?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A solid-phase microextraction (SPME) sampling device, called a needle trap samplers (NTS) that were packed with 60–80 mesh divinylbenzene (DVB) particles, was used to extract indoor volatile organic compounds (VOCs) that were emitted in an oil painting studio. This work compared the sampling performances of a passive NTS and an active charcoal desorption tube that was connected to a personal sampling pump (Method 1501), developed by the National Institute of Occupational Safety and Health, USA. The experimental results reveal that the NTS is a dependable alternative device to Method 1501 for monitoring indoor air quality. 2,2,4,6,6-Pentamethylheptane (isododecane) is the main emitted pollutant when oil painters use odorless thinner as a substitute solvent for turpentine oil, and the mean exposed concentrations of isododecane determined by NTS ranged from 0.83 to 3.10 ppm, which were dependent on whether the indoor ventilation was performed by the natural or mechanic mode. To maintain adequate air exchange rates in an oil painting studio, doors should be opened to increase air circulation, lowering the concentrations of isododecane to which painters are exposed.
Implications: A needle trap sampler (NTS) was used to sample VOCs from oil painting in an indoor studio. Isododecane is the main emitted pollutant when painters use the odorless thinner. The NTS was evaluated to be a dependable alternative to Method 1501 for monitoring indoor air quality. To maintain adequate air exchange rates in a painting studio, doors should be opened to lower concentrations of VOCs.
Introduction
The painting materials that are used in indoor art studios are the main sources of air pollution therein; however, the properties of indoor air pollutants, from corrosive mists of acid etches to low-hazardous sprays of fixatives, vary widely, depending on the method of painting (Ryan, Hart, and Kappler Citation2002). Exposure to volatile organic compounds (VOCs) critically affects human health in an art studio (Al-Awadi Citation2018; Mishra et al. Citation2015; Ryan, Hart, and Kappler Citation2002; Yurdakul et al. Citation2017).
Ryan, Hart, and Kappler (Citation2002) determined exposures of printmakers to VOCs in mixed-use university studios in the United States. The main compounds and their average concentrations were toluene at 17.1 ppb, 1,1,1-trichloroethane at 7.5 ppb and xylenes at 1.8 ppb. Mishra et al. (Citation2015) reported that 41% of indoor VOCs came from air fresheners and art and craft activities in the classrooms of 25 state schools in the Brisbane metropolitan area, Australia. Al-Awadi (Citation2018) measured the concentrations of compounds with aliphatic, aromatic, halogenated and oxygenated functional groups in painting studios in four middle and elementary schools in Kuwait. The total volatile organic compound (TVOC) concentrations ranged from 0.1 to 63.8 mg/m3 (0.15 to 97.6 ppm), and the mean concentration was 3.6 mg/m3 (5.5 ppm). Chang (Citation2011) investigated the ambient VOC emissions in oil painting classrooms of three Taiwanese universities. The results revealed the concentrations of indoor TVOC ranged from 5.6 to 6.6 ppm and indoor turpentine from 0.47 to 1.11 ppm. Except for Chang’s study (Citation2011), the personal exposures of oil painting have rarely been targets in investigations of indoor VOC emissions.
Oil painting originated in tempera painting in the Western world. To avoid the problem of rapid drying, some tempera painting artists added an appropriate amount of volatile oil to their oil paint (National Taiwan Arts Education Center Citation2013). Volatile oils can be used to dilute paint to make it easier to paint and also be used to clean pigments off painters’ brushes; however, the oils will gradually evaporate into the air during painting. Turpentine oil is the most commonly used volatile oil (Chen Citation1993).
Turpentine oil is an essential oil of a plant, which is manufactured from the pine resin. The main chemical constituents of turpentine oil are terpenes. Human exposure to turpentine oil potentially causes irritation, headaches, exanthema and itching. Long-term exposure to turpentine oil potentially causes dermal degreasing and kidney damage (Occupational Safety and Health Administration, Taiwan Citation2019). The OSHA, Taiwan, has set the 8-hr time weighted average permissible exposure limit (8-hr TWA PEL) for turpentine in the workplace at 100 ppm (560 mg/m3). Painting studios currently use odorless thinner (petroleum hydrocarbons) instead of turpentine oil to reduce harm to human health.
In this work, air samples were taken to examine whether odorless thinner affects the indoor air quality in an oil painting studio, in which painting is taught by an artist. Organic compounds in air were sampled using Method 1501 (NIOSH Citation2003) and passive needle trap samplers (NTS), to assess the human exposures to chemical emissions from odorless thinner in the studio. Recommendations concerning indoor ventilation are made based on the results of sampling and analysis.
Methods
Extraction of gaseous compounds using needle trap samplers
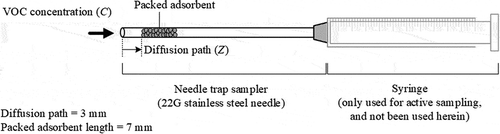
NTS extract gaseous chemical compounds through needles by air diffusion. A linear gaseous concentration profile (C(Z) in ) is obtained along the diffusion path (Z), and the extraction of the analyte is characterized by the area (A) of opening and the length of the diffusion path. The total mass (n) of analyte that is extracted in a time interval (t) is estimated as follows, using Equation (1) (Lord, Zhan, and Pawliszyn Citation2010):
where Dm is the diffusion coefficient of the gaseous compound that is extracted using the NTS. Accordingly, the quantity (n) of the extracted analyte is assumed to be proportional to the mean sample concentration over a time interval (C(t), time weighted average (TWA) concentration) given a constant Dm, a uniform needle opening (A), and a fixed diffusion path distance (Z).
Preparation of needle trap samplers
NTS comprises a 22-gauge stainless steel needle that is packed with DVB adsorbent with a 60–80 mesh particle size. DVB particles were packed by aspiration to the desired length of 7 mm and a diffusion path length of 3 mm (). A very small amount of epoxy glue was applied to the exposed portion of the adsorbent layer to immobilize DVB particles. To prevent blockage of the NTS epoxy-resin plug, air was drawn continuously through the NTS packing phase as the epoxy cured. Finally, the DVB in the NTS was conditioned by heating at the injection port of the GC-FID to 260°C for 30 minutes (min).
The uniformity of the packing phase in an NTS was studied by following the procedures which were established in the earlier works (Cheng et al. Citation2017, Citation2014; Cheng and Wu Citation2019). The desired air flow rate through the NTS was set by drawing air through the packing phase using an aspiration pump. When the relative standard deviation (RSD) of the flow rates across three duplicate tests did not exceed 5%, the packed materials inside the NTS were assumed to be uniformly immobilized. BTEX standard gas samples (analytic grade, Merck, Germany), around 10 ppm, were prepared in a 500 mL Pyrex glass bulb, in which the NTS was inserted for 1–2 hrs to adsorb gaseous BTEX. When the RSD of the sampled mass in triplicate tests was less than 5%, the BTEX adsorption capacities of the tested NTS were qualified and the NTS was ready for use (Cheng et al. Citation2017, Citation2014; Cheng and Wu Citation2019). NTS was checked the adsorption performance using standard gaseous toluene 10 ppm, and then calibrated via GC-FID per five site sampling. If the duplicate test reveals GC-FID analysis deviations exceeding 5%, the NTS should be replaced. Notably, based on Chen and Pawliszyn’s study (Citation2003), ambient temperature (5–35°C) and relative humidity (45–75%) did not significantly affect the adsorption performance of C5–C9 alkanes TWA sampling using NTS.
Chemicals, materials and instruments
Stainless steel needles (22 G, length 7 cm and ID 0.41 mm) were purchased from a local company (Herling Co. Ltd., Pingtung, Taiwan) for use in preparing the NTS. Adsorbent DVB particles were purchased from Supelco (Bellefonte, PA, USA). Aspirating pumps, which were used in examining the NTS sampling flow rates, were purchased from Kitagawa (AP-20, Kawasaki, Japan), and epoxy glue was purchased from Nan-Pao Applied Material Co. Ltd. (Taoyuan, Taiwan). All gases for chromatographic analysis (Jing-De Gas Co., Ltd., Kaohsiung, Taiwan) were of ultra-high purity.
Gaseous samples were analyzed using a GC (6890N, Agilent, Wilmington, DE, USA) that was equipped with a flame ionization detector (FID). The capillary column was HP19091Z-413 HP-1 PDMS (30 m × 320 μm × 0.25 μm) (Agilent Technologies, Inc., Wilmington, DE, USA). The unknown chemical compounds were analyzed to determine the constituents using another gas chromatography-mass spectrum (MS 5973, Agilent) system.
Sampling and analysis
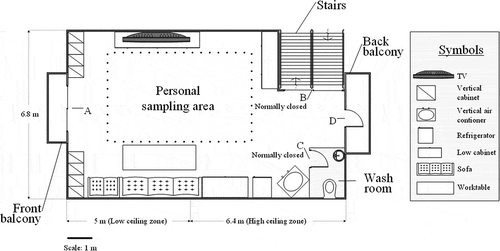
The target sampling site was an oil painting teaching studio in the Fengshan District, Kaohsiung City, Taiwan. This painting studio was located on the top floor of a four-floor building. displays the layout. Notably, the ventilation was either mechanical, using an air conditioner, or natural, through the french windows, which were on the opposite long sides of the studio. Mechanic or natural ventilation was chosen to suit human thermal senses. presents the interior dimensions of the studio and its opening doors. The main organic solvent that is used for oil painting is odorless thinner, which is used primarily to wash out pigments from the artists’ brushes. shows a typical standing postures of artists who were painting and wearing the air samplers. During painting, VOCs are emitted, especially when the artists are washing their brushes using odorless thinner. Generally, the artists do not use any personal protective equipment (PPE) to prevent exposure to VOCs. To measure the artists’ TWA exposure to organic vapors, two types of samplers were worn simultaneously, as shown in . (a) The first is an NTS, which was fixed on a plastic sheet, and (b) the second is a personal sampler, which comprises a charcoal tube (Method 1501 for hydrocarbons), that is connected to a personal air pump for active sampling, and was developed by the U.S. National Institute for Occupational Safety and Health (NIOSH Citation2003).
Table 1. The inner dimensions of the painting studio and the opening doors.
Figure 3. The artist wearing samplers. Left dotted oval: charcoal tube (Method 1501); Right dotted box: NTS fixed on a plastic plate.

At least two or three persons wore the samplers for each site sampling. Totally 28 samples were collected for analysis. After VOC sampling, the NTS were taken to the laboratory and then inserted into the injection ports of the GC-FID to desorb VOCs for analysis. The desorption time and temperature at the injection port were 30 sec and 250°C, respectively. The temperature of the GC was increased from 50°C in increments of 15°C/min to 180°C, which was held for 2 min. The FID detector was heated to 300°C. The flow rate of the carrier gas, nitrogen, was 1.2 mL/min, and the split-off operating mode was used.
Results and discussions
Evaluation of emissions into air from odorless thinner in a painting studio
shows the main gaseous organic compounds (>10 ppb) that are emitted by the odorless thinner during oil painting, collected in 30 sec at 27°C using 500-mL Teflon airbags and then identified by GC/MS analysis. The compounds were divided into two groups. (a) Hydrocarbons with less than six carbons (≤ C6 hydrocarbons) which were propanone (acetone), dichloromethane and n-hexane; (b) Aliphatic hydrocarbons with more than six carbons (> C6 hydrocarbons) which were 3-chloromethylheptane (2-ethylhexyl chloride), 2,2,4,6,6-pentamethylheptane (isododecane), 2,2,3-trimethyldecane, 2,7,10-trimethyldodecane and 2,8-dimethylundecane. Notably, isododecane had the highest concentration, 481 ppb, among all of the gaseous emitted compounds. The result is consistent with the fact that the most dominant constituent of odorless thinners that are used in oil painting studios is petroleum hydrocarbons with many carbon atoms.
Table 2. Main airborne-emitted compounds from odorless thinner.
Based on the data in , most compounds that are emitted by odorless thinners are not as hazardous to human as is turpentine oil vapor (8-hr PEL 100 ppm), except for dichloromethane (8-hr PEL 50 ppm) and n-hexane (8-hr PEL 50 ppm). The PEL values of both dichloromethane and n-hexane are at least 1,700-fold higher than the concentrations in . However, the main emitted compound, isododecane, has irritative and anesthetic effects on human beings, and its emission vapor may burn and even explode if it is mixed with a flame, heat and oxidizing agents. The following section presents the way in which artists’ personal isododecane TWA exposure concentrations were determined using NTS.
Determining personal TWA exposure concentrations of isododecane using NTS
In Equation (1) (Lord, Zhan, and Pawliszyn Citation2010), the total extracted mass (n) of analyte is proportional to the time interval (t) and the concentration (C) of the analyte if the coefficients Dm, Z and A are fixed. Six concentrations (0, 2, 5, 10, 15 and 20 ppm) of isododecane were prepared in separate Pyrex glass bulbs with a volume of 500 mL. The different two or three NTS were inserted in series into the bulbs to perform extraction tests with a sampling period of 15 min. Those procedures were followed according to Cheng and Wu (Citation2019). The extracted masses of isododecane and the corresponding integrated areas under the peaks that were obtained by GC-FID yield the calibration equation:
where R2 is a linear coefficient of regression. The practical TWA exposure concentrations (ppm) of isododecane must be multiplied by the multiplying factor (f) with a time weighting of f = [15 min(practical sampling time)] when the concentrations of isododecane are calculated using Equation (2).
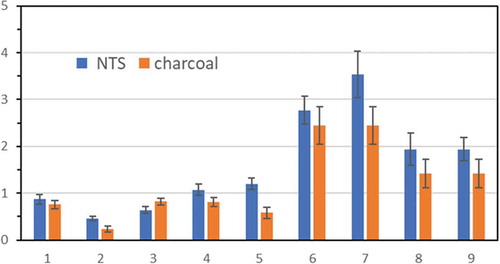
presents the analysis concentrations and the standard deviations of the average of replicate measurements using NTS and charcoal tube (Method 1501, NIOSH) for collecting isododecane in the studio. The distributions of error bars for two methods in are similar; namely, smaller error bars are with the lower concentrations, and bigger error bars are with the higher concentrations. Both sampling methods performed stable collection efficiencies for isododecane in the oil painting studio. The relative differences from the data obtained by analyzing the two sampling approaches, involving NTS and charcoal tubes, were as low as 12–30%, except for samplings #2 and #5 in , in which cases the concentrations sampled by charcoal tubes were extremely low and less than 1.0 ppm. Notably, the analyzed isododecane data that were obtained using NTS mostly were higher than those using charcoal tubes, so the NTS adsorption method is more sensitive than the method that was used with charcoal tubes for isododecane collection. It also follows the performance that the NTS method adsorbed gaseous hydrocarbons more effectively than charcoal tubes at the construction site (Cheng et al. Citation2017) and the medical examination center (Cheng, et al., 2019). Furthermore, NTS is environmentally friendly as the thermal desorption of NTS for the chromatographic analysis requires no solvent, and NTS can be reused for at least ten times for VOC sampling. Therefore, NTS should be used as a lightweight monitor of human exposure to organic chemicals at working places.
Indoor air quality and air ventilation in studios
The air conditioner was operating and doors were open in the studio during the first five site samplings in . The air conditioner was operating and all doors were closed for the subsequent four samplings. reveals that in the closed studio, the personal exposure concentrations of isododecane, which were measured by NTS and Method 1501, exceeded those in the open environment. The carbon dioxide concentration, an index of indoor air quality, was greater when the doors were closed than when the doors were open if the air conditioning was operating. As time spent on oil painting passed, the concentration of CO2 gradually increased in the studio, especially when the doors were closed. According to the data in , when two or three electric fans were running in the studio with open doors, the air was exchanged five to eight times per hour. Four to seven artists were exposed to isododecane concentrations of less than 1 ppm as the concentration of CO2 increased slowly over the painting period around four hours.
Table 3. Personal exposures of isododecane for different ventilation modes and the indoor air quality indexes in the studio.
Conclusion
Based on experimental results, NTS was evaluated as mini-sized samplers to measure personal exposure to organic vapors. NTS are economically efficient as they can be used 10–20 times owing to thermal desorption at the gas chromatograph inlet following the site samplings. Notably, the diffusion path in an NTS will be gradually clogged by particles, which are emitted from the combustion process, like the combustion of incense coils (Cheng et al. Citation2015). The clogged NTS collected less mass of organic compounds than non-clogged NTS and adsorbed no compounds after only 3–5 time samplings for combustion sources.
NTS sampling pen () is a microminiaturized sampler (with a mass of only 200 g and a length of 20 cm) with auxiliary functions of detecting air temperature and humidity. Inside the sampling pen is a stainless steel needle for extracting VOCs. The multifunctionality and lightweight of the NTS sampling pen should increase its acceptability among wearers and make it preferable to the traditional charcoal tube sampling procedure.
Figure 5. Sampling pen with an NTS inside. Detections of humidity and temperature can be seen on the monitoring screen.

Presently, petroleum hydrocarbons are not identified as hazardous organic compounds; however, they are irritative and anesthetic effects on human beings, so effective indoor ventilation is essential when petroleum hydrocarbon solvents are used. It is recommended that the air exchange rate indoors should be considered and the masks with an activated carbon layer should be worn by oil painters.
Acknowledgments
The authors would like to thank the support and assistance in the field sampling from Mr. Bi-Chou Cheng and members of the painting group.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Wen-Hsi Cheng
Wen-Hsi Cheng is a professor at the Department of Occupational Safety and Hygiene, Fooyin University, Kaohsiung City, Taiwan. His research interests are control of VOCs and analysis technology of solid phase microextraction.
Ming-Hsiu Chuang
Hsiao-Lin Huang is a professor and Ming-Hsiu Chuang graduated from the Institute of Industrial Safety and Disaster Prevention, Chia Nan University of Pharmacy and Science, Tainan City, Taiwan.
References
- Al-Awadi, L. 2018. Assessment of indoor levels of volatile organic compounds and carbon dioxide in schools in Kuwait. J. Air Waste Manage. Assoc. 68:54–72. doi:10.1080/10962247.2017.1365781.
- Chang, K. Y. 2011. Indoor air quality and related factors of oil painting classroom in universities. Master Thesis of School of Public Health, Taipei Medical University. (In Chinese).
- Chen, J. R. 1993. The skills of oil painting. Taipei, Taiwan: Lion Arts Co. Ltd. ISBN: 9579420688. (In Chinese).
- Chen, Y., and J. Pawliszyn. 2003. Time-weighted average passive sampling with a solid-phase microextraction device. Anal. Chem. 75:2004–2010.
- Cheng, W. H., C. H. Lai, W. J. Tzeng, C. Her, and Y. H. Hsu. 2015. Gaseous products of incense coil combustion extracted by passive solid phase microextraction samplers. Atmosphere 6:822–33. doi:10.3390/atmos6060822.
- Cheng, W. H., H. L. Huang, K. S. Chen, and Y. C. Chang. 2017. Quantification of VOC emissions from paint spraying on a construction site using solid phase microextraction devices. J. Environ. Sci. Health A52:1158–1163.
- Cheng, W. H., and H. M. Wu. 2019. Assessing organic chemical emissions and workers’ risk of exposure in a medical examination center using solid phase microextraction devices. Aerosol. Air Qual. Res. 19:865–870. doi:10.4209/aaqr.2018.08.0288.
- Cheng, W. H., J. R. Jiang, C. Lin., J. J. Liou, Z. H. Wu, Y. H. Hsu, and Z. Y. Yang. 2014. Preparation of needle trap samplers to extract air compounds from indoor electric-vaporizing sources. J. Air Waste Manage. Assoc. 64:488–93. doi:10.1080/10962247.2013.870941.
- Lord, H., W. Zhan, and J. Pawliszyn. 2010. Fundamentals and applications of needle trap devices. Anal. Chim. Acta 677:3–18. doi:10.1016/j.aca.2010.06.020.
- Mishra, N., J. Bartsch, G. A. Ayoko, T. Salthammer, and L. Morawska. 2015. Volatile organic compounds: Characteristics, distribution and sources in urban schools. Atmos. Environ. 106:485–91. doi:10.1016/j.atmosenv.2014.10.052.
- National Institute for Occupational Safety and Health. 2003. Manual of analytical methods, 4th ed. Atlanta, GA: National Institute for Occupational Safety and Health.
- National Taiwan Arts Education Center. 2013. Understand oil paints to enjoy oil paintings. Taipei, Taiwan: National Taiwan Arts Education Center. ISBN: 9789860360981. (In Chinese).
- Occupational Safety and Health Administration, Ministry of Labor. 2019. Globally harmonized system website. Taipei, Taiwan. Accessed March 20, 2019. https://ghs.osha.gov.tw/cht/intro/search.aspx.
- Ryan, T. J., E. M. Hart, and L. L. Kappler. 2002. VOC exposures in a mixed-used university art building. AIHA J. 63:703–08. doi:10.1080/15428110208984758.
- Yurdakul, S., M. Civan, Ö. Özden, E. Gaga, T. Döğeroğlu, and G. Tuncel. 2017. Spatial variation of VOCs and inorganic pollutants in a university building. Atmos. Pollut. Res. 8:1–12. doi:10.1016/j.apr.2016.07.001.