ABSTRACT
Biological waste from marine sources is discarded into various water bodies which leads to dramatic increase in the water pollution near coastal areas. This animal waste consists of bioactive compounds such as fatty acids, amino acids, and chitin which can be used in agricultural and pharmaceutical sectors. The aim of the current study was to extract chitosan (CS) from the discarded shells of Carinosquilla multicarinata and prepare anti–inflammatory drug diclofenac potassium (DP) encapsulated chitosan nanoparticles (DP-CSNPs). The CS was extracted, purified and physicochemical and morphological properties were characterized such as viscosity (1.44cPs), molecular weight (~57 kDa), degree of deacetylation (83%). The DP-CSNPs were prepared by ionic gelation of extracted chitosan with tripolyphosphate (TPP) anions by varying chitosan, TPP, and drug concentrations. SEM imaging showed that DP-CSNPs were nano-sized (248 nm) along with small, spherical, and uniformity in shape. The endothermic peak appeared at 180°C while performing the thermal analysis of DP-CSNPs by differential scanning calorimetry (DSC). The Loading capacity (LC) and encapsulation efficiency (EE) were determined for all combinations while maximum EE (79.42%), LC (42.08%), and +0.00459 mV for Zeta potential were found for nanoparticles synthesized from CS with 2.5mg/mL concentration and 1mg/mL of TPP and drug concentrations. Moreover, in vitro drug release study was performed at simulated biological fluid (pH 7.4) and at 10th hr maximum (80%) of the drug was released from DP-CSNPs. Therefore, this waste source would be a better model system for the drug release.
Implications: Dumping of marine waste into deep ocean has led to dramatic increase in water pollution leading to the endangerment of various oceanic animals. This discarded waste can be used sustainably for the isolation of various biopolymers into the ultimate use for human community. The work provides a detailed guide into the method of extraction of low molecular weight chitosan and preparation of nanoparticles for the delivery of anti-inflammatory drug diclofenac.
Introduction
Marine sources include abundant amount of fishes, shrimps, and crabs which are used as seafood in different parts of the world. The fresh animals are sold by the fisherman to domestic consumers as well as various restaurant chains while the trash is discarded at the harbor which degrade land and water. This non-edible waste has very high oxygen demand due to which its proper disposal is necessary. When left untreated this waste generates a lot of harmful gases such as hydrogen sulfide which can lead to decreased soil fertility and air pollution as well (Yuvaraj et al. Citation2016). Furthermore, the waste dumped into deep ocean forms a layer on the water surface which affects marine animals and also harms the marine fauna due to inability of the sunlight to penetrate inside. One of the abundant animals found in the dumped areas at the coast was squilla and shrimps which have a high polymer content in their exoskeleton. Dumping of this waste into the ocean without any anaerobic degradation has several harmful effects as explained by Kim et al. (Citation2007) which can be related to the major cause of ocean water pollution and death of many marine organisms. Moreover, marine fisheries constitute about 10% of the total waste produced from the food industry (Khedkar and Singh Citation2018). These marine waste sources such as crab, squilla, and krill shells can be used economically for the extraction of low molecular weight chitosan (CS) by deacetylation process (Bastiaens et al. Citation2019). Also, extract peptides from the muscle and visceral mass of fishes exhibiting various anti–inflammatory, antimicrobial, and antioxidant properties. The fact that many polymers have a characteristic property of protecting drug against the acidic medium of gastric juices and various actions of enzymes has attracted many scientists to work on drug-loaded polymer-based nanoparticles for the past few decades. Controlled release systems such as microparticles, nanoparticles, and liposomes have been prepared to increase the drug bioavailability at particular target sites. The partially deacetylated product of chitin is chitosan which is a copolymer of N-acetylglucosamine and glucosamine. Being least toxic, highly biodegradable and biocompatible, chitosan has increased attention as a promising biomaterial for various purposes (Naskar, Koutsu, and Sharma Citation2019). It finds various applications in the pharmaceutical and biomedical industries such as anti-diabetic agents, anti-cancerous, wound healing, drug delivery agents, and blood anticoagulants. According to Parthiban et al. (Citation2017) the squilla’s chitosan is high in quality and useful for various applications. Squilla as well as crab shells are composed of calcium and other salt compounds which helps them in the defense mechanism. Moreover, due to the presence of these salts the degree of deacetylation is reduced and hence it needs more treatment for removing impurities. The salts interfere with the acetyl group present in the chitin and chitosan polymer which leads to lower deacetylation capacity.
Nonsteroidal anti–inflammatory drugs (NSAIDs) are one of the world’s most commonly used class of medicinal drugs for reducing inflammation due to their antipyretic and analgesic effects which is shown due to suppression of the prostaglandin synthesis owning to feedback inhibition of the cyclooxygenase (COX) enzyme. Diclofenac potassium (DP) (318 Da), a derivative of benzene acetic acid, is a widely used NSAID with anti–inflammatory, antirheumatic, analgesic, osteoarthritis, and antipyretic effects. The biological half-life of DP is about 1–2 hr and the usual oral dose is 50–200 mg per person for 2–4 times a day, depending upon the route of administration (Hinz et al. Citation2005). Therefore, it requires multiple dosages in the body for the maintenance of the therapeutic level of the drug into the blood (pH 7.4).
In this study, chitosan (CS) was extracted and purified from Carinosquilla multicarinata shells collected from discarded trash found near the waterfront and was characterized. Furthermore, a series of chitosan nanoparticles (CSNPs) loaded with diclofenac (DP) were developed using different concentrations of Tripolyphosphate (TPP) crosslinking agent and CS by ionic gelation method. Ionic gelation is the most common and successful technique for the preparation of nanoparticles where several crosslinking agents are used. Crosslinking agents help in formation of spherical structures with determined size. The size of nanoparticles depends on the crosslinker ratio, speed of its addition into polymer solution. Generally, anionic crosslinker agents such as TPP and sodium sulfate are used for the formation of CS-based nanoparticles as CS is a cationic polymer where the polyelectrolyte complex is stabilized at electrostatic interaction (de Carvalho et al. Citation2019). TPP is most commonly used due to its cheap availability and its non-toxicity and hence it is also used in food and beverage industry as a binding agent. Further, the developed DP-CSNPs in the study were characterized by its functional group, morphological observation, and its controlled drug delivery in vitro.
Materials and methods
Materials used and collection of waste shells
DP and TPP (P2O5/Na2O = 0.6; molecular weight = 937.86 ± 0.025 Da) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Commercial chitosan (DD ≥75%) and dialysis membrane-110 (MWCO 12–14 kDa) were obtained from HiMedia, Mumbai, India. All other chemicals used in this study were of reagent grade.
C. multicarinata was collected from discarded waste at Royapuram (13°6ʹ26ʹ’ N and 80°17ʹ43ʹ’E) coast, Chennai, Tamil Nadu, India. The collected samples were properly cleaned, shells were removed and washed several times under running water to remove any remaining dead tissue matter, sun dried and then stored at −20°C until use.
Proximate analysis of the dried shell powder
The moisture and ash contents were estimated for shell powder by heating it at 100°C for 24 h and 420°C for 1 hr, respectively, in a muffle furnace explained by Gaikwad, Koli, and Desai (Citation2015). Carbohydrate content was estimated for powdered shells by the method described by Albalasmeh, Berhe, and Ghezzehei (Citation2013) using 5% phenol solution and sulfuric acid. Further, the absorbance was measured at 490 nm and the carbohydrate percentage was calculated.
Extraction and purification of CS from shells
The shells were washed with distilled water, dried at 50°C for 6 hr and powdered using a grinder. To obtain chitin from the shell powder 100 gm of it was used and various steps were followed including demineralization (2N HCl for 6 hr at 37°C), deproteinization (2M NaOH for 3 hr at 90°C) and decolorization step by mixing with acetone at a solid/solvent ratio of 1:10 for 10 min as explained by Nessa et al. (Citation2010). The pellet was collected after centrifugation and dried. The obtained chitin was further deacetylated to produce chitosan (CS). The process of deacetylation is carried out by the addition of 50% NaOH into the chitin solution and gentle stirring for 12 hr at 90°C over hot plate and further washed with neutral water until pH is neutralized. The extracted CS was purified by the method previously described by Divakar et al. (Citation2018) using 10% SDS and 5% (w/v) EDTA in extracted CS solution.SDS is detergent which is used for protein precipitation and EDTA acts as a chelating agent for the removal of mineral impurities. The samples were dried in hot air oven at 60°C and the dry weight was measured to calculate the yield percentage.
Material characterization of purified CS
The functional group peak analysis was done using Fourier transformation infrared (FTIR) spectrophotometer (Cary 660 FTIR, Agilent Technologies, USA) for extracted CS from shells of squilla at a frequency of 4000–400 cm−1. To record FTIR spectra, CS powder was mixed with an appropriate amount of KBr and compressed under higher pressure in pelletizer to obtain a thin pellet of 10 mm diameter with a concentration of the sample of 0.1% w/w (Hassan et al. Citation2018). Scanning electron microscopy (SEM) (JSM-5600LV; JEOL, Tokyo, Japan) analysis was done to determine the structural morphology of the CS, which was also equipped with Energy dispersive X-ray spectroscopy (EDS) to determine the presence of elemental impurities in samples. X-ray diffraction (XRD) analysis was done using PANalytical X’PERT PRO powder diffractometer to find the physical nature of extracted CS using the position of characteristic peaks. Diffraction graph was obtained using Cu Kα radiation (λ = 1.54 A°) over the range 5–40° 2θat a scan rate of 2° 2θ per minute. The spectra were obtained at 45 kV, 25 mA at 25°C (Hussein‐Al‐Ali et al. Citation2016). Furthermore, the crystallinity index (CrI) was calculated for crude and purified CS.
Physicochemical characterization of purified CS
Intrinsic viscosity was assessed using Brookfield viscometer (Brookfield Engineering Labs, Stoughton, MA, USA). In brief, 1% CS solution was prepared in the solvent acetic acid (0.5 M), sodium acetate (0.2 M) and intrinsic viscosity were determined using No. 18 spindle at 100 rpm at 25°C. Intrinsic viscosity was further used for the calculation of the average molecular weight for the prepared samples using the Mark-Houwink equation (Wang et al. Citation1991). Degree of acetylation (DA) and deacetylation (DD) was obtained for CS using the equations described by Takarina et al. (Citation2017). The water and fat binding capacity were determined using a modified procedure described by Kumari, Rath, and Kumar (Citation2016). Briefly, 15 ml centrifuge tube containing the known amount of extracted CS sample was added and weighed. To determine the WBC, 10 ml of milliQ water were added into the centrifuge tube and vortexed for 1 min to disperse CS powder in water. Later tubes were kept at 37°C for 30 min with constant mixing after every 10 min and further centrifugation for 25 min at 6000 rpm. For evaluating FBC, olive oil was used instead of water and the percentage WBC and FBC were calculated.
Preparation of DP-CSNPs
Extracted CS was used for the synthesis of Blank-CSNPs and DP-CSNPs using crosslinking ionic gelation method where different concentrations (0.5–3.0 mg/mL) of chitosan solution were prepared in acetic acid (1%, v/v) and the pH was maintained at 5. Furthermore, different concentrations (0.5–2.5mg/mL) of tripolyphosphate solution were prepared and the pH was maintained at 2 (Sreekumar et al. Citation2018). TPP solution was added dropwise using a syringe into the chitosan solution under continuous stirring at 600 rpm at ambient temperature until an opalescent solution was obtained which states the formation of nanoparticles (Hassan et al. Citation2018). Similarly, diclofenac encapsulated chitosan nanoparticles (DP-CSNPs) were also prepared using ionic gelation method in which different concentrations of CS were prepared along with varying TPP-DP concentrations (0.5–2.5mg/mL) and used for the synthesis of DP-CSNPs described by Alqahtani et al. (Citation2019).
Characterization of nanoparticles
FTIR, XRD analysis, and SEM imaging were done for drug, blank NPs and DP-CSNPs to find out the functional group peaks, physical state, and morphological features, respectively. The size was determined through dynamic light scattering (DLS) measurement, performed on a Zetasizer (Nano-ZS90, Malvern Instru. Ltd) at a scattering angle of 173° (Sotelo-Boyás et al. Citation2017). Zeta potential distribution on DP-CSNPs was evaluated by instrument Zetasizer (Ver. 6.32, MAL1066495, Malvern Instruments Ltd) at 25°C taking water as a dispersant and placing it in Zeta dip cell where the electric field was applied (Hussein‐Al‐Ali et al. Citation2016). The thermal analysis was done for DP loaded CSNPs to find out any possible drug–polymer interaction by doing differential scanning calorimetry (DSC) (2910 DSC, TA Instruments, New Castle, DE) (Sarwar et al. Citation2015).
Evaluating the effectiveness of nanoparticle synthesis
The evaluation was performed by calculating encapsulation efficiency (EE), percentage yield, and the loading capacity (LC). DP-CSNPs were separated from its suspension using the centrifugation method as described by Hassani et al. (Citation2015) with slight modifications. Briefly, the suspension was centrifuged using Kubota cooling centrifuge at 18,000 rpm in 4°C for 30 min and pellet was freeze-dried overnight at −20°C using Freeze dryer (Penguin Classic Plus (Lark)). EE was studied by the method shown by Alqahtani et al. (Citation2019). For calculating EE and LC, 2 mg of DF-CSNPs were suspended in milliQ water, stirred using magnetic stirrer and sonicated for 10 min at 2 cycles and at 50 magnitudes. The samples were again centrifuged at 17,000 rpm and drug concentration was calculated using UV method at 275 nm using Double beam Spectrophotometer (SL 210 UV VIS, ELICO, India). All the experiments were performed in triplicates (n = 3).
In vitro drug release studies
In vitro release of the drug DP through the synthesized NPs was done using dialysis membrane with a molecular weight cut off (MWCO) 12–14 kDa in phosphate buffer saline (PBS, pH 7.4) as described by Sun et al. (Citation2019) with slight modifications. The dialysis membrane works on the principle of selective diffusion which will allow the drug to be released slowly from the small pores with respect to time as the molecular size of DP is 296.14 Da which is less than the MWCO of dialysis membrane. Briefly, a known amount of freeze-dried DP-CSNPs was suspended in 5 mL of 1X PBS and filled in the dialysis tube. It was later dipped in 250 mL conical flask filled with 150 mL PBS and was maintained at 37°C with 100 rpm rotation. At regular time interval, 2 mL of sample was withdrawn from a flask and optical density was measured at 275 nm to find the concentration of drug released. Moreover, fresh PBS was replaced at every withdrawal and samples were analyzed in triplicates.
Results and discussion
Proximate composition and CS yield
The proximate analysis reveals the percentage of moisture, ash content, and total carbohydrate which was found to be 1.67 ± 0.026%, 2.22 ± 0.21%, and 29.09 ± 1.59%, respectively, as per dry weight basis. Similarly, the ash and carbohydrate content found by Lawal-Are, Moruf, and Afolayan (Citation2018) in Squilla aculeatacalmani which was around 2.12 to 4.22 and 28.11 to 33.01, respectively. Ash content of the squillashell was found to be more than the ash content found in the shells of Horseshoe Crab (Tachypleus gigas) (Kassim et al. Citation2018). The high content of shell ash reveals the presence of higher percentage of mineral impurities in the form of calcium carbonate and phosphates, which are the main constituents of an exoskeleton. Hence, the yield percentage for CS was reduced from 58.25 ± 1.089% to 35.265 ± 1.127% after the purification step due to the removal of mineral and salt form it.
Characterization of extracted chitosan
Material characterization of CS
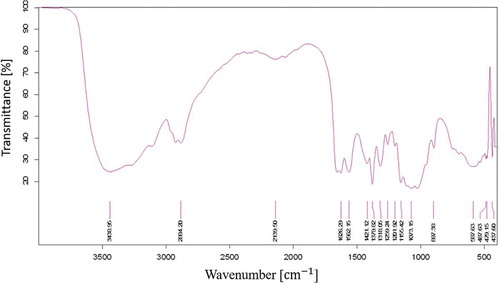
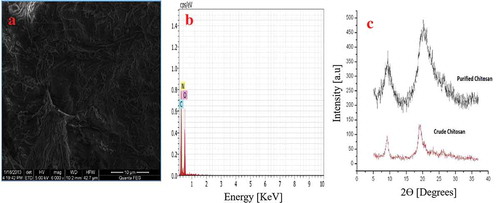
The FTIR spectra for extracted CS indicate the presence of OH group stretching at 3438.96 cm−1 and C-H stretching at 2884.20 cm−1 (). FTIR investigation further shows the characteristic absorption bands at 1626.29 cm−1 attributed to atomic vibration of NH of R-NH2, indicating deacetylation of chitosan, 1421.12 cm−1 (C-H deformation vibrations), 1418.06 cm−1 (C-N stretching in 2° amide) and 1073 cm−1 (C − O − C stretching). Similarly, the presence of amide I and II group was seen at 1652 and 1592 cm−1 for low molecular weight CS (Sarwar et al. Citation2015). SEM imaging for the extracted CS () showing smooth, homogenous, nonporous crystalline surface. EDS graphs () confirm that the extracted CS is free from any mineral impurities such as calcium and phosphorus. Furthermore, XRD analysis was done to find out the physical nature of purified and crude chitosan by 2θ values, which were close to 10° and 20° (). The percentage crystallinity index was calculated as 62 ± 0.2318% which confirms the crystalline nature of the extracted CS.
Physicochemical characterization of CS
Intrinsic viscosities were determined to be 1.44 cP and 55.06 cP for squilla and commercial CS, respectively. It clearly depicts that the extracted CS is less viscous than commercially available CS. The average molecular weight was calculated as 932.4 kDa, ~57 kDa for commercial and extracted CS, respectively. The molecular weight of squilla CS was very less than the commercial CS which makes it more suitable for the synthesis of small uniform nanoparticles. The DD of extracted CS was found to be around 83% calculated through FTIR method described by Takarina et al. (Citation2017) which lies in the range for the synthesis of the required size of nanoparticles. Viscosity, molecular weight, and DD depend on the steps of extraction and purification. Moreover, water binding capacity was found as 637.73 ± 0.71% which lies in the range 458 – 805% while fat binding capacity, 176.91 ± 1.25% lies in the range 170–315%, respectively, given by Sarbon et al. (Citation2015). Commercial CS shows higher values for water (717.26 ± 1.72%) and fat binding capacity (463.2 ± 0.97%). Possible reasons for different values may be the crystalline structure, salt-forming capacity, and different protein content in the CS samples (Kumari et al. Citation2017).
Material characterization of DP-CSNPs
FTIR spectra of CS showed functional group peaks of TPP at 1093.25 cm−1 and 1099 cm−1 which were found in blank and DP-CSNPs, respectively, were related with the phosphate group (P = O) which indicates the cross-linking of CS with TPP. The intense peak of C = O stretching in 2° amide (bending vibration of amide I) shifted from 1626.29 cm−1 to 1638.20 cm−1 in blank CSNPs and 1641 cm−1 in DP-CSNPs, respectively, indicating interactions between – NH3 groups of chitosan with TPP and DP within DP-CSNPs. Moreover, on the formation of nanoparticles, hydroxyl (OH) stretching peak at wavelength 3438.96 cm−1 found in extracted CS shifted to 3324.25 cm−1, 3419 cm−1 in blank CSNPs and DP-CSNPs, respectively (). The FTIR spectra obtained for drug-loaded CSNPs shows close similarity with the data reported by He et al. (Citation2019) for ovalbumin delivery through cyclodextrin/chitosan NPs where the intensity increase in C-H band was seen due to presence of methylene group in side chain of carboxymethyl cyclodextrin. Similarly, the high absorption peak was obtained at 1328 cm−1 and 3440 cm−1 which can be attributed to ether and alcoholic hydroxyl group, respectively.
Figure 3. (a) FTIR spectra of Blank CSNP, Drug (DP), DP-CSNPs and (b) X-Ray diffraction graph for A. Blank CSNPs, B. Drug (DP) and C. DP-CSNP
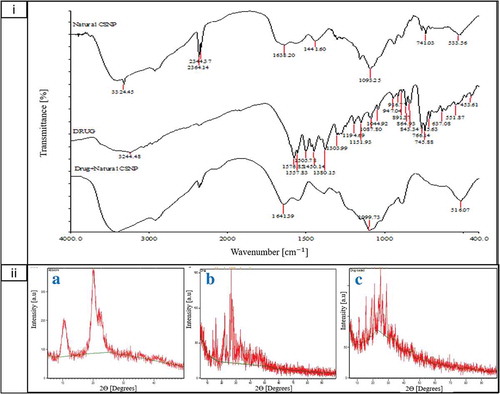
XRD of the blank CSNPs, drug (DP) and DP-CSNPs was done and found that drug was crystalline in nature since it has a number of peaks between 10-30° of 2θ values (). DP-CSNPs showed all high-intensity peaks which were observed in the drug at 2θ range from 10-30° indicating high degree of crystallinity and encapsulation of drug in nanoparticles. Similar results were obtained by Hussein‐Al‐Ali et al. (Citation2016) for perindopril erbumin loaded CSNPs where the diffraction peaks were at 10.4° and 21.8° as well as the CrI was found as 37%. Moreover, the crystallinity index (CrI) in our study for drug, blank CSNPs, and DP-CSNPs was calculated as 20%, 42%, and 59%, respectively.
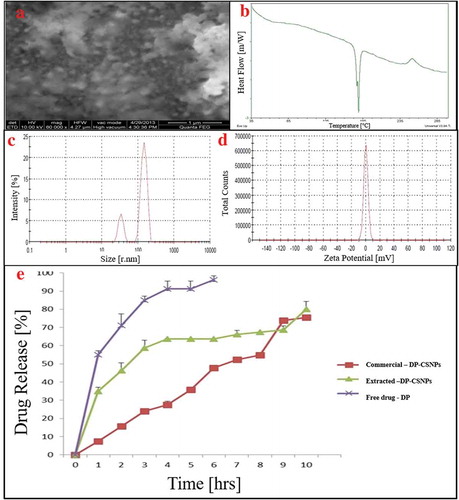
SEM image of DP-CSNPs () showed the spherical morphology of nanoparticles. Moreover, DSC thermograms of DP-CSNPs showed an endothermic peak sharply at 180°C (). Since the temperature of DP is at 178.12°C and the endothermal maximum of melting is at 178.99°C. Moreover, it was clear that water may form intermolecular hydrogen bonds in CS polymer (Sarwar et al. Citation2015). Moreover, Intensity average size was found as 247.6 nm for CSNPs (). Charge of the DP-CSNPs was studied by measuring zeta potential () which was around +0.0459 mV for extracted DP-CSNPs due to low availability of +NH3 ions and more interaction of drug to CS polymer as explained by Sotelo-Boyás et al. (Citation2017) for lime essential oil loaded CSNPs.
Figure 4. Material characterization of extracted CS derived DP-CSNPs showing A. Scanning electron micrograph image, B. Differential scanning calorimetry thermogram, C. Particle size distribution D. Zeta potential and E. In vitro drug release profile at pH 7.4 for commercial CS synthesized DP-CSNPs, squilla CS synthesized DP-CSNPs and free drug
Encapsulation efficiency (EE), loading capacity (LC) and % yield
EE, LC, and % yield of nanoparticles synthesized from different CS to TPP weight ratios of 5:1, 5:2, 5:3, 5:4, and 5:5, respectively, were calculated. By obtained results, it was found that the 5:2 ratio showed maximum EE which is 40.33 ± 1.09%. Hence, the same ratio was used for the further preparation of drug-loaded nanoparticles for the different set of combination. Effect of different concentration of drug (0.5–2.5 mg/mL) on %EE, %LC, and %Yield while keeping TPP and chitosan concentration as 1 mg/mL was studied from which it was clear that nanoparticles synthesized from chitosan (2.5 mg/mL), TPP (1 mg/mL) and drug (1 mg/mL) concentrations gave maximum EE and LC for DP-CSNPs produced from purified CS. EE for different CS:drug ratio was obtained for tacrine loaded CSNPs in a study performed by Hassani et al. (Citation2015).
In vitro drug release study
The drug release profile at pH 7.4 was obtained for free drug, synthesized DP-CSNPs from commercial CS and extracted CS using dialysis membrane diffusion method (). The maximum release was obtained at 6th hr for the free drug release while the release of DP from the synthesized nanoparticles was found to show sustained release up to 10th hr with cumulative drug release of 80% and the drug was sufficiently stable into the medium. Moreover, the diffusion of the drug from NPs prepared from commercially available CS was fast at 4th hr which became slow and sustained and can be correlated to the high molecular weight of commercial CS and low encapsulation efficiency. Although the number of pores and size determines the rate of diffusion through the dialysis membrane. Similar burst release was observed for the release of Albendazole and Praziquantel from CSNPs where the initial burst release was due to the surface attached drug while the later release of the drug was due to the entrapped drug molecules in the middle of CSNPs which were released gradually (Torabi, Dobakhti, and Haniloo Citation2018).
Conclusion
The CS was successfully extracted from Carinosquilla multicarinata shells from the discarded waste in waterfront and was used for the synthesis of anti–inflammatory drug diclofenac encapsulated CSNPs. The CSNPs loaded with the drug were prepared using different CS and TPP concentrations and was found that 2.5:1 ratio of CS: TPP provided the highest encapsulation efficiency. The physicochemical characterization was done to evaluate the functional properties such as ash, moisture, and carbohydrate content, WBC, FBC, viscosity, average molecular weight, LC and % yield. Furthermore, CS and prepared DP-CSNPs were characterized using FTIR, XRD, SEM-EDS, and DSC showing in small uniform spherical nanoparticles with characteristic endothermic and crystallinity peaks. The in vitro drug release profile at pH 7.4 suggests that the release from nanoparticles was sufficiently stable and sustained release as compared to the free drug. Hence, the marine scrap from the harbor and fisheries can be used economically for the synthesis of specific drug incorporated nanoparticles to cure various anti–inflammatory diseases.
Acknowledgment
The authors extend their gratitude to the management of SRM Institute of Science and Technology, Kattankulathur, Chennai, India for providing the facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Akshad Balde
Akshad Balde is a B. Tech. Biotechnology student.
Abshar Hasan
Abshar Hasan is a M. Tech. Biotechnology student.
Ila Joshi
Ila Joshi is a Ph.D. scholar.
R.A. Nazeer
R.A. Nazeer is a Professor in the Department of Biotechnology at SRM Institute of Science and Technology, Chennai.
References
- Albalasmeh, A. A., A. A. Berhe, and T. A. Ghezzehei. 2013. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 97 (2):253–61. doi:10.1016/j.carbpol.2013.04.072.
- Alqahtani, F. Y., F. S. Aleanizy, E. El Tahir, B. T. Alquadeib, I. A. Alsarra, J. S. Alanazi, and H. G. Abdelhady. 2019. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 27 (1):82–87. doi:10.1016/j.jsps.2018.08.001.
- Bastiaens, L., L. Soetemans, E. D’Hondt, and K. Elst. 2019. Sources of Chitin and Chitosan and their Isolation. Chitin and Chitosan Properties and Applications, 1–34. John wiley and Sons Ltd. doi:10.1002/9781119450467.ch1.
- de Carvalho, F. G., T. C. Magalhães, N. M. Teixeira, B. L. C. Gondim, H. L. Carlo, R. L. Dos Santos, A. R. de Oliveira, and Â. M. L. Denadai. 2019. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C 109885. doi:10.1016/j.msec.2019.109885.
- Divakar, D. D., N. T. Jastaniyah, H. G. Altamimi, Y. O. Alnakhli, A. A. Alkheraif, and S. Haleem. 2018. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 108:790–97. doi:10.1016/j.ijbiomac.2017.10.166.
- Gaikwad, B. V., J. M. Koli, and A. S. Desai. 2015. Isolation and characterization of chitosan from crab (Scylla serrata) shell waste. Int. J. Sci. Appl. Res. 2:6.
- Hassan, U. A., M. Z. Hussein, N. B. Alitheen, S. A. Y. Ariff, and M. J. Masarudin. 2018. In vitro cellular localization and efficient accumulation of fluorescently tagged biomaterials from monodispersed chitosan nanoparticles for elucidation of controlled release pathways for drug delivery systems. Int. J. Nanomedicine 13:5075. doi:10.2147/IJN.S164843.
- Hassani, S., A. Laouini, H. Fessi, and C. Charcosset. 2015. Preparation of chitosan–TPP nanoparticles using microengineered membranes–Effect of parameters and encapsulation of tacrine. Colloids Surf. A Physicochem. Eng. Asp. 482:34–43. doi:10.1016/j.colsurfa.2015.04.006.
- He, M., C. Zhong, H. Hu, Y. Jin, Y. Chen, K. Lou, and F. Gao. 2019. Cyclodextrin/chitosan nanoparticles for oral ovalbumin delivery: Preparation, characterization and intestinal mucosal immunity in mice. Asian J. Pharm. Sci. 14 (2):93–203. doi:10.1016/j.ajps.2018.04.001.
- Hinz, B., J. Chevts, B. Renner, H. Wuttke, T. Rau, A. Schmidt, I. Szelenyi, K. Brune, and U. Werner. 2005. Bioavailability of diclofenac potassium at low doses. Br. J. Clin. Pharmacol. 59 (1):80–84. doi:10.1111/bcp.2005.59.issue-1.
- Hussein‐Al‐Ali, S. H., A. Kura, M. Z. Hussein, and S. Fakurazi. 2016. Preparation of chitosan nanoparticles as a drug delivery system for perindopril erbumine. Polym. Compos. 39 (2):544–52. doi:10.1002/pc.23967.
- Kassim, Z., W. N. A. W. Murni, M. R. Razak, and S. B. Adam. 2018. Chitosan isolated from horseshoe crab Tachypleus gigas from the Malay Peninsula. Orient. J. Chem. 34 (2):928. doi:10.13005/ojc.
- Khedkar R., Singh K., 2018. Food Industry Waste: A Panacea or Pollution Hazard? In Paradigms in pollution prevention. Springer briefs in environmental science, eds. Jindal T, 35–47. Springer: Cham. doi:10.1007/978-3-319-58415-7_3.
- Kim, H. W., H. S. Shin, S. K. Han, and S. E. Oh. 2007. Response surface optimization of substrates for thermophilic anaerobic codigestion of sewage sludge and food waste. J. Air Waste Manage. Assoc. 57 (3):309–18. doi:10.1080/10473289.2007.10465334.
- Kumari, S., S. H. K. Annamareddy, S. Abanti, and P. K. Rath. 2017. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 104::1697–1705. doi:10.1016/j.ijbiomac.2017.04.119.
- Kumari, S., P. Rath, and A. S. H. Kumar. 2016. Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeo rohita): Extraction and characterization Suneeta. Afr. J. Biotechnol. 15 (24):1258–68. doi:10.5897/AJB2015.15138.
- Lawal-Are, A. O., R. O. Moruf, and O. A. Afolayan. 2018. Proximate composition, mineral profile and cholesterol level in whole and fillet of the Guinean Mantis Shrimp, Squilla aculeate calmani (Holthuis, 1959) (Crustacea: Stomatopoda). Albanian J. Agric. Sci. 17 (3):160–65.
- Naskar, S., K. Koutsu, and S. Sharma. 2019. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 27 (4):379–93. doi:10.1080/1061186X.2018.1512112.
- Nessa, F., S. M. Masum, M. Asaduzzaman, S. K. Roy, M. M. Hossain, and M. S. Jahan. 2010. A process for the preparation of chitin and chitosan from prawn shell waste. Bangladesh J. Sci. Ind. Res. 45 (4):323–30. doi:10.3329/bjsir.v45i4.7330.
- Parthiban, F., S. Balasundari, A. Gopalakannan, K. Rathnakumar, and S. Felix. 2017. Comparison of the quality of chitin and chitosan from shrimp, crab and squilla waste. Curr. World Environ. 12 (3):672. doi:10.12944/CWE.
- Sarbon, N. M., S. Sandanamsamy, S. F. S. Kamaruzaman, and F. Ahmad. 2015. Chitosan extracted from mud crab (Scylla olivicea) shells: Physicochemical and antioxidant properties. J. Food Sci. Technol. 52 (7):4266–75. doi:10.1007/s13197-014-1522-4.
- Sarwar, A., H. Katas, S. NoradilaSamsudin, and N. M. Zin. 2015. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS One 10 (4):e0123084. doi:10.1371/journal.pone.0123084.
- Sotelo-Boyás, M. E., Z. N. Correa-Pacheco, S. Bautista-Baños, and M. L. Corona-Rangel. 2017. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT 77:15–20. doi:10.1016/j.lwt.2016.11.022.
- Sreekumar, S., F. M. Goycoolea, B. M. Moerschbacher, and G. R. Rivera-Rodriguez. 2018. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 8 (1):4695. doi:10.1038/s41598-018-23064-4.
- Sun, X., J. Shen, D. Yu, and X. K. Ouyang. 2019. Preparation of pH-sensitive Fe3O4@ C/carboxymethyl cellulose/chitosan composite beads for diclofenac sodium delivery. Int. J. Biol. Macromol. 127:594–605. doi:10.1016/j.ijbiomac.2019.01.191.
- Takarina, N. D., A. B. Indah, A. A. Nasrul, A. Nurmarina, A. Saefumillah, A. A. Fanani, and K. D. P. Loka. 2017. Optimisation of deacetylation process for chitosan production from red snapper (Lutjanus sp.) scale wastes. J. Phys. Conf. Ser. 812 (1):012110. IOP Publishing.
- Torabi, N., F. Dobakhti, and A. Haniloo. 2018. Albendazole and praziquantel chitosan nanoparticles: Preparation, characterization, and in vitro release study. Iran. J. Sci. Tech. Trans. A: Sci. 42 (3):1269–75. doi:10.1007/s40995-017-0150-z.
- Wang, W., B. Shuqin, L. Shuqing, and W. Qin. 1991. Determination of the Mark-Houwink equation for chitosan with different degrees of deacetylation. Int. J. Biol. Macromol. 13 (5):281–85. doi:10.1016/0141-8130(91)90027-R.
- Yuvaraj, D., B. Bharathiraja, J. Rithika, S. Dhanasree, V. Ezhilarasi, A. Lavanya, and R. Praveenkumar. 2016. Production of biofuels from fish wastes: An overview. Biofuels 10 (3):301–07. doi:10.1080/17597269.2016.1231951.