ABSTRACT
Discarded electronic devices (E-waste) have historically been found to exceed US Toxicity Characteristic hazardous waste thresholds for lead. Research was conducted to assess whether global and national lead reduction initiatives in the past decade translate to reduced toxicity characteristic leaching procedure (TCLP) lead leaching from E-waste. Nine categories of devices were subjected to TCLP and in all devices except one (smoke detectors), mean TCLP lead concentration results decreased by an order of magnitude or more (to levels below regulation thresholds). Mean TCLP lead concentrations decreased from 29.1 mg/L (2000–2005) to 0.224 mg/L (2008+) for cell phones and 1.26 mg/L (2000–2005) to 0.060 mg/L (2008+) for PCs. Most recently manufactured electronic devices (of those types tested here) comply with the definition of non-hazardous waste under US regulations.
Implications: Discarded electronic devices (E-waste) have often been tested as hazardous waste in the US because of lead leaching. Toxicity characteristic leaching procedure (TCLP) testing on more recently manufactured devices reveals that global lead reduction efforts have resulted in newer devices complying with US non-hazardous waste definitions. While these results highlight the success of lead reduction efforts, they raise policy questions regarding how best to incentivize E-waste recycling going forward.
Introduction
The management of discarded electronic devices, referred to E-waste, E-scrap or waste electronic and electrical equipment (WEEE), remains a challenge; the magnitude and diversity of this waste stream continues to grow (Awasthi et al. Citation2018; Borthakur and Govind Citation2017; Musson et al. Citation2006; Nnorom and Osibanjo Citation2008; Townsend Citation2011) and the multitude of potential toxic elements contained within warrant appropriate handling measures (Chen et al. Citation2016; Garlapati Citation2016; Hira et al. Citation2018). In the European Union (EU), member nations are required to maintain WEEE recycling programs that meet specific recycling targets and to limit the amount of select hazardous chemicals found in the electronic devices (EU Citation2003; Official Journal of the European Communities, Citation2015). In addition, EU policy directives have been handed down that require EU member states to minimize the disposal of WEEE in household waste and ensure proper treatment of WEEE; appropriate inspection and monitoring is required and there are potential penalties for noncompliance with the directive (EU Citation2012). The US, however, does not have a similar encompassing regulatory scheme for management of E-waste at the federal level; existing recycling efforts are bolstered through a combination of state regulations, industry efforts, non-regulatory federal initiatives, and in some cases favorable economics.
US federal regulations that do have a major effect on how E-waste is managed are those in place for hazardous waste management under the Resource Conservation and Recovery Act (RCRA). Under the RCRA rules, any solid waste not otherwise exempted by definition will be considered hazardous waste if the leachate from the toxicity characteristic leaching procedure (TCLP) exceeds specified toxicity characteristic (TC) limits. Previous research found that cathode ray tubes (CRTs) and an array of other devices often “fail” the TCLP for the element lead (Jang and Townsend Citation2003; Kang, Chen, and Ogunseitan Citation2013; Lim et al. Citation2010, Citation2012; Lincoln et al. Citation2007; Musson et al. Citation2006; Townsend Citation2004, Citation2011; Yoshida et al. Citation2016). This observation prompted many state and local governments to ban or limit E-waste from landfill disposal (Townsend Citation2011), and the hazardous waste status of E-waste has often been referenced as motivation in public advertising campaigns or government policy initiatives (Kumar, Teichman, and Timpernagel Citation2012; USEPA Citation1999, Citation2013a).
E-waste has been proven to be an effective means to recover valuable resources (Kumar, Teichman, and Timpernagel Citation2012; Townsend Citation2011). The potentially recoverable resources should be considered in the context of the sheer magnitude of discarded electronics; close to 1 billion small electronic devices are expected to be disposed of within 5 years (Kumar, Holuszko, and Espinosa Citation2017). Recoverable gold from waste mobile phones alone in China, for example, is estimated to exceed 9 metric tons in the year 2025 (Tan et al. Citation2017). Recycling revenue from as-is waste electronics in the European market is estimated to exceed 2 billion euros (Cucchiella et al. Citation2015). However, the recycling potential of E-waste is also related to the content of hazardous elements contained within, and it is important to understand how evolving technology will impact the toxic element content of E-waste. For example, studies have found that some E-waste may contain toxic emerging contaminants that are not governed by any current legislation (Chen et al. Citation2016).
The original study examining the TC hazardous waste status of E-waste was conducted on devices manufactured from 1987 to 2003 (Townsend Citation2004). Since this time, in addition to the natural evolution of device configuration and component composition because of technological advances and consumer demands, other factors have led to changes in the content of potentially hazardous elements, including lead. In the US, the US Environmental Protection Agency (EPA) and various public and private stakeholder groups raised awareness of the E-waste lead issue as part of the lead-free solder partnership (Abtew and Selvaduray Citation2000; Geibig and Socolof Citation2005; Napp Citation1995; Rödel et al. Citation2009; Townsend Citation2011). The EU’s restriction of hazardous substances (RoHS) regulations limit the amount of lead, mercury, cadmium, and hexavalent chromium contained in new electronic devices in their jurisdiction (Official Journal of the European Communities, Citation2015). While rules similar to RoHS do not exist in the US, many manufactures now meet RoHS standards for all devices, regardless of where they are marketed. California adopted rules similar to RoHS by limiting the concentrations of lead, mercury, cadmium, and chromium in products sold in the state (Rödel et al. Citation2009).
Here we present research that follows up on our previous study characterizing the TC status of E-waste (Musson et al. Citation2006). The same categories of devices originally tested (manufactured from 1987 to 2003) are characterized for more recently manufactured devices (2000 to 2015). Two primary objectives are addressed. First, information on TC status of more recent devices provides important data for regulators, policy makers, and original equipment manufacturers (OEMs). Second, this work allows an examination of the efficacy of lead reduction initiatives by examining changes in the TCLP lead leaching as a function of device manufacture date.
Materials and methods
Sample collection and processing
Nine categories of discarded electronic devices (cell phones, remote controls, computer mice, smoke detectors, keyboards, personal computers (PCs), laptop computers, computer monitors, and printers) were collected from a local household hazardous waste collection center and classified in three groups based on the year the electronic devices were manufactured (2000–2005, 2006–2007, and after year 2008). We selected these time periods with consideration of RoHS adoption and implementation. RoHS was adopted by the EU in 2003 and took effect in June 2006 (2000–2005 = pre-RoHS, 2006–2007 = RoHS transition, and 2008 = post-RoHS). summarizes the different devices tested, their number, and include the number of samples analyzed in the original study. The total number of individual devices tested and reported here for each category device was equal to or greater than the amount examined in the original study. All of the electronic devices were first disassembled, separated into six categories (plastics, ferrous metals, non-ferrous metals, printed wire boards (PWBs), wires, and other), with each category weighed. Representative samples were then tested using the TCLP as described in the following sections. When laptop computers and computer monitors were disassembled, the fluorescent lamps were removed and excluded from the waste composition, as mercury was not a focus in this study.
Table 1. Number of electronic devices tested.
Leaching and analysis methods
Target elements for analysis included seven of the RCRA TC metals (arsenic, barium, cadmium, chromium, lead, selenium, and silver) and three other common elements (Fe, Cu, and Zn) also measured in the original study. Mercury in this study was not emphasized as it was always found below the TC limit (0.2 mg/L) in the first study and has generally been of minimal use in new electronic devices. However, mercury was measured randomly in leachates from every device type to verify similar results.
Two approaches, the standard TCLP and a modified TCLP, were employed. Both approaches used TCLP fluid 1 and a liquid to solid ratio (L:S) of 20:1. The standard TCLP was performed on smaller devices easily size-reduced to meet the TCLP requirement of less than 0.95 cm (cell phones, remote controls, computer mice, smoke detectors, and keyboards). The modified TCLP methodology uses a scaled-up apparatus that permits a larger amount of material to be leached (greater than 100 g) while maintaining the same leaching solution, contact time and liquid-to-solid ratio. Because of the difficulty in size reducing some devices (in this study these included PCs, laptop computers, computer monitors, and printers), the modified large-scale TCLP was conducted such that the devices were disassembled and tested in entirety (or near entirety) without size reduction (Musson et al. Citation2006; Townsend Citation2004). The validity and limitations of this approach have been previously discussed (Musson et al. Citation2006; Townsend Citation2004; Vann, Musson, and Townsend Citation2006a, Citation2006b).
Toxicity characteristic leaching procedure
The TCLP involves leaching 100 g of size-reduced (< 0.95 cm) sample with 2 L of leaching solution. As the devices tested using the standard methodology (cell phones, remote controls, computer mice, smoke detectors, and keyboards) were generally more than 100 g, each device was first disassembled into component parts (plastic, ferrous metal, non-ferrous metal, PWB, wire, and other). As necessary, the devices were size-reduced by hand (using shears or bolt cutters). A 100-g sample representative of each device was then recreated by randomly selecting components from each category and recombining them for a total weight of 100 g in the same proportion as the original composition. The only exception were remote controls (devices were less than 100 g); for this device type, multiple similar devices were combined using the same approach.
The prepared 100-g samples were placed into 2.34-L HDPE (high density polyethylene) bottles. The electronic device samples were evaluated to determine appropriate extraction solution following EPA Method 1311; every sample required use of TCLP fluid 1 (acetic acid mixed with sodium hydroxide). Two liters of TCLP fluid 1 were added to each TCLP bottle. The initial pH for TCLP fluid 1 was 4.93 ± 0.05, and the samples were placed on the rotator at a speed of 30 ± 2 rpm for 18 ± 2 hrs. After rotation, leachate pH was measured using a multi-probe meter (YSI Inc. model 556 MPS). The TCLP leachates were filtered through a 0.70-μm pore size glass fiber filter and preserved with nitric acid (trace metal grade) to a pH of less than 2. Filtered samples were digested using an auto-block (Environmental Express Inc. auto-block plus) following EPA method 3010A (USEPA Citation2013b). The digested samples were analyzed for trace metals using Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (Thermo-Scientific Inc. model iCAP 6000) in accordance with EPA Method 6010B (USEPA Citation2013b). Mercury was analyzed separately from other trace metals. Filtered TCLP leachate samples of each device type were randomly selected and digested using a Titan MPS microwave (Perkin Elmer Co. Ltd.) following EPA Method 3015A (USEPA Citation2013b). The microwave-digested samples were analyzed for mercury with the ICP-AES in the same manner as the other trace metals, except for a gold solution of approximately 0.4 mg/L was added in the ICP-standard, ICP-rinse, and sample solutions.
Modified large-scale TCLP
The modified large-scale TCLP was performed on four electronic device categories (PCs, laptop computers, computer monitors, and printers). This method permitted the entire device to be placed in a 210-liter HDPE drum for a maximum sample weight of 10 kg or a 110-liter HDPE drum for a maximum sample weight of 5 kg, depending on the mass of each electronic device (maintaining a L:S = 20:1). The rationale for using two HDPE drum sizes was to reduce differences in leach test results because vessel head space can affect metal leachability via an oxidation/reduction mechanism resulting from the presence of oxygen in the headspace air (Townsend Citation2004; Vann, Musson, and Townsend Citation2006a). For devices weighing more than 10 kg, sample masses were reduced to 10 kg while maintaining their original component compositions. For example, a PC manufactured in 2000–2005 with a total weight of 11.3 kg and a composition of plastic = 15.4%, ferrous metals = 60.6%, non-ferrous metal = 7.0%, PWB = 13.5% and wire = 3.6%, was reduced to a weight of 10 kg by adjusting component masses to 1.5 kg of plastic, 6.1 kg of ferrous metals, 0.7 kg of nonferrous metals, 1.3 kg of PWB, and 0.4 kg of wires. A similar approach was used for with masses slightly above 5 kg. If any of the larger electronic devices had a total weight less than 5 kg, multiple devices of the same manufacturer and model were combined to reach a weight of 5 kg. The disassembled samples were placed in 210-liter or 110-liter HDPE vessels depending on the total weight of sample tested. TCLP extraction fluid 1 was used and the volume of the extraction solution (100 liters or 200 liters) was prepared to maintain a L:S of 20:1. The vessel was placed on a drum rotator (Morse Inc. model 1–300 series) and rotated at a speed of 13 rpm for 18 hrs., the speed used in previous studies (Townsend Citation2004; Vann, Musson, and Townsend Citation2006a) (speed was found not to effect leachability). Leachate was measured upon completion and analyzed for trace metals in the same manner as previously described. A simple statistical analysis of variance (ANOVA) and t-test were introduced to determine the different lead concentrations for each period for every device by assuming all these data are normal distribution.
Results and discussion
Nine types of electronic devices were subjected to TCLP; a total of 211 TCLP assays were performed. The only element that ever exceeded its respective TC limit was lead. As expected, mercury results from the subset of devices tested (Table A.2 in Appendix A) were all lower than 0.002 mg/L. Thus, lead results are the primary discussion here. Results for the other elements are included in the Appendix A (Supplementary data).
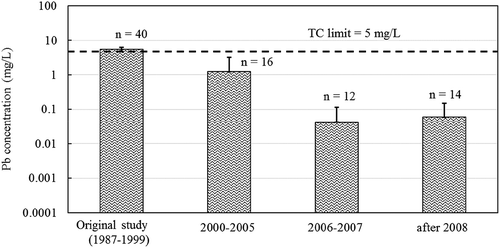
presents lead concentrations of cell phones; the average lead concentration for the 2000–2005 samples (29.1 mg/L) was statistically similar to the previous study (20. 5 mg/L), but the newer models were statistically lower (0.305 and 0.224 mg/L for the 2006–2007 and the 2008+ devices). TCLP lead concentrations for the PCs tested are displayed in . The average lead concentration for the 2000–2005 PC samples (1.25 mg/L) was statistically lower than the previous study (5.44 mg/L) and higher (though statistically similar) to the 2006–2007 and present (0.041 and 0.06 mg/L). The lead concentrations from both devices went from above the TC threshold in older devices to lower than the TC limit (5 mg/L) in newer devices. Similar trends were observed for other devices (Figure A.1 and A.2 in Appendix section) with the exception of the smoke detectors (Figure A.1 (d)); lead concentrations of the smoke detectors tested continued to exceed the TC limit (40.1 mg/L in 2000–2005 and 12.2 mg/L in 2008+).
Figure 1. TCLP lead concentrations for cell phones manufactured during different time periods. Error bars represent standard deviations.
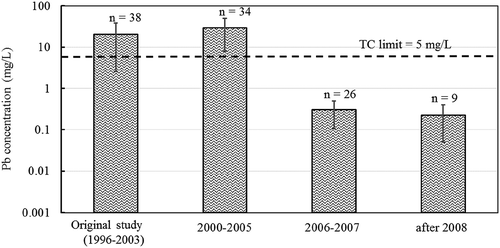
Figure 2. TCLP lead concentrations for PCs manufactured during different time periods; modified TCLP utilized. Error bars represent standard deviations.
The results suggest that for most electronic devices, lead contents were sufficiently reduced to translate into a significant decrease in TCLP-leachable lead. While no single factor can be definitely ascribed as the cause of lead reduction, the product stewardship initiatives implemented by OEM, whether in response to government policies and regulations or otherwise, manifested themselves in the results presented here. The OEM of most of the devices are large international electronics companies that market their products globally, and thus would be sensitive to pressures such as RoHS. The one device not demonstrative of a reduction, smoke detectors, tend to be manufactured by smaller companies (or subsidiaries), and in this study they marketed predominantly to US consumers. These devices are thus likely still manufactured using conveniently available, traditional lead-containing parts, such as lead solder in printed circuit boards, without concerning for mandated lead-reduction initiatives.
Conclusion
The data clearly show a decreasing trend of TCLP leachable lead concentrations in most electronic devices, notably after RoHS took effect in 2006. Most electronic devices manufactured in the past decade passed the US TC threshold limit for lead. These results will be welcome news for those advocating lead reduction, but they raise several policy questions that may need to be addressed. In the US, the rationale for stringent management requirements for E-waste beyond those for typical municipal waste has been the accepted status of E-waste as a likely frequent hazardous waste. Our results suggest that this status may no longer be appropriate; newer devices of the type tested here will in most cases not be hazardous. The benefits of E-waste recovery are well recognized, and even though these devices may be classified as non-hazardous in the US, they do still contain multiple elements that pose concern from an environmental perspective. In addition, several potentially toxic (to humans or otherwise) elements and compounds found in E-waste, such as Cu, Co, Sb, polychlorinated biphenyls (PCBs), or brominated flame-retardants, are not included in the TC hazardous waste list. E-waste recycling may be sufficiently well-entrenched in the US to continue in the future, but if not, alternative regulations and policy initiatives may become necessary to sustain growth in E-waste recycling.
Supplemental Material
Download PDF (1 MB)Acknowledgment
The authors thank the Alachua County household hazardous waste center, Kurt Seaburg, and his colleagues for providing materials. Also, the authors acknowledge the Royal Thai Government for providing the first author’s PhD scholarship. Moreover, the authors would like to thank Ryan Hundersmarck, as well as Assist. Prof. Pongsak Noophan from Kasetsart University (Thailand) and his internship students (Kanokwan Chumpolbunchorn, Kanin Songartigamas, Supawat Satjawathee, Pongpol Wangveeramitr, Thanapat Limsomboon, Peerason Pensoot) for helping prepare the samples. This work was partially supported by the Hinkley Center for Solid and Hazardous Waste Management.
Supplementary material
The supplemental information file provides an additional 3 tables and 11 figures.
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Vicharana Intrakamhaeng
Vicharana Intrakamhaeng received his Ph.D. in Environmental Engineering Sciences from the University of Florida in Gainesville, Florida, USA. He currently works for Thailand’s Ministry of Natural Resources and Environment.
Kyle A. Clavier
Kyle A. Clavier is a graduate research assistant in Environmental Engineering Sciences at the University of Florida in Gainesville, Florida, USA.
Timothy G. Townsend
Timothy G. Townsend is a Professor of Environmental Engineering Sciences at the University of Florida in Gainesville, Florida, USA. He teaches and conducts research on solid and hazardous waste management.
References
- Abtew, M., and G. Selvaduray. 2000. Lead-free solders in microelectronics. Mater. Sci. Eng. 27:95–141. doi:10.1016/S0927-796X(00)00010-3.
- Awasthi, A. K., F. Cucchiella, I. D’Adamo, J. Li, P. Rosa, S. Terzi, G. Wei, and X. Zeng. 2018. Modelling the correlations of e-waste quantity with economic increase. Sci. Tot. Environ. 613-614:46–53. doi:10.1016/j.scitotenv.2017.08.288.
- Borthakur, A., and M. Govind. 2017. Emerging trends in consumers’ E-waste disposal behaviour and awareness: A worldwide overview with special focus on India. Resour. Conserv. Reycl. 117B:102–13. doi:10.1016/j.resconrec.2016.11.011.
- Chen, M., O. Ogunseitan, J. Wang, H. Chen, B. Wang, and S. Chen. 2016. Evolution of electronic waste toxicity: Trends in innovation and regulation. Environ. Int. 89-90:147–54. doi:10.1016/j.envint.2016.01.022.
- Cucchiella, F., I. D’Adamo, S. C. L. Koh, and P. Rosa. 2015. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sust. Energ. Rev. 51:263–72. doi:10.1016/j.rser.2015.06.010.
- EU. 2003. Directive 2002/96/EC of the European parliament and of the council of 27 January 2003 on waste electrical and electronic equipment (WEEE) - joint declaration of the European parliament, the council and the commission relating to article 9. Directive 2002/96/EC. L037:0024-0039. Brussels, Belgium: European Parliament
- EU. 2012. Directive 2012/19/EU of the European parliament and of the council of 4 July 2012 on waste electrical and electronic equipment (WEEE). Off. J. Eur. Union. L. 197:38, 24.7.2012.
- Garlapati, V. K. 2016. E-waste in India and developed countries: Management, recycling, business and biotechnological initiatives. Renew. Sust. Energ. Rev. 54:874–81. doi:10.1016/j.rser.2015.10.106.
- Geibig, J. R., and M. L. Socolof. 2005, August. Solders in electronics: A life-cycle assessment. Washington, DC: US Environmental Protection Agency: Design for the Environment.
- Hira, M., S. Yadav, P. Morthekai, A. Linda, S. Kumar, and S. Anupam. 2018. Mobile phones- An asset or a liability: A study based on characterization and assessment of metals in waste mobile phone components using leaching tests. J. Hazard Mater. 342:29–40. doi:10.1016/j.jhazmat.2017.08.008.
- Jang, J. C., and T. G. Townsend. 2003. Leaching of lead from computer printed wire boards and cathode ray tubes by municipal solid waste landfill leachates. Environ. Sci. Technol. 7 (20):4778–84. doi:10.1021/es034155t.
- Kang, D. H. P., M. Chen, and O. A. Ogunseitan. 2013. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol. 47:5495–503. doi:10.1021/es400614y.
- Kumar, A., M. Holuszko, and D. C. R. Espinosa. 2017. E-waste: An overview on generation, collection, legislation, and recycling practices. Resour. Conserv. Recycl. 122:32–42. doi:10.1016/j.resconrec.2017.01.018.
- Kumar, S., S. Teichman, and T. Timpernagel. 2012. A green supply chain is a requirement for profitability. Int. J. Prod. Res. 50:1278–96. doi:10.1080/00207543.2011.571924.
- Lim, S., D. Kang, O. A. Ogunseitan, and J. M. Schoenung. 2010. Potential environmental impacts of light-emitting diodes (LEDs): Metallic resources, toxicity, and hazardous waste classification. Environ. Sci. Technol. 45:320–27. doi:10.1021/es101052q.
- Lim, S., D. Kang, O. A. Ogunseitan, and J. M. Schoenung. 2012. Potential environmental impacts from the metals in incandescent, compact fluorescent lamp (CFL), and light-emitting diode (LED) bulbs. Environ. Sci. Technol. 47:1040–47. doi:10.1021/es302886m.
- Lincoln, J. D., O. A. Ogunseitan, A. A. Shapiro, and J. M. Saphores. 2007. Leaching assessments of hazardous materials in cellular telephones. Environ. Sci. Technol. 41:2572–78.
- Musson, S. E., K. N. Vann, Y. Jang, S. Mutha, A. Jordan, B. Pearson, and T. G. Townsend. 2006. RCRA toxicity characterization of discarded electronic devices. Environ. Sci. Technol. 40 (8):2721–26.
- Napp, D. 1995. Lead free interconnect materials for the electronics industry. 238–44. Institute of Electrical and Electronics Engineers (IEEE). doi:10.1109/IEMT.1995.526121.
- Nnorom, I., and O. Osibanjo. 2008. Overview of electronic waste (e-waste) management practices and legislations, and their poor applications in developing countries. Resour. Conserv. Recycl. 52 (6):843–58. doi:10.1016/j.resconrec.2008.01.004.
- Official Journal of the European Communities, EC. 2015. Commission delegated directive (EU) 2015/863 of 31 March 2015 amending Annex II to Directive 2011/65/EU of the European parliament and of the council as regards the list of restricted substances. EUR-Lex. L. 137: 10–12.
- Rödel, J., W. Jo, K. T. Seifert, E. Anton, T. Granzow, and D. Damjanovic. 2009. Perspective on the development of lead‐free piezoceramics. J. Am. Ceram. Soc. 92:1153–77. doi:10.1111/j.1551-2916.2009.03061.x.
- Tan, Q., Q. Dong, L. Liu, Q. Song, Y. Lian, and J. Li. 2017. Potential recycling availability and capacity assessment on typical metals in waste mobile phones: A current research study in China. J. Clean. Prod. 148:509–17. doi:10.1016/j.jclepro.2017.02.036.
- Townsend, T. G. 2004. RCRA toxicity characterization of computer CPUs and other discarded electronic devices. Gainesville, Florida: US Environmental Protection Agency.
- Townsend, T. G. 2011. Environmental issues and management strategies for waste electronic and electrical equipment. J. Air Waste Manage. Assoc. 61:587–610. doi:10.3155/1047-3289.61.6.587.
- USEPA. 1999. Environmental technology verification report: Rechargeable alkaline household battery system rayovac corporation, renewal. EPA/600/R-99/005. USEPA Office of Research and Development, Washington, DC.
- USEPA. 2013a. Improved information could better enable EPA to manage electronic waste and enforce regulations. 13-P-0298. USEPA Office of Inspector General, Washington, DC.
- USEPA. 2013b. Test methods for evaluating Solid Waste (SW-846). Washington, DC: United States Environmental Protection Agency.
- Vann, K. N., S. Musson, and T. G. Townsend. 2006b. Evaluation of a modified TCLP methodology for RCRA toxicity characterization of computer CPUs. J. Hazard. Mater. 129:101–09. doi:10.1016/j.jhazmat.2005.08.011.
- Vann, K. N., S. E. Musson, and T. G. Townsend. 2006a. Factors affecting TCLP lead leachability from computer CPUs. Waste Manage. 26:293–98. doi:10.1016/j.wasman.2005.06.016.
- Yoshida, A., A. Terazono, F. C. Ballesteros Jr., D. C. Nguyen, S. Sukandar, M. Kojima, and S. Sakata. 2016. E-waste recycling processes in Indonesia, the Phillipines, and Vietnam: A case study of cathode ray tube TVs and monitors. Resour. Conserv. Recycl. 106:48–58. doi:10.1016/j.resconrec.2015.10.020.
