box signifies the period selected for modeling. Lower panel presents a time-series plot of daily mean PM2.5 concentrations for November 2008. The dashed-line box signifies the period selected for modeling.
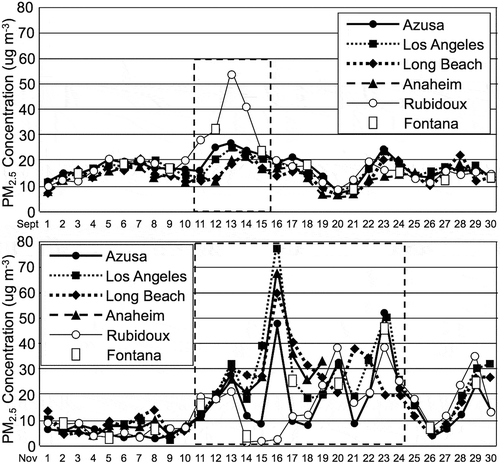
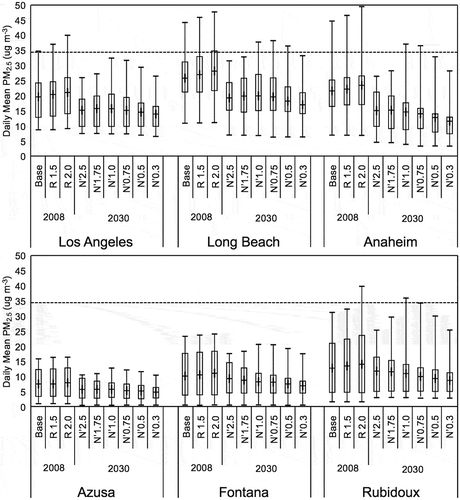
Figure 8. Range of simulated daily mean mass of PM2.5 at six monitoring sites in the SoCAB. The adjustments to VOC emissions were made for 2008 and the adjustments to NOx emissions were made for 2030. The box and whisker plots indicate the minimum, first and third quantile, maximum, and mean (+) values during two high PM2.5 episodes, September 12–15, 2008, and November 11–24, 2008. The red line shows the 35 μg m−3 NAAQS standard.
For the September episode Rubidoux had the highest PM2.5 concentrations. The PM2.5 concentration was over 50 μg m−3 on September 13, 2008, at that site. At the other stations the PM2.5 concentrations were greater than 20 μg m−3 during the period chosen for modeling. The PM2.5 concentrations were greater during the November episode. The PM2.5 concentrations reached 80 μg m−3 at the Central Los Angeles site and at or above 50 μg m−3 at the other sites except for Rubidoux on November 16, 2008. Rubidoux became the site with the greatest PM2.5 concentrations on November 20, 2008. On November 23, 2008, Azusa became the site with the highest PM2.5 concentration, with Central Los Angeles and Fontana nearly as high.
A January 2008 episode was eliminated due to the lack of available speciated PM2.5 data due to the every-sixth-day PM monitoring schedule used at most measurement sites. There was another high PM2.5 episode that occurred during the ozone season, from Friday July 4, 2008, to Monday July 7, 2008. This episode was not selected because it was driven by higher OC concentrations due to the holiday weekend activities and not by NH4NO3.
Modeling methodology
The Community Multi-scale Air Quality model (CMAQ; (Binkowski Citation1999; Binkowski and Shankar Citation1995; Byun and Ching Citation1999; Byun and Schere Citation2006) was used to simulate the selected September and November episodes. Eighteen days were simulated (September 12–15, 2008, and November 11–24, 2008). Meteorological, emission data and CMAQ input files were obtained from the SCAQMD to simulate the episodes. The 2008 CMAQ simulated PM values were compared with available measurements to evaluate the model’s performance. New CMAQ sensitivity simulations for these two episodes were for the 2030 future year with the same meteorology but with different levels of NOx, VOC, and NH3 emissions.
SCAQMD modeling protocol was followed strictly to allow our results to be compared with theirs. The 2008 meteorology, provided by the SCAQMD, was used for simulations of the base year and the 2030 future year. The SCAQMD provided meteorology that was used to make these simulations more comparable with SCAQMD simulations. The version of CMAQ used by the SCAQMD and in this project was CMAQ 4.7. The modeling grid had a spatial resolution of 4 × 4 km. The gas-phase chemical mechanism was SAPRC99; the aerosol module was CMAQ 5 with the saprc99_ae5_aq aqueous chemical mechanism. CMAQ script codes provided by the SCAQMD were modified to allow CMAQ to run in parallel on 96 processors. For initialization of the multiday simulations, CMAQ profile files were used and two days were simulated as initialization spin-up days before each simulation. For successive days, CMAQ output files were used to initialize the next day. Appendix V (Modeling and Attainment Demonstrations) of the 2012 AQMP (SCAQM, Citation2013) presents more details of the modeling procedures followed by SCAQMD. In Fujita et al. (Citation2016) our CMAQ modeling simulations of 91 simulated days for summer 2008 were compared with SCAQMD simulations and we successfully reproduced their results. Therefore, the methodology used here, including the spin-up procedure, is equivalent to the modeling procedure used by SCAQMD.
A series of CMAQ simulations was performed with adjustments made to the emissions of NOx, VOC, and NH3 to investigate the effects of these emissions changes on ozone and PM2.5. The effects of these emission changes on O3 were presented by Fujita et al. (Citation2016) and are not repeated here. shows a summary of NOx, VOC, and NH3 emissions in the SoCAB for 2008 and those projected for 2030. The adjustments were made by multiplying the 2008 and the 2030 emissions by the factors shown in . VOC emissions for 2008 were adjusted by the multiplicative factors of 1.0, 1.5, and 2.0 (Fujita et al. Citation2016). The adjustments to VOC emissions were made to the 2008 base-year emissions to examine the effects of an underestimate in VOC emissions on PM2.5 concentrations. The primary PM emissions for the 2008 simulations were kept at 2008 levels, 80 tpd (tons per day; SCAQMD Citation2013) and were not changed between the 2008 simulations with differing VOC emissions. Likewise, the primary PM emissions for the 2030 simulations were kept at 2030 levels, 75 tpd (SCAQMD Citation2013), and were not changed between the 2030 simulations with differing NOx emissions. This procedure for the primary PM emissions helped ensure that any changes in the PM2.5 simulations within the 2008 series were due to the changes in the VOC inventory only and any changes in the PM2.5 simulations within the 2030 series were due to the changes in the NOx inventory only.
Table 1. The 2008 and 2030 model simulations with baseline total VOC, NOx, and NH3 emissions and sensitivity cases with varying adjustments to baseline emissions. The VOC/NOx ratios are given in units of moles carbon/moles nitrogen.
The effect of changes in NOx emissions on PM2.5 concentrations was examined by multiplying the NOx emission inventory for 2030 by the adjustment factors of 2.5, 1.75, 1.0, 0.75, 0.5, and 0.3, as shown in (Fujita et al. Citation2016). These emission multipliers correspond to reductions from the 2008 base year of 2%, 31%, 61%, 70%, 81%, and 88%, respectively. The 88% reduction in the NOx emission inventory brings these emissions down to 85 tpd, which is below the SCAQMD and CARB estimates of the reduction required to reach ozone attainment of the U.S. National Ambient Air Quality Standards (NAAQS; Fujita et al. Citation2016).
CMAQ simulations were made for each of the NOx adjusted inventories. This allowed SCAQMD’s NOx-focused reduction strategy to be evaluated. Direct emissions of primary PM are somewhat reduced between the 2008 and the 2030 inventories, and this effect was included in the CMAQ modeling. Finally, to examine the effects of the NH3 emissions inventory on PM2.5 concentrations the 2030 NH3 emissions were multiplied by the factors given in and CMAQ simulations were performed. Greater detail on the modeling methodology may be found in Stewart (Citation2017) and Stockwell et al. (Citation2017).
Results
CMAQ model evaluation for the 2008 September and November episodes
CMAQ air quality simulations for the September and November 2008 base year high PM episodes were evaluated. There are published methodologies for model performance evaluation of PM simulations (e.g. Eder and Yu Citation2006; Yu et al., Citation2018), which were used in this study. In general, air quality model performance for simulating PM2.5 is not as high as it is for simulating ozone due to the limited current state of the science for measuring and modeling PM.
Our goal was to follow SCAQMD modeling protocols to allow our simulations to make our study more comparable with theirs, and therefore major changes to their modeling protocol to improve model performance were not made. Our PM episodic modeling evaluation is consistent with longer term statistics (SCAQMD Citation2013). The analysis showed that CMAQ underpredicted PM2.5 mass concentrations and the shared variance between the observed concentrations and the simulated concentrations was not extremely high.
Comparison of time series of observed and modeled PM2.5 concentrations for the two high PM2.5 episodes, September 12–15 and November 11–24, 2008, showed that during the September 12–15 episode PM2.5 was relatively well modeled for the Los Angeles, Long Beach, and Anaheim sites but the modeled PM2.5 was low at the other sites. During the November 11–24 episode the CMAQ-modeled PM2.5 was usually lower that the measurements and several maxima were missed (Stockwell et al. Citation2017).
A number of statistics were calculated for measured and simulated daily average PM2.5 mass concentrations (Eder and Yu Citation2006). The statistics used here are mean bias (MB), normalized mean bias (NMB), root mean square error (RMSE), normalized mean error (NME), unpaired peak ratio (UPRR), paired mean normalized gross error (PMNGE) and paired mean normalized bias (PMNB). These statistics are commonly used to evaluate air quality models. The statistics were calculated for each of the six stations individually and for all stations together.
Overall for the CMAQ simulations of the 2008 base year the mean bias was −8.87 μg m−3, the normalized mean bias was −34.53%, and the normalized mean error was 47.25%. shows that CMAQ underpredicted PM2.5 mass concentrations at all the sites. The underprediction was least at Long Beach and the site with the second least underprediction was Anaheim. The statistics show that it is difficult to simulate PM and there is no generally accepted statistic for acceptable model performance for PM. Eder and Yu (Citation2006) suggest that an NME within 50% is reasonable performance for PM. This metric is met at Los Angeles, Long Beach, Anaheim, and Rubidoux but it is not met at Azusa and Fontana. However, the performance statistics and time-series plots of PM2.5 mass concentrations and its components at a number of sites give some confidence that CMAQ performed well enough for a weight-of-evidence argument (U.S. EPA Citation2007), in that the conclusions drawn from our two episodes and multiple measurements will be seen to reinforce each other.
Table 2. Statistics for the CMAQ simulated PM2.5 mass concentrations: mean bias (MB), normalized mean bias (NMB), root mean square error (RMSE), normalized mean error (NME), unpaired peak ratio (UPR), paired mean normalized gross error (PMNGE), and mean paired normalized bias (MPNB).
Simulated Differences in Primary PM, Organic Carbon and Elemental Carbon Concentrations Due to Changes in Emission Inventories between 2008 and 2030
The projected changes between 2008 and 2030 in primary PM emissions do not have a strong effect on CMAQ simulated PM concentrations in the SoCAB. shows the results of CMAQ simulations of organic matter (OM), EC, unknown PM, and unspecified anthropogenic mass for 2008 (1R1N simulation) and for 2030 (1R’1N’ simulation). Given that the concentrations of OM and EC are due to primary emissions, it can be inferred from that primary PM2.5 emissions do not change by a large amount between 2008 and 2030. However, there is a small decrease in OM and EC. This decrease in OM and EC is caused presumably by projected decreases in mobile source emissions (particularly light-duty vehicles). The increase in unspecified anthropogenic mass (UAM) is similar in magnitude to the total of the decrease in primary OM and EC.
Table 3. Differences between 2008 and 2030 base case predictions of primary PM, organic carbon, and elemental carbon concentrations at Rubidoux on November 20 for the 2008 and the 2030 future-year simulations.
The daily average coarse aerosol mass concentrations are only slightly affected by changes in VOC emissions for the base year. At Rubidoux for 2030, the daily average coarse aerosol mass concentrations are higher than for 2008 (Stockwell et al. Citation2017). At the other sites the daily average coarse aerosol mass concentrations show a slight dependence on the NOx emissions and tend to decrease with lower NOx emissions.
The daily average total PM2.5 OM concentrations showed a slight increase with increases in basin-wide VOC emissions for 2008 (Stockwell et al. Citation2017). The projected reductions in emissions reduce the total PM2.5 OM concentrations for the 2030 future year, but variations in the future-year NOx emissions have little effect on these concentrations. Daily average total EC concentrations showed behavior similar to that of OM. Daily average total EC concentrations decreased between the 2008 and 2030 simulations, while variations in the future-year NOx emissions showed little effect on EC (Stockwell et al. Citation2017).
Evaluation of the response of PM2.5 concentrations to VOC and NOx emission changes
shows plots of the range of simulated daily mean mass concentrations of PM2.5 at six SoCAB monitoring sites. Increasing the VOC emissions for 2008 leads to increases in the extrema and mean PM2.5 concentrations at Central Los Angeles, Long Beach, Anaheim, and Rubidoux, but the extrema and mean PM2.5 concentrations at Azusa and Fontana are not greatly affected by increases in VOC emission levels. Given that our CMAQ simulations yielded a negative bias, this increase is a marginal improvement in model performance. However, the effect of increasing the VOC on CMAQ performance for 24-hr PM2.5 is not as striking as it was for ozone (Fujita et al. Citation2016).
also shows that for 2030 the maxima and the mean PM2.5 mass concentrations are reduced from 2008 for all of the NOx reduction cases, but for the stations at Long Beach, Anaheim, and Rubidoux, the maximum PM2.5 daily mean mass concentrations remain above the NAAQS standard with the target N’1.0 NOx emissions. The 2030 maximum PM2.5 daily mean mass concentrations are highest for the N’1.0 case at Central Los Angeles, Anaheim, Azusa, Fontana, and Rubidoux. There is a slight increase between the maxima for the N’1 and N’0.75 cases at Long Beach.
The mean values of the mean PM2.5 mass concentrations are less sensitive to NOx emissions levels than the maximum values. The mean values show similar responses to NOx emission reductions at Central Los Angeles and Long Beach; at these two stations decreasing NOx emissions from N’2.5 down to N’1.0 increases mean PM2.5 mass concentrations and further NOx emission reductions reduce the concentrations. At Anaheim the mean PM2.5 mass concentrations are little reduced until NOx emissions are reduced down to the target N’1.0 simulation and decrease further with greater NOx reductions, while at Fontana and Rubidoux the response of the mean is more linear to greater NOx reductions. shows that the mean PM2.5 mass concentrations are not greatly affected by NOx reductions.
The response of simulated daily mean mass concentrations of PM2.5 and that for particulate nitrate are similar. shows the range of simulated daily average total NH4NO3 mass concentrations during the two modeled PM episodes for various adjustments to the VOC and NOx emissions. In the particulate NH4NO3 concentration was estimated from the sum of CMAQ Aitken and accumulation mode aerosol nitrate, assuming it had all converted to NH4NO3. The daily average NH4NO3 mass concentrations increase as VOC emissions are increased for 2008, with the greatest increases occurring at Los Angeles, Long Beach, and Anaheim.
Figure 9. Range of simulated daily average total ammonium nitrate mass concentrations for various adjustments to VOC and NOx emissions at six monitoring sites in the SoCAB. The adjustments to VOC emissions were made for 2008 and the adjustments to NOx emissions were made for 2030. The box and whisker plots indicate the minimum, first and third quantile, maximum, and mean (+) values during two high PM2.5 episodes, September 12–15 and November 11–24. Ammonium nitrate was estimated from the sum of Aitken and accumulation mode aerosol nitrate, assuming it had all converted to NH4NO3.
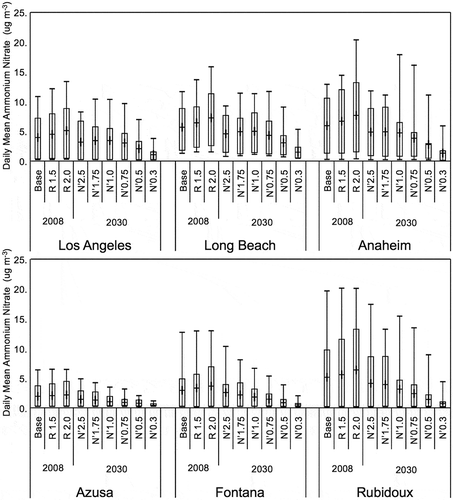
For 2030 with the NOx emissions the response of the NH4NO3 mass concentration to NOx reductions is much more complicated and nonlinear. The maxima of the NH4NO3 mass concentration are not greatly reduced at Central Los Angeles, Long Beach, Anaheim, and Rubidoux until the reductions reach the N’0.50 and N’0.3 cases, while at Azusa and Fontana the response of the maxima is much more linear to NOx emission reductions. The mean values of the NH4NO3 mass concentration at Central Los Angeles, Long Beach, and Anaheim are relatively constant, or they may increase slightly as NOx emissions are reduced between the N’2.5 and N’1.0 case and the mean values of the NH4NO3 mass concentration at all sites decrease as the NOx emissions are decreased further in the N’1.0, N’0.75, N’0.50, and N’0.30 cases. At Azusa, Fontana, and Rubidoux the mean values of the NH4NO3 mass concentration decrease as the NOx emissions are reduced for all cases.
The upper panel of shows that most of the variation in PM2.5 with respect to the changes in the NOx emissions inventory is due to variations in the concentrations of NH4NO3(s) for the simulated episodes. is a stack plot of simulated daily-average particulate matter mass concentrations by component at Los Angeles on November 20 for 2008 and 2030 and it is typical of the other sites and days. November 20, 2008, was a day with high measured PM2.5 mass concentrations. Gas-phase HNO3 is a necessary precursor of NH4NO3(s). The major source of HNO3 is the reaction of the hydroxyl radical (HO) with NO2:
The reaction of HNO3 with NH3 is the source of NH4NO3, which may exist in solid form depending on atmospheric humidity and temperature (Kim and Seinfeld Citation1995; Kim, Seinfeld, and Saxena Citation1993a; Citation1993b):
The concentrations of HO are very dependent on NOx and VOC concentrations. Maximum HO concentrations tend to follow the ridgeline of the O3 isopleth (Calvert et al. Citation2015, 478). This is because while NOx is necessary to produce O3, HO radicals and HNO3, its reaction with HO (Reaction 1) terminates free radical chains. Reaction 1 will dominate the gas-phase chemistry if the concentration of NO2 becomes too high for the ambient VOC concentration, and this will reduce HO concentrations. The competing roles of NOx as a catalyst for gas-phase atmospheric chemistry and its role as a free radical-chain terminator make HNO3 production very dependent on NOx and VOC concentrations. This chemistry is the basis for the O3 isopleth and the response of HNO3 production to NOx and VOC concentrations (Stockwell et al. Citation2012).
Figure 10. Upper panel presents a stack plot of simulated daily-average particulate matter mass concentrations by component on November 20 for 2008 and 2030 at Los Angeles. The components are secondary organic aerosol (SOA), ammonium nitrate (AmNit), ammonium sulfate (AmSul), unidentified PM2.5 (unid PM2.5), elemental carbon (EC), primary organic matter (primary OM), and coarse mass. Lower panel presents daily HNO3 production for differing initial VOC and NOx concentrations.
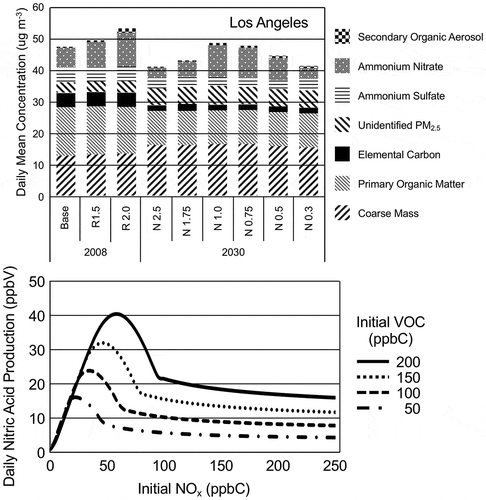
The dependence of HNO3 on NOx and VOC concentrations is illustrated by the lower panel of . A series of box model simulations were made with a chemical box-model using the Regional Atmospheric Chemical Mechanism, version 2 (RACM2; Goliff, Stockwell, and Lawson Citation2013). The box-model simulation procedures are described in Fujita et al. (Citation2016). The simulations were made for a range of initial concentrations of NOx and VOC and they presented the box-model simulations as isopleths. Here the simulated daily production of HNO3 are plotted in as a function of the initial NOx for four initial VOC concentrations.
shows that at low NOx concentrations the production of HNO3 is limited by the available NOx. The production of HNO3 increases almost linearly until it reaches a peak where the production is no longer NOx limited. The concentration of NOx where HNO3 production becomes VOC-limited is determined by the available VOC. Increases in the available NOx decreases HNO3 production when the production of HNO3 becomes VOC limited. This is reflective of the behavior of PM2.5 seen in the CMAQ simulations shown in and and the upper panel of .
Particulate sulfate and secondary organic aerosol
Particulate ammonium sulfate was estimated from the sum of CMAQ simulated Aitken and accumulation mode aerosol sulfate, assuming it had all converted to (NH4)2SO4. The daily average total ammonium sulfate mass concentrations were not strongly affected by the basin-wide VOC or NOx emissions. Relative to 2008, the daily average total ammonium sulfate mass concentrations were substantially lower at Los Angeles, Long Beach, and Anaheim, a little lower at Azusa and Fontana, and unchanged at Rubidoux for 2030 (Stockwell et al. Citation2017).
The daily average secondary organic aerosol concentrations are relatively small in our CMAQ simulations, as shown in the upper panel of . The simulations show that greater reductions in NOx emissions lead to higher daily average secondary organic aerosol concentrations (Stockwell et al. Citation2017). The same relationship holds for SOA produced from both biogenically and anthropogenically emitted VOC. However, there is literature that suggests that SOA simulations might not be accurate (e.g., Murphy et al. Citation2017).
Evaluation of the response of PM2.5 concentrations to ammonia emission changes
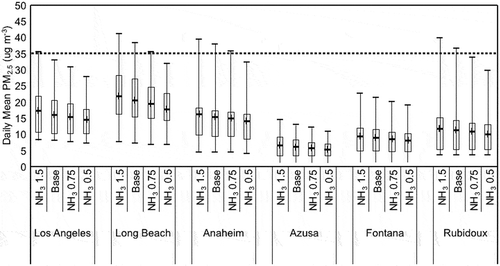
The effects of variations in NH3 emissions are shown in . Daily average PM2.5 concentrations, NH4NO3 mass concentrations, and ammonium sulfate mass concentrations decrease with reductions in NH3 emissions at all sites. Reductions in these particulate matter concentrations are seen for the first and third quantile, maximum, and mean values at all sites. shows that the PM2.5 concentrations are below the NAAQS regulations at most sites except for a few extreme events. Most of the reductions in PM2.5 concentrations are due to the effect of the NH3 reductions on the formation of NH4NO3 and ammonium sulfate (Stockwell et al. Citation2017). The limitation of PM2.5 concentrations to NH3 emissions in the SoCAB is in contrast to the San Joaquin Valley, where NH4NO3 formation is more limited by NOx. However, much of the upwind reactive nitrogen emissions are converted to nitrate prior to entering the San Joaquin Valley (Kleeman, Ying, and Kaduwela Citation2005). One limitation of our result is that Kelly et al. (Citation2014) have shown there are multiple uncertainties in NH3 emissions models and inventory. This uncertainty may affect simulations of PM2.5 for the SoCAB.
Figure 11. Range of predicted daily average PM2.5 mass concentrations for several adjustments to NH3 emissions at six monitoring sites in the SoCAB. The box and whisker plots indicate the minimum, first and third quantile, maximum, and mean (+) values during two high PM2.5 episodes, September 12–15 and November 11–24. The dashed line is the level of the current NAAQS.
Conclusion
Primary PM species, elemental carbon, and organic mass are not affected by changes to the NOx and VOC emission inventories, but coarse aerosol mass particle concentrations decrease slightly due to reductions in the NOx emissions inventory. Ammonium nitrate particle concentrations show a strong dependence on the NOx emissions inventory. The projected 2030 NOx emission inventory results in lower ammonium nitrate concentrations in 2030 than in 2008. However, the projected 2030 NOx emission inventory is not the most effective in reducing NH4NO3 concentrations of the scenarios that were simulated. Under some conditions, higher NOx emissions (greater than the projected 2030 inventory) can yield lower maximum PM2.5 and NH4NO3 concentrations in the SoCAB, but greater NOx reductions may be required to reduce both PM2.5 and ozone. At Azusa and Fontana, the sites located farthest upwind from the coast, reductions in NOx emissions decrease NH4NO3 for all of the 2030 cases. Ammonium sulfate is not greatly affected by changes in the NOx and VOC emissions inventory. Secondary organic aerosol increases with increases in the VOC emissions inventory. Decreases in NOx emissions lead to increases in secondary organic aerosol concentrations. Reductions in NH3 emissions reduce the formation of PM2.5 through reductions in the formation of NH4NO3 and ammonium sulfate, but additional efforts are needed to improve both NH3 emission inventories and model treatment of this chemistry. We suggest on the basis of our simulations that a more comprehensive multipollutant plan be developed for improving air quality that focuses on ozone and PM together and that policymakers consider a more optimum strategy involving reductions in NOx, VOC, and NH3. This conclusion is supported by the previous study of ozone and PM in the SoCAB by Nguyen and Dabdub (Citation2002).
Acknowledgment
The authors gratefully acknowledge the South Coast Air Quality Management District for providing its CMAQ modeling input and output files for our use.
Additional information
Funding
Notes on contributors
Devoun R. Stewart
Devoun R. Stewart is a professor in the Department of Chemistry at Sacramento City College.
Emily Saunders
Emily Saunders is a senior research scientist in the Global Modeling and Assimilation Office at the NASA Goddard Space Flight Center.
Roberto Perea
Roberto Perea is with the Department of Information Technology at UTEP.
Rosa Fitzgerald
Rosa Fitzgerald is a professor in the Physics Department of the University of Texas El Paso (UTEP).
David E. Campbell
David E. Campbell is an associate research scientist in the Division of Atmospheric Sciences at the Desert Research Institute (DRI) (Nevada System of Higher Education).
William R. Stockwell
William R. Stockwell is an affiliated faculty member in the Division of Atmospheric Sciences at DRI and a research professor in the Physics Department at UTEP.
References
- Binkowski, F. S. 1999. Chapter 10. Aerosols in MODELS-3 CMAQ. In Science algorithms of the EPA models-3 Community Multiscale Air Quality (CMAQ) modeling system, eds. Q. W. Byun and J. K. S. Ching. Washington, DC, USA: EPA Report EPA-600/R-89-030, U.S. Environmental Protection Agency.
- Binkowski, F. S., and U. Shankar. 1995. The regional particulate model 1. Model description and preliminary results. J. Geophys. Res. 100:26191–26209. doi:10.1029/95JD02093.
- Brunekreef, B., and S. T. Holgate. 2002. Air pollution and health. The Lancet 360:1233–1242. doi:10.1016/S0140-6736(02)11274-8.
- Byun, D., and K. L. Schere. 2006. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) modeling system. Appl. Mech. Rev. 59:51–77. doi:10.1115/1.2128636.
- Byun, Q. W., and J. K. S. Ching. 1999. Science Algorithms of the EPA Models-3 Community Multiscale Air Quality (CMAQ) Modeling System. EPA Report EPA-600/R-89-030, U.S. Environmental Protection Agency, Washington, DC, USA.
- Calvert, J. G., J. J. Orlando, W. R. Stockwell, and T. J. Wallington. 2015. The mechanisms of reactions influencing atmospheric ozone. Oxford, UK: Oxford University Press, Oxford.
- Chen, D., Q. Li, J. Stutz, Y. Mao, L. Zhang, O. Pikelnaya, J. Y. Tsai, C. Haman, B. Lefer, B. Rappenglück, S. L. Alvarez, J. A. Neuman, J. Flynn, J. M. Roberts, J. B. Nowak, J. de Gouw, J. Holloway, N. L. Wagner, P. Veres, S. S. Brown, T. B. Ryerson, C. Warneke, and I. B. Pollack. 2013. WRF-Chem simulation of NOx and O3 in the L.A. basin during CalNex-2010. Atmos. Environ. 81:421–432. doi:10.1016/j.atmosenv.2013.08.064.
- Chow, J. C., J. G. Watson, L.-W. A. Chen, M. C. O. Chang, N. F. Robinson, D. Trimble, and S. Kohl. 2007. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manage. Assoc. 57:1014–1023. doi:10.3155/1047-3289.57.9.1014.
- Cohen, A. J., M. Brauer, R. Burnett, H. R. Anderson, J. Frostad, K. Estep, K. Balakrishnan, B. Brunekreef, L. Dandona, R. Dandona, V. Feigin, G. Freedman, B. Hubbell, A. Jobling, H. Kan, L. Knibbs, Y. Liu, R. Martin, L. Morawska, C. A. Pope, H. Shin, K. Straif, G. Shaddick, M. Thomas, R. van Dingenen, A. van Donkelaar, T. Vos, C. J. L. Murray, and M. H. Forouzanfar. 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. The Lancet 389:1907–1918. doi:10.1016/S0140-6736(17)30505-6.
- Eder, B., and S. Yu. 2006. A performance evaluation of the 2004 release of Models-3 CMAQ. Atmos. Environ. 40:4811–4824. doi:10.1016/j.atmosenv.2005.08.045.
- Fujita, E. M., D. E. Campbell, W. R. Stockwell, E. Saunders, R. Fitzgerald, and R. Perea. 2016. Projected ozone trends and changes in the ozone-precursor relationship in the South Coast Air Basin in response to varying reductions of precursor emissions. J. Air Waste Manage. Assoc. 66:201–214. doi:10.1080/10962247.2015.1106991.
- Goliff, W. S., W. R. Stockwell, and C. V. Lawson. 2013. The regional atmospheric chemistry mechanism, version 2. Atmos. Environ. 68:174–185. doi:10.1016/j.atmosenv.2012.11.038.
- Grahame, T. J., R. Klemm, and R. B. Schlesinger. 2014. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manage. Assoc. 64:620–660. doi:10.1080/10962247.2014.912692.
- Kelly, J. T., K. R. Baker, J. B. Nowak, J. G. Murphy, M. Z. Markovic, T. C. VandenBoer, R. A. Ellis, J. A. Neuman, R. J. Weber, J. M. Roberts, P. R. Veres, J. A. de Gouw, M. R. Beaver, S. Newman, and C. Misenis. 2014. Fine-scale simulation of ammonium and nitrate over the South Coast Air Basin and San Joaquin Valley of California during CalNex-2010. J. Geophys. Res. 119:3600–3614.
- Kim, Y. P., and J. H. Seinfeld. 1995. Atmospheric gas–Aerosol equilibrium III. Thermodynamics of crustal elements Ca2+; K+; and Mg2+. Aerosol Sci. Technol. 22:93–110. doi:10.1080/02786829408959730.
- Kim, Y. P., J. H. Seinfeld, and P. Saxena. 1993a. Atmospheric gas–Aerosol equilibrium I. Thermodynamic model. Aerosol Sci. Technol. 19:157–181. doi:10.1080/02786829308959628.
- Kim, Y. P., J. H. Seinfeld, and P. Saxena. 1993b. Atmospheric gas–Aerosol equilibrium II. Analysis of common approximations and activity coefficient calculation methods. Aerosol Sci. Technol. 19:182–198. doi:10.1080/02786829308959629.
- Kleeman, M. J., Q. Ying, and A. Kaduwela. 2005. Control strategies for the reduction of airborne particulate nitrate in California’s San Joaquin Valley. Atmos. Environ. 39:5325–5341. doi:10.1016/j.atmosenv.2005.05.044.
- Makar, P. A., M. D. Moran, Q. Zheng, S. Cousineau, M. Sassi, A. Duhamel, M. Besner, D. Davignon, L.-P. Crevier, and V. S. Bouchet. 2009. Modelling the impacts of ammonia emissions reductions on North American air quality. Atmos. Chem. Phys. 9:7183–7212. doi:10.5194/acp-9-7183-2009.
- Murphy, B. N., M. C. Woody, J. L. Jimenez, A. M. G. Carlton, P. L. Hayes, S. Liu, N. L. Ng, L. M. Russell, A. Setyan, L. Xu, J. Young, R. A. Zaveri, Q. Zhang, and H. O. T. Pye. 2017. Semivolatile POA and parameterized total combustion SOA in CMAQv5.2: Impacts on source strength and partitioning. Atmos. Chem. Phys. 17:11107–11133. doi:10.5194/acp-17-11107-2017.
- Nguyen, K., and D. Dabdub. 2002. NOx and VOC and its effects on the formation of aerosols. Aerosol Sci. Technol. 36:560–572. doi:10.1080/02786820252883801.
- Sicard, P., A. Mangin, P. Hebel, and P. Mallea. 2010. Detection and estimation trends linked to air quality and mortality on French Riviera over the 1990–2005 period. Sci. Total Environ. 408:1943–1950. doi:10.1016/j.scitotenv.2010.01.024.
- South Coast Air Quality Management District. 2013, February. Final 2012 air quality management plan. Diamond Bar, CA: SCAQMD.
- South Coast Air Quality Management District. 2017, March. Final 2016 air quality management plan. Diamond Bar, CA: SCAQMD. https://www.aqmd.gov/home/air-quality/clean-air-plans/air-quality-mgt-plan/final-2016-aqmp.
- Spada, N. J., and N. P. Hyslop. 2018. Comparison of elemental and organic carbon measurements between IMPROVE and CSN before and after method transitions. Atmos. Environ. 178:173–180. doi:10.1016/j.atmosenv.2018.01.043.
- Stewart, D. 2017. Air quality modeling and health and economic assessment of projected ozone and particulate matter response to varying reductions of oxides of nitrogen and volatile organic compound emissions in the south coast air basin. Ph.D. Dissertation, Howard University.
- Stewart, D., E. Saunders, R. A. Perea, R. Fitzgerald, D. E. Campbell, and W. R. Stockwell. 2017. Linking air quality and human health effects models: An application to the Los Angeles Air Basin. Environ. Health Insights 11:1–13. doi:10.1177/1178630217737551.
- Stockwell, W. R., C. V. Lawson, E. Saunders, and W. S. Goliff. 2012. A review of tropospheric atmospheric chemistry and gas-phase chemical mechanisms for air quality modeling. Atmosphere 3:1–32. doi:10.3390/atmos30100012011.
- Stockwell, W. R., R. Fitzgerald, R. Perea, D. E. Campbell, D. Stewart, and E. Saunders. 2017. Air quality modeling of the relationship between projected ozone and pm trends and changes in precursor relationships in the south coast air basin in response to varying reductions of precursor emissions. Final Report for CRC Project A-101, Coordinating Research Council, Atlanta, GA.
- U.S. EPA. 2007. Guidance on the Use of Models and Other Analyses for Demonstrating Attainment of Air Quality Goals for Ozone, PM2.5, and Regional Haze. U.S. Environmental Protection Agency, Research Triangle Park, North Carolina. Accessed August 13, 2018. https://www3.epa.gov/scram001/guidance/guide/final-03-pm-rh-guidance.
- Yu, H., A. Russell, J. Mulholland, T. Odman, Y. Hu, H. H. Chang, and N. Kumar. 2018. Cross-comparison and evaluation of air pollution field estimation methods. Atmos. Environ. 179:49-60.
