ABSTRACT
Liquid manure storages are a significant source of methane (CH4) emissions. Farmers commonly agitate (stir) liquid manure prior to field application to homogenize nutrients and solids. During agitation, manure undergoes mechanical stress and is exposed to the air, disrupting anaerobic conditions. This on-farm study aimed to better understand the effects of agitation on CH4 emissions, and explore the potential for intentional agitation (three times) to disrupt the exponential increase of CH4 emissions in spring and summer. Results showed that agitation substantially increased manure temperature in the study year compared to the previous year, particularly at upper- and mid-depths of the stored manure. The temporal pattern of CH4 emissions was altered by reduced emissions over the subsequent week, followed by an increase during the second week. Microbial analysis indicated that the activity of archaea and methanogens increased after each agitation event, but there was little change in the populations of methanogens, archaea, and bacteria. Overall, CH4 emissions were higher than any of the previous three years, likely due to warmer manure temperatures that were higher than the previous years (despite similar air temperatures). Therefore, intermittent manure agitation with the frequency, duration, and intensity used in this study is not recommended as a CH4 emission mitigation practice.
Implications: The potential to mitigate methane emissions from liquid manure storages by strategically timed agitation was evaluated in a detailed farm-scale study. Agitation was conducted with readily-available farm equipment, and targeted at the early summer to disrupt methanogenic communities when CH4 emissions increase exponentially. Methane emissions were reduced for about one week after agitation. However, agitation led to increased manure temperature, and was associated with increased activity of methanogens. Overall, agitation was associated with similar or higher methane emissions. Therefore, agitation is not recommended as a mitigation strategy.
Introduction
Liquid dairy manure storages emit substantial amounts of methane (CH4), which has a high global warming potential (Myhre et al. Citation2013). The production of CH4 in anaerobic environments, including manure storages, is the result of complex microbial communities involving hydrolysis, fermentation, and ultimately methanogenesis. Despite the recognized importance of CH4 emissions in manure management chains, the processes leading to its production are not fully characterized, which limits the ability to model and predict emissions under real-world conditions (Baldé et al., Citation2016a; Baral et al. Citation2018).
Farmers commonly agitate liquid manure storages prior to field application to facilitate pumping and to homogenize nutrients and solids. Agitation brings manure from depth to the surface, and in some cases sprays the manure onto the surface, which involves a high level of mechanical force and provides aeration. The effects of this process on CH4 emissions are not well understood. On one hand, pilot-scale and on-farm measurements show the immediate effect of agitation is a rapid increase in CH4 emissions due to the release of dissolved gas and trapped bubbles (~10-fold increase in the first hour; Kaharabata, Schuepp, and Desjardins Citation1998; Leytem et al. Citation2017; VanderZaag et al. Citation2014, Citation2010a). Manure surface crusts are disrupted by agitation, removing potential methanotrophy and a physical barrier to CH4 emissions. The longer-term effects on manure CH4 production are not clear, however, because agitation is typically immediately followed by manure removal, measurement studies often stop after agitation. On the other hand, data from other disciplines suggest there could be an inhibitory effect of agitation. Laboratory studies with anaerobic incubation of rice paddy soil demonstrated that agitation led to 80% lower CH4 production overall. This decrease was attributed to the disruption of the conversion of fatty acids and alcohols, a reduced conversion of acetate, and the physical destruction of acetotrophic methanogens caused by mechanical forces (Dannenberg, Wudler, and Conrad Citation1997). Laboratory studies on manure anaerobic digestion point in a similar direction, whereby CH4 yield was reduced and microbial activity was lower as a result of a greater manure mixing speed (Turker et al. Citation2013). Ong, Greenfield, and Pullammanappallil (Citation2002) found a 28% decrease of methane production from cattle slurry in a continuously stirred reactor compared to an unstirred reactor. Mixing disrupted the production of extracellular polymeric substances which help form larger microbial aggregates. Studies on aeration of pig slurries prior to storage found that aeration significantly reduced CH4 emissions compared to unaerated slurry (Loyon et al. Citation2007; Martinez et al. Citation2003). Calvet, Hunt, and Misselbrook (Citation2017) observed an increased pH as well as a 40% decrease in CH4 emission as a result of low frequency aeration of pig slurries at a pilot-scale.
A manure temperature model suggests that agitation can significantly increase manure temperature (Rennie et al. Citation2018), which would in-turn increase CH4 production. In a modelling study, when manure was removed in autumn, monthly agitation the following spring was associated with increased peak manure temperature and increased annual average manure temperature compared to a non-agitated control (Rennie et al. Citation2018).
Methane emissions are characterized by a low emission period (lag phase) in spring followed by an exponential increase in late spring and early summer. Previous on-farm measurements noted a reduction in CH4 emissions coinciding with a brief manure mixing event during the exponential phase (Baldé et al. Citation2016b). It has been hypothesized that strategic agitation during the exponential phase could lead to reduced CH4 emissions. If so, it could be an interesting option for CH4 emission mitigation because farmers already possess agitation equipment and it is generally available for use during early summer. Intermittent agitation is a practical option because the cost and time required for intense agitation over several days is manageable.
In light of the previous studies, we sought to determine if a reduction in CH4 emission could be achieved through targeted agitation during the exponential phase of emissions. To this end, the particular timing of the agitation events for this study (late spring – early summer) was selected to target a disruption of methanogenic archaeal communities at a time when CH4 emissions would be in the exponential phase, expecting that the agitation events would disrupt the progression of this phase. The objective of the present study is therefore to characterize the impact of planned intermittent agitation using farm-scale equipment at a liquid manure storage tank. The response was measured in terms of CH4 emissions, manure temperature, manure characteristics, and microbial communities.
Materials and methods
Experimental site and agitation
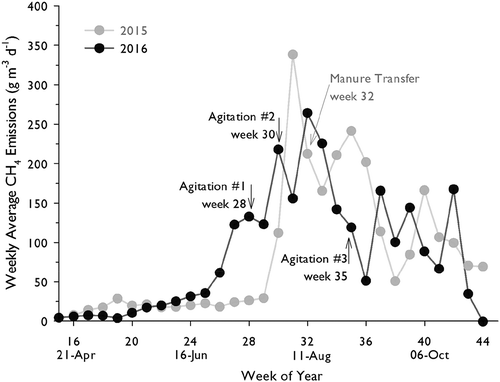
This study took place on a commercial dairy farm near Ottawa, Ontario (45°15ʹ N, 75°49ʹ W), which had ~150 milking cows, and ~110 heifers and dry cows. Data was collected from January to October 2016. Manure solids from the barns were separated by a screw-press (DariTech, Lynden, Washington) and the liquid fraction was sent to a large concrete storage tank (1,257 m2 surface area, 2.44 m depth). The farm also had a smaller secondary tank (314 m2 surface area, 3.05 m depth) which was used when the large tank was full (during most of the study the small tank was empty). Further details of the farm management and setup, including a detailed diagram of the storage location and configuration of the sensor location, is described by Baldé et al. (Citation2016a). Field application of the liquid manure took place in the spring and fall. A manure contractor was hired to agitate the large storage tank using a 140 horsepower tractor (7720 John Deere, Moline, IL, USA) and a 12.8 m manure agitator pump with a 15.2 cm diameter discharge pipe (Houle Super Pump; GEA Group, Düsseldorf, Germany). The manure in the large concrete storage tank was vigorously mixed for 7 to 13 consecutive hours on three dates: July 12, 2016 (DOY 194; AG1), July 26 (DOY 208; AG2), and September 1 (DOY 245; AG3). These dates were chosen to target the period when CH4 emissions had increased exponentially and reached a maximum in previous years of measurements at this farm (Baldé et al. Citation2016b). Dates and the number of hours of agitation were also subject to availability of the contractor.
Methane emissions
Methane emissions from the liquid manure storage system were determined from April to November, 2016 using an inverse dispersion method. The method is based on the increase in downwind CH4 concentration above the background level. Emission calculations were done using the backwards Lagrangian Stochastic (bLS) model implemented in WindTrax (version 2.0.8.9). Inputs to the model consisted of synchronized CH4 concentrations measured by open-path lasers (GasFinder 2.0 OP, ColdFire Processor V1.10.h, Boreal Laser In., Edmonton, Canada) and wind speed, direction, and turbulence statistics (i.e. variances and covariances of wind speed components u, v, w, and temperature) calculated by a CR1000 datalogger based on 10 Hz measurements from a CSAT3 sonic anemometer (Campbell Scientific, Edmonton, AB). Friction velocity (u*), Monin-Obukhov length (LMO), and surface roughness (Z0) were calculated by WindTrax. The site layout was the same as multiple previous years of measurements at the same site (described in Baldé et al. Citation2016a, Citation2016b). Lasers were placed upwind and downwind of the manure storage to sample and record CH4 concentration about every second. Concentration data was then filtered to remove periods of abnormal laser operation (e.g. laser quality code ≠ 1 or 4001). The remaining data were averaged in 15-min intervals to match the 15-min time intervals of wind and turbulence statistics. After CH4 emissions were calculated using WindTrax, filters were applied to remove unsuitable atmospheric conditions (i.e. u* < 0.15 m sec−1, −5 < LMO < +5 m, Z0 > 0.25 m) and unsuitable wind direction when the source fraction covered by touchdowns was <65%.
Environmental parameters and manure characteristics
Air temperature (Ta) was measured using a type T (copper-constantan) thermocouple in a radiation shield. Manure temperature (Tm) was measured at three depths using thermistors (CS-109 L, Campbell Scientific, Edmonton AB) attached to a chain and a float, resulting measurements being 10 cm above the bottom, at mid-depth (1 m below the float), and 15-cm below the surface float. Manure temperature sensors were removed from the storage during the agitation events to prevent physical damage. Because of logistical reasons (scheduling the agitation equipment; personnel availability) there were delays before the sensors were returned to the tank after agitation.
Manure depth was measured with a SR50AT Sonic Ranging sensor (Campbell Scientific, Logan, UT). Data was measured at 5-sec intervals and 15-min averages were recorded with a CR1000 data logger (Campbell Scientific, Edmonton, AB). Digital photographs of the large storage tank surface were taken every 30-min using a time-lapse camera (Wingscapes, EBSCO Industries, AL, USA).
Manure was sampled before (1–2 days prior), during (after 6 hours of mixing) and after (5–7 days later) each agitation using a sampling pipe to obtain a sample from top to bottom at eight locations around the tank perimeter. Samples were homogenized in a 20 L pail and three groups of composite samples were obtained and refrigerated immediately. One 500 mL sample was frozen for analysis of TS (drying to constant weight at 110°C), VS (loss on ignition at 550°C), TN (Dumas method of combustion), ammonium N (ion specific electrode), and pH (ion specific electrode), following recommended methods of analysis (Peters et al. Citation2003). The second sample group consisted of duplicate 1 L samples that were frozen and sent for analysis of Volatile Fatty Acids (VFAs) at a commercial lab (Innotech Alberta, Vegreville, AB) by headspace gas chromatography using a DB-FFAP column (30 m × 0.25 mm × 0.5 µm) on a Varian CP-3800 gas chromatograph equipped with a flame ionization detector (Agilent Technologies, Santa Clara, CA). Helium was used as carrier gas and the head-space was sampled with 2.5 mL HS syringe using a CombiPaL auto-sampler (CTC Analytic; Zwingen, Switzerland). The third sample group was retained for microbial analysis, as described below.
Nucleic acid extractions, reverse transcriptions, and PCR
For microbial analysis, ~2 g of triplicate slurry sub-samples were mixed with 5 mL of LifeGuard® Soil Preservation Solution (MoBio Laboratories Inc., Carlsbad CA, USA) and frozen (−20°C) until ready for nucleic acid extraction. DNA and RNA were co-extracted using RNA PowerSoil® Total RNA Isolation with DNA Elution Accessory Kits (MoBio Laboratories Inc.) according to the manufacturer protocol. DNA contaminants in RNA were digested using RNase-free DNase I (Promega, Madison, WI). Total RNA was reverse transcribed into complementary DNA (cDNA) according to the recommended protocol using a high capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems, California, USA). After pooling products of three reverse transcription reactions for each sample, DNA and cDNA were used to quantify the abundances and activities of methanogenic, archaeal, and bacterial communities. Methanogens were targeted using mlas-mod F (5ʹ-ggyggtgtmggdttcacmcarta-3ʹ) and mcrA-rev-mod R (5ʹ-cgttcatbgcgtagttvggrtagt-3ʹ) primers, which amplify the gene encoding the alpha subunit of methyl-coenzyme M reductase (mcrA), an enzyme that catalyzes the final step in methanogenesis (Angel, Claus, and Conrad Citation2012; Steinberg and Regan Citation2009). Archaeal and bacterial communities were targeted using the universal 16S rRNA gene primers (A934bR/A364aF and EUB338/EUB518, respectively). Target genes from each sample were quantified using quantitative real-time PCR (qPCR) on a thermal cycler (CFX96; BioRad Laboratories Inc., CA). PCR reaction mixes and conditions for methanogens and total archaeal communities were performed as per Habtewold et al. (Citation2017). Two 10× dilution of plasmids with the respective target gene inserted were used to make qPCR standard curves. Efficiencies and R2 of the standard curves for mcrA and 16S rRNA gene quantifications were 91–102% and ≥ 0.99, respectively. CFX ManagerTM software version 3.1 (Bio-Rad Laboratories, Inc.) was used to analyze the qPCR data.
Calculations and statistical analyses
Since greater amounts of CH4 emission data are filtered out during night time periods (low wind conditions), average CH4 emissions (kg d−1) were calculated from binned hourly averages for each hour of the day (0:00 to 23:00 h), and used to calculate weekly averages, as per VanderZaag et al. (Citation2014). Tank volume (m3) was calculated by multiplying the average weekly depth (m) of the large and small tanks by their respective surface areas (1257-m2 and 314-m2). Average CH4 emissions scaled to volume (g m−3 d−1) were calculated by dividing the average weekly CH4 emissions (g d−1) by the combined (large and small tank) average weekly tank volume (m3).
As a farm-scale case study, the advantage is that emissions represent the reality of farm conditions which cannot be replicated in a laboratory. The drawback with a farm-scale study is there was no true control; rather, emissions in the study year (2016) were compared to a baseline condition in the previous year (2015). Statistical comparisons between years were performed using a paired t-test on weekly manure depth, temperature (Ta, Tm), and emissions. Statistical comparisons of manure physical and chemical properties before vs during agitation, and during vs after agitation were conducted using paired t-tests. Changes in microbial DNA and RNA were analysed by a two-way ANOVA on log-transformed values, followed by Tukey multiple comparison test. Differences were considered significant at a p-value < 0.05.
Results and discussion
Manure characteristics
A summary of the manure characteristics are provided in . Over the entire study, the Dry Matter (DM) averaged 4.1 ± 0.4% and volatile solids (VS) averaged 2.9 ± 0.3%. These values were similar to the average over the previous 3 years (~4% and ~3%, respectively), which reflects consistent management of the dairy herd over these years (Baldé et al. Citation2016b).
Table 1. Manure characteristics: Dry Matter (DM), Volatile Solids (VS), Organic Carbon (OC), pH, Electrical Conductivity (EC), Nitrogen (N), Ammonia (NH3), Phosphorus (P), Potassium (K), total Volatile Fatty Acids (VFA), and Acetic Acid (AA) before, during, and after each agitation event (AG1-AG3), and on average.
During agitation, concentrations of DM, VS, and OC consistently increased compared to before agitation, a difference which was statistically significant (). These increases were likely due to the agitation activity re-suspending settled sludge which is difficult to sample, even with a column sampling pipe. Nitrogen (N), however, did not follow this trend and was present in significantly greater concentration before agitation compared to during and after, perhaps due to enhanced ammonia (NH3) volatilization during agitation. However, the NH3 concentration did not change significantly during or after agitation.
For AG1 and AG2, a numerical increase in pH (0.1 to 0.2 units) was observed after the agitation event, however, this change was not statistically significant. No pH increase was observed after AG3. The observed pH increase may be related to the release of trapped CO2 from within the liquid (Hafner, Montes, and Rotz Citation2012). Our findings also suggest that acetic acid (AA) concentrations significantly increased after each agitation (). There was also a decreasing trend in AA over time (from AG1 to AG3) which may be attributed to methanogens becoming more efficient at using AA relative to the rate of hydrolysis. The significant increase in AA after each agitation could be linked to the homogenization of substrates from the sludge throughout the tank, as evidenced by increased VS and OC during and after agitation.
Depth and temperature
The manure depth time series of the large storage tank show roughly the same emptying and filling patterns between 2015 and 2016 (). Spring manure removal occurred around the same time in both years (completed during week 18), but in 2016, less residual manure was left in the tank (~0.3 m) compared to 2015 (~0.6 m). Average weekly depths from week 18 to 38 were not significantly different between the two years (p = .98). A notable difference was that in 2016, manure was retained in the large storage tank for an additional ~ 4 weeks before fall emptying ().
Figure 1. Weekly average quantity of manure in storage during 2015 and 2016. Top panel: depth (m) in the small tank (314 m2; dashed lines) and large tank (1257 m2; solid lines). Bottom panel: total volume (m3) of stored manure in both tanks combined.
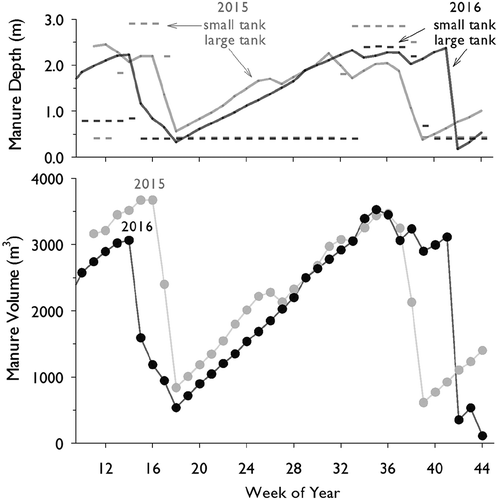
Air temperature (Ta) was similar in 2015 and 2016 (). The highest monthly temperatures were in July and August in both years. In August, average Ta was warmer in 2016 (21.7°C) compared to 2015 (19.0°C). Considering the period when manure depth was > 1.5m (week 30–38; mid-July to mid-September) the average Ta was not significantly different between 2015 and 2016 (20.1°C and 20.5°C, respectively, p = .71; ).
Table 2. Weekly average depth in the large tank (m), air temperature (°C, Ta), and stratified manure temperatures (°C, Tm) for weeks 30–38 in 2015 and 2016.
Figure 2. Weekly time series (weeks 24–44) of: air temperature (°C, top panel), manure temperature at 10 cm depth (°C, 2nd panel), manure temperature at mid-depth (°C, 3rd panel), and manure temperature at the bottom of the large storage tank (°C, bottom panel).
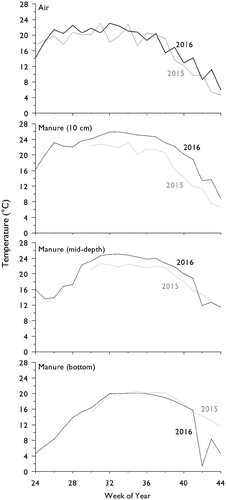
Manure Temperature (Tm) was higher in 2016 than 2015, despite the similarities in Ta and manure (; ). Considering only weeks 30 to 38, the average top and middle Tm were higher by ~3°C and ~2°C (respectively) in 2016 compared to 2015 (p < .001) (). Temperature at the bottom of the tank was similar in both years (from week 30 to 41). When averaged across the tank profile, 2016 saw some of the highest Tm readings recorded over four-years at the research site (Baldé et al. Citation2016b noted that Tm was higher in 2015 than the two previous years).
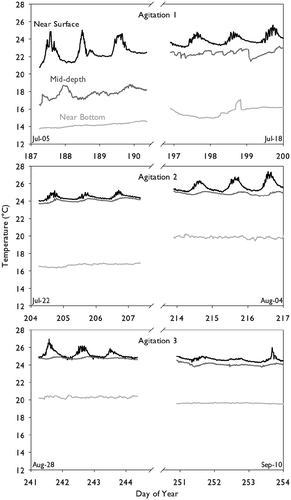
A deeper analysis of manure temperatures pre- and post- agitation shows that Tm increased sharply as a result of agitations, especially earlier in the warming phase of the year (AG1 and AG2). In the case of AG1, Tm in the middle depth strata saw an increase post-agitation. In the case of AG2, it was the bottom strata that saw a significant increase in Tm (). After AG3 all temperatures decreased slightly (). These findings are generally in agreement with model simulations of manure temperature by Rennie et al. (Citation2018) that show agitation in spring (when large vertical temperature gradients exist) leads to increased tank-average temperatures as the warmer surface manure is mixed with the cooler manure at lower depths. On the other hand, the model simulations predicted a cooling effect on the manure surface temperature, which were not evident in the measurements perhaps because of the delay in returning the temperature probes after agitation ().
Figure 3. Stratified (top, middle, bottom) manure temperature (Tm, °C) profile of the large storage tank before and after agitation events. Top panel: Agitation 1 (AG1), DOY 196; middle panel: Agitation 2 (AG2), DOY 208; bottom panel: Agitation 3 (AG3), DOY 245. Each panel shows approximately 3d of data before and after each agitation event. Note: Gap between pre- and post- agitation data segments is not to scale.
Microbial communities
Analysis of bacterial 16S rRNA genes and transcripts shows that bacterial abundance (number of gene copies) was not significantly different across the 3 agitation events (p = .06) while bacterial activity (transcript copies) did change significantly across the 3 agitation events (). Within each agitation event, bacterial gene copy numbers were significantly greater after agitation than before in every case, and transcript copy numbers were significantly greater after agitation for AG1 and AG2, but not AG3.
Table 3. Summary of qPCR (gene copies and transcript copies) targeting Bacterial 16S rRNA (first), Archaeal 16S rRNA (second), and mcrA (third) from samples taken before, during and after three agitation events (AG1, AG2, AG3).
Archaeal populations significantly increased in abundance with time from AG1 to AG3 (from 1010 to 1011) while the archaeal activity significantly increased exponentially (from 107 to 1011). Archaeal activity (transcript copies) increased significantly after each of the first two agitations compared to before (). Methanogen abundance did not change significantly from AG1 to AG3, but did increase after the first two agitations compared to before. On the other hand, methanogen activity (mcrA transcript copies) increased significantly with time from AG1 to AG3, and was significantly higher after the second and third agitations than before. Increased activity may have been caused by disintegration of aggregates and suspension in the manure profile thereby supplying microbes with more substrates. Although there were too few measurement time points to correlate methanogen activity with CH4 emissions as was done by Ma, Conrad, and Lu (Citation2012), our results agree that transcript copy numbers (hence activities) are more dynamic than gene copies numbers.
Methane emissions
Cumulative methane emissions
Of the last four years at the farm site, cumulative annual CH4 emissions were greatest during this study, compared to 33–50 Mg y−1 for 2013–2015 (; Baldé et al. Citation2016b). Comparing the cumulative CH4 emissions between 2015 and 2016 for weeks 18–44 we see that emissions were higher in 2016. After scaling by VS, CH4 emissions were also greater for 2016 during weeks 18–44 (). Considering weeks 18–37, when both years had similar manure volume, cumulative emissions were more similar, and emissions scaled by VS were identical (2.6 g kg−1 d−1 for both years; ).
Table 4. Summary of methane (CH4) emission for 2014, 2015, and 2016 during the main period of methane emissions, which occur between spring and fall manure removal.
Methane emission dynamics
Despite a smaller volume of residual inoculum (30 cm in 2016; 60 cm in 2015), the break from lag-phase emissions (~ 20–30 g m−3 d−1) occurred several weeks earlier in 2016 (i.e. doubling from week 25 to week 26) compared to 2015 (i.e., tripling from week 29 to week 30) as shown in . This was, perhaps linked to warmer Ta in 2016 during weeks 26–28 than 2015 (), combined with slightly shallower depth of manure at that time in 2016, which is expected to warm up faster (Rennie et al. Citation2018). The CH4 emission time series for 2016 showed decreases in daily emissions in the week following each agitation event (). In each case, however, emissions subsequently increased beyond pre-agitation levels in the following week. Time-lapse photography of the large storage tank shows that prior to agitation, there was a buildup of surface foam, which typically formed overnight before dissipating after daybreak (, AG1). After agitation, the foam did not form for several days and gradually began to return to pre-agitation levels (). These visual observations reflect the time required for methanogens to re-establish trapped gasses and soluble CH4 back to pre-agitation levels, and at least partially explains why emissions were lower in the week following an agitation, as has been observed previously (Citation2014; VanderZaag et al. Citation2010a). These findings support the notion that farm-scale agitation primarily alters CH4 transport to the atmosphere, with less effect on CH4 production.
Figure 5. Daily time-lapse series showing surface foam formation of the large storage tank the day before first agitation event [AG1 (−1d)], during the first 5 hours of agitation (AG1 0h and 5h), the day after agitation [(AG1 (+1d)], until 5 days after agitation [AG1 (+5)]. Except during agitation, pictures were captured between 06:30 a.m. and 07:00 a.m. (before foam dissipated in the late morning).
![Figure 5. Daily time-lapse series showing surface foam formation of the large storage tank the day before first agitation event [AG1 (−1d)], during the first 5 hours of agitation (AG1 0h and 5h), the day after agitation [(AG1 (+1d)], until 5 days after agitation [AG1 (+5)]. Except during agitation, pictures were captured between 06:30 a.m. and 07:00 a.m. (before foam dissipated in the late morning).](/cms/asset/f92bd6c6-49c3-4e94-b0be-d8bc816a6eb7/uawm_a_1629359_f0005_oc.jpg)
It is important to note that any CH4 emission abatement observed in the week following agitation may have been counteracted by high emissions that occur during agitation (Leytem et al. Citation2017, Citation2014; VanderZaag et al. Citation2010a), but that were not captured in this study due to the lack of suitable wind conditions for the inverse-dispersion method during the agitation events. Adding these emissions would further increase the annual 2016 CH4 emissions. For context, pilot-scale results from VanderZaag et al. (Citation2010b) found fall agitation contributed ~ 2% of cumulative emissions over a 6-mo period. Likewise, Kaharabata, Schuepp, and Desjardins (Citation1998) measured CH4 emissions during pumping operations at a swine manure tank and concluded the brief emission spikes made a negligible contribution to annual emissions.
Methane emissions started to decrease before AG3, and after the agitation continued their downward trend to a level lower than the subsequent 6 weeks (). Although we cannot conclusively say, it is possible that emissions would have increased in week 35 had it not been for the effect of AG3. A similar trend was observed in 2015 when, after a minor agitation event (manure transfer from the large to small tank), the CH4 emissions were depressed the following week before increasing again. However, despite this observed (temporary) lowering effect that agitation had, no appreciable difference in scaled CH4 emissions () were observed between 2016, which received 3 vigorous agitations (in addition to minor agitation due to manure transfers) and 2015, which received only minor agitations due to manure transfers.
The timing of the agitation events was selected with the goal of disrupting methanogenic archaeal communities and thus specifically targeting and breaking the (late spring) exponential phase in CH4 emissions observed in previous studies. This did not occur; rather, the exponential phase continued, largely unhindered. Our results support previous research that has observed a temporary suppression of CH4 emission post-agitation due to the release of dissolved and trapped gases. It is also possible that homogenization and intense aeration of the slurry disrupted methanogens for a brief period of time post-agitation before they re-established. When they re-established, methanogens had increased transcript copies indicating greater synthesis of enzymes for methane production, perhaps reflecting an increase in the availability of methanogenic substrates.
A potential trade-off of intermittent aeration is the production of nitrous oxide. A previous study on swine manure found nitrous oxide emissions occurred when the aerobic to anoxic ratio was 0.625, but was avoided by extending the anoxic period between aeration to create a ratio of 0.375 (Béline and Martinez Citation2002). In our study the aerobic to anoxic ratio was quite low (e.g., 0.035 for AG1 where 12 hours of aeration was followed by 336 hours without aeration), which is favourable for complete denitrification and minimal nitrous oxide production.
Conclusion
Manure agitation significantly increased manure temperature at upper- and mid-depths, and changed the temporal pattern of CH4 emissions over the subsequent 1–2 weeks. Overall, intentionally agitating the manure three times in the spring and summer had little effect on average CH4 emissions per m3 of manure, which were similar to the previous year at the same farm. Methane emissions were lowered for about one week after agitation, which was likely the amount of time required to re-saturate the manure with dissolved CH4 and bubbles that had been liberated during agitation. Increased activity of methanogens was associated with agitation, suggesting that agitation did not adversely impact this anaerobic community (by oxygenation or physical damage to cells), rather, perhaps redistribution of manure substrates was beneficial for methanogens. Therefore, intermittent agitation as conducted in the present study is not recommended as an emission mitigation practice.
Acknowledgment
The cooperating farmers are greatly appreciated.
Additional information
Funding
Notes on contributors
Hambaliou Baldé
Andrew C. VanderZaag and Ray L. Desjardins are research scientists and Hambaliou Baldé and Stephen Burtt are research technicians at the Science and Technology Branch of Agriculture and Agri-Food Canada in Ottawa, Ontario, Canada.
Jemaneh Habtewold
Jemaneh Habtewold was a Ph.D. student at the University of Guelph, and is now a post-doctoral fellow at Agriculture and Agri-Food Canada in Ottawa, Ontario, Canada.
Etienne L. Le Riche
Etienne L. Le Riche is a research associate at Wilfrid Laurier University in Ontario, Canada.
Kari Dunfield
Kari Dunfield is an associate professor in the School of Environmental Sciences at the University of Guelph, in Ontario Canada. She holds the Canada Research Chair in Environmental Microbiology of Agro-Ecosystems.
Robert J. Gordon
Robert J. Gordon is a professor and Vice-President Research at Wilfrid Laurier University in Ontario, Canada.
Earl Jenson
Earl Jenson, P.Eng., is a research scientist and Team Lead of Bio-Thermo-Chemical Processing at Innotech Alberta, in Alberta, Canada.
References
- Angel, R., P. Claus, and R. Conrad. 2012. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. Isme J 6:847–62. doi:10.1038/ismej.2011.141.
- Baldé, H., A. C. VanderZaag, S. Burtt, L. Evans, C. Wagner-Riddle, R. L. Desjardins, and J. D. MacDonald. 2016a. Measured versus modeled methane emissions from separated liquid dairy manure show large model underestimates. Agric. Ecosyst. Environ. 230:261–70. doi:10.1016/j.agee.2016.06.016.
- Baldé, H., A. C. VanderZaag, S. Burtt, R. J. Gordon, and R. L. Desjardins. 2016b. Does complete fall removal of the dairy manure sludge in a storage tank reduces subsequent methane emissions? J. Environ. Qual. 45 (6):2038–43. doi:10.2134/jeq2016.03.0083.
- Baral, K. R., G. Jégo, B. Amon, R. Bol, M. H. Chantigny, J. E. Olesen, and S. O. Petersen. 2018. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 166:26–35. doi:10.1016/j.agsy.2018.07.009.
- Béline, F., and J. Martinez. 2002. Nitrogen transformations during biological aerobic treatment of pig slurry: Effect of intermittent aeration on nitrous oxide emissions. Bioresour. Technol. 83 (3):225–28.
- Calvet, S., J. Hunt, and T. H. Misselbrook. 2017. Low frequency aeration of pig slurry affects slurry characteristics and emissions of greenhouse gases and ammonia. Biosyst. Eng. 159:121–32. doi:10.1016/j.biosystemseng.2017.04.011.
- Dannenberg, S., J. Wudler, and R. Conrad. 1997. Agitation of anoxic paddy soil slurries affects the performance of the methanogenic microbial community. FEMS Microbiol. Ecol. 22:257–63. doi:10.1111/j.1574-6941.1997.tb00378.x.
- Habtewold, J., R. J. Gordon, J. D. Wood, C. Wagner-Riddle, A. C. VanderZaag, and K. E. Dunfield. 2017. Dairy manure total solid levels impact CH4 flux and abundance of methanogenic archaeal communities. J. Environ. Qual. 46 (1):232–36. doi:10.2134/jeq2016.11.0451.
- Hafner, S. D., F. Montes, and C. A. Rotz. 2012. The role of carbon dioxide in emission of ammonia from manure. Atmos. Environ 66:63–71. doi:10.1016/j.atmosenv.2012.01.026.
- Kaharabata, S. K., P. H. Schuepp, and R. L. Desjardins. 1998. Methane emissions from above ground open manure slurry tanks. Global Biogeochem. Cycles. 12 (3):545–54. doi:10.1029/98GB01866.
- Leytem, A. B., D. L. Bjorneberg, A. C. Koehn, L. E. Moraes, E. Kebreab, and R. S. Dungan. 2017. Methane emissions from dairy lagoons in the western United States. J. Dairy Sci. 100:1–19. doi:10.3168/jds.2017-12777.
- Loyon, L., F. Guiziou, F. Beline, and P. Peu. 2007. Gaseous Emissions (NH3, N2O, CH4 and CO2) from the aerobic treatment of piggery slurry – Comparison with conventional storage system. Biosys. Eng. 97:472–80. doi:10.1016/j.biosystemseng.2007.03.030.
- Ma, K., R. Conrad, and Y. Lu. 2012. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl. Environ. Microbiol. 78 (2):445–54. doi:10.1128/AEM.06934-11.
- Martinez, J., F. Guiziou, P. Peu, and V. Gueutier. 2003. Influence of treatment techniques for pig slurry on methane emissions during subsequent storage. Biosys. Eng. 85 (3):347–54. doi:10.1016/S1537-5110(03)00067-9.
- Myhre, G., D. Shindell, F.-M. Bréon, W. Collins, J. Fuglestvedt, J. Huang, D. Koch, J.-F. Lamarque, D. Lee, B. Mendoza, et al. 2013. Anthropogenic and natural radiative forcing. In Climate change 2013: The physical science basis. Contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change, ed. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley, 659–740. Cambridge, United Kingdom and New York, NY: Cambridge University Press.
- Ong, H. K., P. F. Greenfield, and P. C. Pullammanappallil. 2002. Effect of mixing on biomethanation of cattle-manure slurry. Environ. Technol. 23:1081–90. doi:10.1080/09593332308618330.
- Peters, J., S. Combs, B. Hoskins, J. Jarman, J. Kovar, M. Watson, A. Wolf, and N. Wolf, 2003. Recommended methods of manure analysis. University of Wisconsin. A3769. http://learningstore.uwex.edu/assets/pdfs/A3769.PDF (accessed 18 March 2019).
- Rennie, T. J., R. J. Gordon, W. N. Smith, and A. C. VanderZaag. 2018. Liquid manure storage temperature is affected by storage design and management practices—A modelling assessment. Agric. Ecosyst. Environ. 260:47–57. doi:10.1016/j.agee.2018.03.013.
- Steinberg, L. M., and J. M. Regan. 2009. mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Appl. Environ. Microbiol. 75:4435–42. doi:10.1128/AEM.02858-08.
- Turker, G., O. Ince, E. Ertekin, C. Akyol, and B. Ince. 2013. Changes in performance and active microbial communities due to single and multiple effects of mixing and solid content in anaerobic digestion process of OTC medicated cattle manure. Int. J. Renew. Energ. Res. 3:144–48.
- VanderZaag, A. C., T. K. Flesch, R. L. Desjardins, H. Baldé, and T. Wright. 2014. Measuring methane emissions from two dairy farms: Seasonal and manure-management effects. Agr. Forest Meteorol. 194:259–67. doi:10.1016/j.agrformet.2014.02.003.
- VanderZaag, A. C., R. J. Gordon, R. C. Jamieson, D. L. Burton, and G. W. Stratton. 2010a. Effects of winter storage conditions and subsequent agitation on gaseous emissions from liquid dairy manure. Can. J. Soil Sci. 90 (1):229–39. doi:10.4141/CJSS09040.
- VanderZaag, A. C., R. J. Gordon, R. C. Jamieson, D. L. Burton, and G. W. Stratton. 2010b. Permeable synthetic covers for controlling emissions from liquid dairy manure. Appl. Eng. Agric. 26 (2):287–97. doi:10.13031/2013.29544.