ABSTRACT
Beaded activated carbons (BACs) were derived from waste bamboo tar through carbonization (500°C for 2 hr) followed by physical activation using carbon dioxide (800–900°C for 2–4 hr). The adsorbent was examined for their physical and chemical properties, adsorption capacities toward methylethylketone (MEK) and toluene, and regenerabilities under microwave heating. It was found that the maximum total surface area reached for bamboo-tar-derived BAC after physical activation was 1364 m2 g−1, and more than 95% of the area was attributed to the microporous structures. Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) isotherm models were applied to the adsorption isotherm fitting, and the minimum R2 for each model was 0.986, 0.915, and 0.943, respectively. The isosteric heats of adsorption calculated based on D-R parameters for methylethylketone and toluene were 44.04 to 51.50 and 45.88 to 73.27 KJ mol−1, respectively. They were slightly over the range of physisorption and increased with adsorbate loading, which might be related to the micropore filling mechanism. Microwave regeneration under 600 W of power output removed most of the adsorbate (>93.03%) within 8 min. The results of this study are intended to benefit future study on waste-derived adsorbent in environmental applications.
Implications
Recycling waste bamboo tar for the novel adsorbent preparation is shown feasible in this study. Beaded activated carbon (BAC) synthesized from this waste bamboo tar possessed a high specific surface area, which aided in the capturing of volatile organic compounds (VOCs). Three adsorption isotherms, Langmuir, Freundlich, Dubinin-Radushkevich (D-R) models can be applied in interpreting the experimental adsorption data, providing information on adsorption heat and possible adsorption mechanism. A potential microwave regeneration method for BAC is tested, showing high desorption efficiencies with minimum heel formation. These findings can provide a new pathway for waste bamboo tar management and VOC abatement using adsorbents.
Introduction
Air pollution kills millions of people yearly around the world (Lelieveld et al. Citation2015). Among the anthropogenic air pollutants, volatile organic compounds (VOCs) are emitted in tremendous amounts and found ubiquitously in modern society, causing adverse effects to both human beings and our environment (Zhang et al. Citation2017). For stationary VOCs emission sources, plenty of control strategies can be utilized, including absorption (Ozturk and Yilmaz Citation2006), oxidization, and catalytic conversion (Kamal, Razzak, and Hossain Citation2016), biotreatment (Mudliar et al. Citation2010), and adsorption using porous adsorbents such as activated carbon (AC) (Ghoshal and Manjare Citation2002; Zhang et al. Citation2017). Specifically, the adsorption removal of VOCs has the merit that economically recovering high-priced solvent can be fulfilled by regenerating the adsorbent, namely, reversing the adsorption process by heating the adsorbent followed by condensation (Johnsen et al. Citation2016; Wang et al. Citation2012).
The adsorption performance of AC has been studied for years. Factors influencing the adsorption capacity include physical properties of the adsorbent such as the pore structure, pore shape, and pore size distribution (Chiang, Chiang, and Huang Citation2001), and that of the adsorbate such as the molecular weight, concentration, dynamic diameter, and polarity (Li et al. Citation2012). The surface chemical properties of the adsorbent also play important roles in the adsorption processes, as the modification by oxidation or heat treatment of the surface of AC is typically made to increase the number of functional groups, enhancing the adsorption capacities toward certain compounds (Romero-Anaya, Lillo-Ródenas, and Linares-Solano Citation2015).
AC can be tailored into different morphologies (Luo et al. Citation2006), among which is the beaded activated carbon (BAC) that has several advantages including high fluidity, good packing ability, more controllable pore structures, high mechanical strength, and great adsorption performance (Qi et al. Citation2017; Romero-Anaya, Lillo-Ródenas, and Linares-Solano Citation2010). BAC can be derived from numerous polymeric precursors, including divinylbenzene-based polymer (Zhu et al. Citation2008), copolymer of vinylidene chloride and styrene (Qian et al. Citation2015), polystyrene-based resin (Wang et al. Citation2009), and phenol-formaldehyde (PF) resin (Qi et al. Citation2017; Zhang et al. Citation2018). However, the costs of these polymeric materials vary with fluctuating oil price, therefore, encouraging scientists to seek out possible replacements for BAC precursors.
A recent study has demonstrated that BAC could potentially be derived from waste bioresources such as waste bamboo tar (Huang, Hsi, and Liu Citation2013). It was found that the vast amount of waste bamboo tar generated during the bamboo charcoal production was rich in phenolic content, suitable to be recovered, applied in the synthesis of beaded phenolic resin (Lee et al. Citation2011), and subsequently activated to produce BAC (Singh and Lal Citation2008, Citation2010). The method was further improved in the study by Chen, Huang, and Hsi (Citation2019), yet the qualities of BAC still demand improvements in total surface area, microporous structures, and most importantly, the adsorption performance of some widely used organic solvents, in order to be qualified as a potential replacement for the commercial BAC. As bamboo remains one of the predominant bioresources in several tropical and subtropical regions of Asia, Africa, and Latin America, the problem of a proper management for waste bamboo tar is still challenging. Moreover, the related research on developing valuable materials such as BAC, carbon fiber, and carbon quantum dots from waste bamboo tar is still in its infant stage, requiring more thorough investigations (Liang et al. Citation2017; Qiao et al. Citation2005).
The objective of the current study aims at valorizing waste bamboo tar and improving the qualities of BAC derived from it. For this purpose, suspension polymerization and physical activation using carbon dioxide (CO2) were applied to derived BAC from waste bamboo tar. The properties of BAC were examined using an elemental analyzer (EA), scanning electron microscope (SEM), and nitrogen adsorption at 77 K. The BAC was then tested in a bench-scale adsorption system to evaluate its capability of recovering organic solvent, where methylethylketone (MEK) and toluene were selected as targeted compounds. To better understand the adsorption performance of BAC, the results of VOCs adsorption were fitted with the Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) isotherm model and the isosteric heat of adsorption was obtained analytically. The last part of this study devotes to the regenerabilities of the derived adsorbent since this characteristic is equally important for an ideal adsorbent. Comparing to the conductive heating method, microwave heating has been extensively studied in recent years due to its rapid temperature response, homogeneous heating, high energy efficiency, and less direct contact between heating source and targeted materials (Foo and Hameed, Citation2012; Foo, Lee, and Hameed Citation2013). The technology has been adopted in the heating process of material preparation (Cheng et al. Citation2018, Citation2017; Mao et al. Citation2015b), and regeneration of dielectric materials such as AC (Hashisho, Rood, and Botich Citation2005; Mao et al. Citation2015a). Moreover, the inherent capability of BAC to be operated in a fluidized configuration favors the utilization of microwave heating (Cherbanski and Molga Citation2009; Price and Schmidt Citation1998) and lowers the heel buildup (irreversible adsorption) after cycles of regeneration (Kamravaei et al. Citation2017). The saturated BAC was therefore regenerated at the end of the experiment using microwave heating.
Material and methods
Sample preparation and activation
The rectification was carried out to separate phenolic compounds from other substances. The rectified bamboo tar contained 73.7 wt% phenolic compounds, suggested by the examination of a high-resolution gas chromatography/mass spectrometer (JEOL JMS-700 and SHIMADZU QP2010). This rectified bamboo tar served as the main component participating in the suspension polymerization.
The suspension polymerization was conducted in a 1-L four-neck round-bottom glass reactor equipped with a thermometer, a reflux condenser, and a Teflon stirrer. The rectified bamboo tar was blended with reagent-grade phenol (95% phenol) to adjust the formaldehyde to free phenol ratio (F/P). For a typical synthesis process, 1 wt% aqueous solution of polyvinyl alcohol (hydrolysis 98.5–99.2 mol%; degree of polymerization: 1700) as the stabilizer was added into the mixture of 1.6 F/P ratio. The pH value of the mixture was adjusted by adding trimethylamine (basic catalyst) to 7–8. After 90 min of homogenizing, hexamine was added to alter the F/P ratio to 2.9, and the crosslinking reaction occurred. The reaction ceased after around 4 hr and the mixture was cooled. The PF beads were collected on the filter and washed with deionized water and acetone. Cleaned PF beads were dried in an oven at 105°C and beads with the size of 20 × 30 mesh were sieved for the following experiment.
The BAC synthesis procedures involved carbonization under inert conditions and physical activation of PF beads using CO2. All experiments were conducted in a cylindrical quartz tube with an inner diameter of 30 mm, heated by a conventional temperature-programmed furnace (DENGYNG, model D-35). The furnace utilized conductive heating method, and the heat was directed from outside to inside of the quartz tube. The PF beads were placed in the center of the quartz tube, carbonized under flowing of nitrogen (purity = 99.999%, flow rate = 200 mL min−1) in a tubular furnace using a heating rate of 10°C min−1 up to 500°C, dwell for 2 hr. The subsequent physical activation using high purity CO2 (99.999%) as a mild oxidative agent removed the impurities blocking the pores generated after the carbonization step. The activation step was carried out under flowing of nitrogen (purity = 99.999%, flow rate = 200 mL min−1) in the same tubular furnace using a heating rate of 10°C min−1 up to 800°C, 850°C, and 900°C. Upon reaching the activation temperature, the CO2 gas stream was directed into the furnace for 2, 3, and 4 hr of activation and then was switched back to nitrogen during the cooling step.
Physical and chemical characterization of materials
Morphology and pore structure
The morphologies and pore structures of the PF beads before and after the activation along with the commercial BAC were revealed using a scanning electron microscope (Hitachi, model TM3000) and nitrogen adsorption at 77 K (Micromeritics, model ASAP2020), respectively. Before the analysis, all samples were degassed at 10–20 Torr vacuum under 150°C for 24 hr. The total surface area (Stotal) was calculated by the Brunauer-Emmett-Teller equation according to ASTM D4820-96a. The micropore surface area (Smicro) and micropore volume (Vmicro) were obtained from a t-plot analysis using Jura-Harkins equation: t = [13.99/(0.0340 – log(P/P0))]0.5. The range of relative pressures used to determine Smicro and Vmicro was based on thickness t values between 0.45 and 0.8 nm. Pore size distributions (PSDs) were determined using nonlocal density function theory (NLDFT) and Barrett-Joyner-Halenda (BJH) methods.
Elemental analysis
The chemical composition of the adsorbent was measured by an elemental analyzer (Elementar, model Vario EL Cube). The mass fractions of C, H, N, O, and S for the beads were obtained by complete oxidization of the samples followed by the detection of gaseous compounds with a thermal conductivity detector (TCD).
VOC adsorption test and isotherm construction
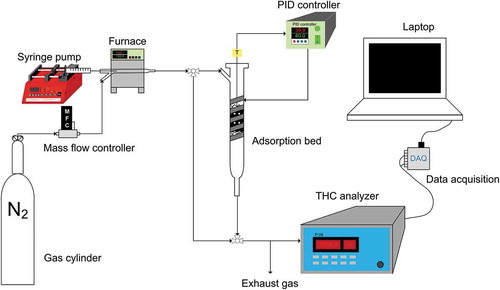
The adsorption testing system consisting of a VOCs vapor generating system, a temperature-controlled adsorption bed, a total hydrocarbon (THC) analyzer, and a data acquisition system (DAQ) is illustrated in . A syringe pump (New Era Pump Systems Inc., model NE-1000) loaded with a gastight syringe (Hamilton Company) constantly injected MEK and toluene solvents into a small furnace. The temperature of the furnace was monitored by the thermocouple, K-type, and set well above the boiling point of both solvents (79.6°C and 110.6°C for MEK and toluene, respectively) where they immediately vaporized and mixed with the inert carrier gas (N2, purity = 99.999%). The injection rates were adjusted according to the ideal gas law to generate a VOC gas stream with a desired concentration.
The on-line THC detecting system was a flame ionization detector (FID) (Ratfisch Analysensysteme GmbH, model RS53-T) continuously measuring the outlet concentrations of the adsorption bed. The concentrations of VOCs were recorded every second via a DAQ system consisted of a LabView software (National Instruments) and a data logger (National Instruments, model USB-6000).
The adsorption bed in this study was a tube made of Pyrex glass with an inner diameter of 1 cm. A K-type thermocouple (Omega) was inserted from one of the openings and extended into the center of the bed while the heating tape (Omega) wrapping around the tube. A proportional-integral-derivative (PID) controller connected to the thermocouple and the heating tape measured and controlled the temperature of the adsorption bed to the accuracy of ± 0.1°C. The experiments were conducted in a fixed-bed configuration. VOCs vapor came from the top to avoid expansions of the bed. Typically, BAC was filled in the tube with both sides covered by glass wool and fine glass meshes. The adsorption of VOCs onto the coverings was assumed negligible during the test.
The concentrations of VOCs ranged from 0.001 to 0.2 P/P0 for MEK and from 0.004 to 0.28 P/P0 for toluene, respectively. The operating temperature was set at 30°C, 40°C, or 50°C. The total flow rate was set at 1.6 SLPM. For each run, 1 g of BAC was loaded in the adsorption tube. The saturated adsorption capacity was calculated using weighted method and the following equation:
where Wf is the weight of BAC after adsorption and Wi is the weight of BAC before adsorption.
Microwave regeneration
The saturated BAC was regenerated in a microwave muffle furnace (Milestone, model Pyro 260). Prior to the microwave regeneration, 1 g of virgin BAC was saturated with MEK under the inlet concentration of 500 ppmv and operating temperature of 40°C. Once the BAC was saturated, it was transferred to an air-tight quartz reactor with a total volume of 1.5 L placed inside the microwave furnace. During the desorption step, the microwave furnace operated at 2.45 GHz with a constant power output of 600 W. A K-type thermocouple (Omega) measured the temperature change of the BAC, while 2.0 SLPM of inert purging gas (N2, 99.999%) was directed into the reactor to purge the desorbed adsorbate from the reactor immediately. Desorption efficiencies were obtained by weighing the mass of BAC before and after the microwave regeneration.
Results and discussion
Adsorbent characterization
The surface morphologies and shapes of commercial BAC, termed “KBAC” (K stands for Kureha CORP) and self-prepared BAC with various synthesize conditions, termed “SBAC” were compared based on the SEM analysis. The beads which possess smooth surfaces and round shapes are considered suitable for mechanical interactions and fluidization. As can be seen from the SEM images (), all the prepared adsorbents were in beaded shape. Macroscopically, the suspension polymerization reaction can successfully derive the beaded adsorbent so that both its packing in the fixed-bed and its fluidity in a fluidized system are superior to those in irregular shapes. However, except for SBAC-T800-t3 and KBAC, PF beads after the activation process appeared to have apparent fractures on the surface, microscopically. The extents of the fractures developed generally followed the order: SBAC-T900-t4 > SBAC-T900-t3 > SBAC-T900-t2 > SBAC-T850-t3 > SBAC-T800-t3 (T and t represent activation temperature in °C and time in hour, respectively.). This is in consistence with the results found in literatures, as the thermal degradation starts to enhance from 800°C. The vigorous degradation of the PF beads is attributed to the effective gasification agent, CO2, and the suitable reaction temperature, especially above 800°C (Kim et al. Citation2004, Citation2002).
Figure 2. Scanning electron micrograph under 100× (upper) and 1500× (lower) magnification for (a) SBAC-T800-t3, (b) SBAC-T850-t3, (c) SBAC-T900-t2, (d) SBAC-T900-t3, (e) SBAC-T900-t4, and (f) KBAC.

The result of the elemental analysis indicates the degree of devolatilization and transformation from a raw material to a porous AC (). The carbonization process decomposed the raw material and increased its carbon content to 81.01 wt%. The subsequent activation further raised the carbon content up to 85.36– 89.47 wt%, consistent with the elemental composition of a typical AC.
Table 1. Physical and chemical properties of the adsorbents examined in this study.
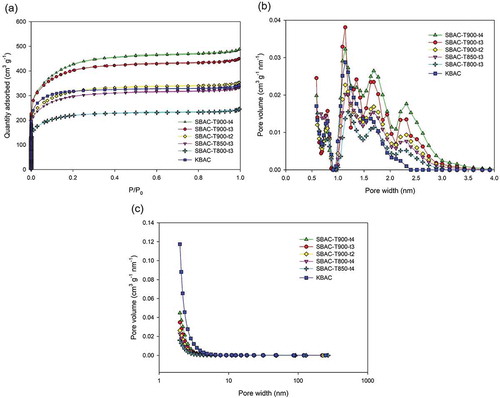
The pore structures of BAC samples were evaluated by using nitrogen adsorption at 77 K. The nitrogen adsorption and desorption curves for KBAC and SBACs are presented in ). All the adsorbents followed the type I(b) adsorption isotherm classified by the latest version of the IUPAC definition, indicating a microporous material having a broader pore size distribution including wider micropores (of width >0.7 nm) (Thommes et al. Citation2015). The results of pore structure analysis are also shown in . High-level trends were found that the surface area and the pore volume increased with activation temperature and duration, with SBAC-T900-t4 having the optimal Stotal of 1364 m2 g−1 and Vtotal of 0.751 cm3 g−1. The PSDs of BAC samples were also examined based on NLDFT and BJH methods ()). It was found that the activation temperature and duration increased the fractions of pores with larger pore widths (revealed by two sharp peaks at 1.5–2 nm and 2.1–3.0 nm in ) and curves in )). This might be due to the overgasification by CO2 that causes the widening of micropores into mesopores.
Adsorption and desorption performance
Isotherm construction
MEK and toluene were selected to compare the influence of activation condition on the adsorption performance of the SBACs. These two solvents have distinct physical and chemical properties. A preliminary evaluation of SBACs adsorption was conducted at 40°C and 500 ppmv of MEK and toluene. The results are shown in the Supplemental Data. The adsorption capacities of toluene were up to 85% greater than that of MEK under the same conditions, suggesting the stronger interaction between the toluene molecules and the adsorbent. This strong interaction can be attributed to two reasons. First, both toluene and the AC’s surface are nonpolar, which strongly enhances their mutual interaction (Li et al. Citation2012). Toluene’s aromatic structure also facilitates π-π interactions with the aromatic structure of the AC surface, increasing the amount adsorbed. It was also found that both MEK and toluene adsorption capacities increased linearly with the surface area and pore volume, indicating that these two are the dominating factors determining the overall adsorption performance (Ouzzine et al. Citation2019; Yu et al. Citation2018).
A great way to investigate and predict adsorption characteristics under various conditions is using the adsorption isotherm constructed based on the experimental results. The data of MEK and toluene adsorption by two bamboo-tar-derived BACs, SBAC-T900-t3, and SBAC-T900-t4, and the commercial BAC were fitted with Langmuir, Freundlich, and D-R isotherm models. The two SBAC samples were selected not only because of their high Stotal but also of their abundant microporous structures, which have been demonstrated to facilitate the capture of gaseous molecules especially under a lower pressure range (Ghafari and Atkinson Citation2018; Lillo-Ródenas, Cazorla-Amoros, and Linares-Solano Citation2005; Romero-Anaya, Lillo-Ródenas, and Linares-Solano Citation2014, Citation2010).
The Langmuir model is one of the most widely applied theoretical isotherms. It assumes monolayer adsorption on the surface of the adsorbent and has the expression as follows:
where q (mg g−1) is the adsorbed amount (or loading), P (Pa) the partial pressure of the adsorbate in the bulk phase, qs (mg g−1) the saturated adsorbed amount, and b (Pa−1) the Langmuir constant representing the strength of adsorbate-adsorbent interactions (Do Citation1998).
The Freundlich model is an empirical one having the following form:
where KF and nF are empirical constants that are generally temperature dependent (Do Citation1998).
D-R isotherm has been shown to be able to reveal the extent of adsorbate-adsorbent interactions (Ghafari and Atkinson Citation2018; Mao et al. Citation2015b). It is derived based on Polanyi potential theory and expressed as:
and
where W is the cumulated volume of the adsorbed space, W0 the limiting volume of the adsorbed space, E0 the characteristic energy of adsorption for a reference vapor (usually benzene), β the similarity constant characterizing the polarizability of the adsorbate (β = 1 for the reference vapor), R the universal gas constant, T the temperature, and P0 the saturated vapor pressure of the adsorbate. The fitting results are tabulated in Tables S1-S3. We found that the minimum R2 for Langmuir, Freundlich, and D-R isotherm was 0.986, 0.915, and 0.943, respectively.
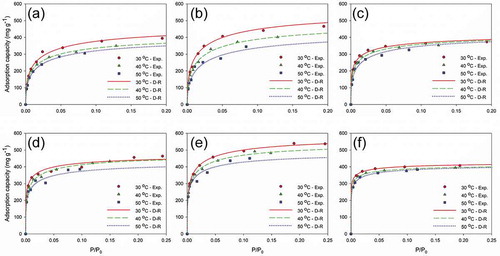
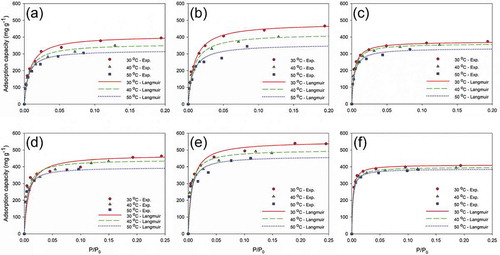
The plots of experimental data and results fitted by the Langmuir isotherm are presented in . For the Langmuir isotherm model, qs is greater in SBACs than in KBAC, both for MEK and toluene. This is mainly due to the drastic increase of adsorbed amounts for SBACs in higher inlet concentration ranges, which is attributed to the larger pore volumes of SBACs. Song et al. (Citation2005) have pointed out that, in general, the larger pore volume leads to more adsorption of molecules due to the adsorbate condensation in pores.
Figure 4. Experimental and Langmuir-modeled isotherms; MEK adsorption on (a) SBAC-T900-t3, (b) SBAC-T900-t4, and (c) KBAC; toluene adsorption on (d) SBAC-T900-t3, (e) SBAC-T900-t4, and (f) KBAC.
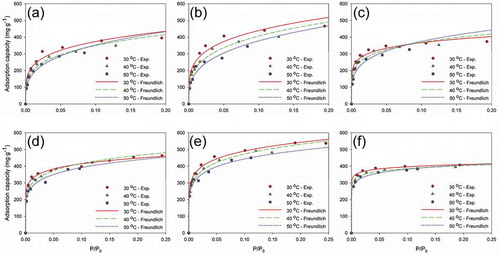
Judging from the R2 value, the Freundlich isotherm did not fit as good as the other two models. The heterogeneity of the carbon surface was revealed by nF > 1 for all scenarios. The plots of the experimental and the Freundlich isotherm modeled data are presented in . The Freundlich isotherm can hardly obtain a good fit for such a broad concentration range, as it does not conform to Henty’s law at lower concentration and fails to account for the more complicated adsorption mechanisms involved at higher concentration of adsorbate (Pei and Zhang Citation2012).
Figure 5. Experimental and Freundlich-modeled isotherms; MEK adsorption on (a) SBAC-T900-t3, (b) SBAC-T900-t4, and (c) KBAC; toluene adsorption on (d) SBAC-T900-t3, (e) SBAC-T900-t4, and (f) KBAC.
For both MEK and toluene adsorption, the micropore volumes (W0) obtained in the D-R isotherm model are in the order of SBAC-T900-t4 > SBAC-T900-t3 > KBAC, which is identical to that obtained from nitrogen adsorption test. A slight difference between the values obtained might be due to different kinetic diameters among the probing molecules (N2: 4.02 Å, MEK: 5.25 Å, and toluene: 5.85 Å) (Lashaki et al. Citation2012).
Generally, the adsorption capacity under lower vapor pressure (<0.1 P/P0) is attributed to micropore filling mechanism (Lillo-Ródenas, Cazorla-Amoros, and Linares-Solano Citation2005), whereas the amount adsorbed under higher vapor pressure can be accounted for by surface layering or capillary condensation mechanism due to the presence of meso- and macropores (Mosher et al. Citation2013). The increase of adsorption capacities above P/P0 = 0.05 should be considered as adsorption in meso- and macropores region, which was found to be much higher in SBACs than in KBAC (). This is in consistence with PSD analysis shown previously, where SBACs developed higher proportions of larger pores compared with KBAC. The characteristic energy, E (or E0), is a parameter indicating the solid properties (Nguyen and Do Citation2001) and affinity between adsorbent and adsorbate (Ghafari and Atkinson Citation2018). The resulting E was consistently found decreasing with increasing adsorbent’s davg, which is the average pore size measured by the nitrogen adsorption. This implies that a narrower average pore size results in a higher adsorption potential and stronger tendencies for the molecules to adsorb. It can be utilized to estimate the slit-pore half-width, x0, by adopting an empirical equation proposed by Dubinin (Citation1989):
As shown in Table S3, SBACs had a larger x0 than KBAC. Results for SBACs showed good agreement using MEK or toluene as a probing molecule, while those for KBAC were deviated. This could be due to the fact that smaller and narrower pore sizes in KBAC might hinder diffusion of larger molecules, while wider PSDs found in SBACs compensate these effects (Hsi et al. Citation2011). It was also noticed that W0 calculated for KBAC was less dependent on temperature than that calculated for SBACs. The “activated entry effect” defined as adsorption phenomena that the diffusion of molecules through narrow pores can be enhanced by increasing the surrounding temperature, which could explain this observation (Chiang, Chiang, and Huang Citation2001). The plots of experimental and D-R isotherm-modeled data are presented in . A good agreement was found for the D-R modeling, and the results can be applied in predicting adsorption capacities under various conditions (Cal, Rood, and Larson Citation1997).
Isosteric heat of adsorption
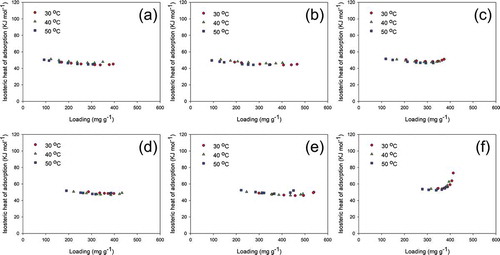
The isosteric heat of adsorption, ΔHs, can be calculated analytically using the D-R parameters and Clausius-Clapeyron (C-C) equation (Ramirez, Qi, and Rood Citation2005). The derivation is shown in the Supplemental Data. ΔHs for all the adsorbate-adsorbent systems is presented in . It was found that ΔHs for MEK adsorption onto all BACs was within 44.04 to 51.50 KJ mol−1, which was similar to the results found in Long et al. (Citation2012). On the other hand, ΔHs for toluene adsorption ranged from 45.88 to 73.27 KJ mol−1, which increased drastically at a higher loading. Such phenomena are not rare in other studies regarding the adsorption of organic compound in porous materials. Similar trends can be found in Long et al., where ΔHs first decreased and then increased for both benzene and MEK adsorption onto the activated carbon. Ramirez, Qi, and Rood (Citation2005) demonstrated that, as the loading increased, increasing lateral interactions due to the molecule aggregation within the micropores could possibly lead to a higher ΔHs. Zheng et al. (Citation2018) showed that due to the preferential adsorption of molecules with the adsorption sites, ΔHs tended to decrease initially, and increase as the effect of intermolecular interaction became significant. This intermolecular interaction was negligible under lower partial pressure, but contributed greatly to the adsorption heat, as molecules needed to overcome the interactive forces with other molecules to reach certain adsorption sites under high pressure. Moreover, ΔHs obtained in this study was all higher than typical bonding energy for physisorption (i.e., approximately 40 KJ mol−1), suggesting that adsorption in the porous medium could involve complex interactions between molecules and higher energy released during the process.
Microwave regeneration
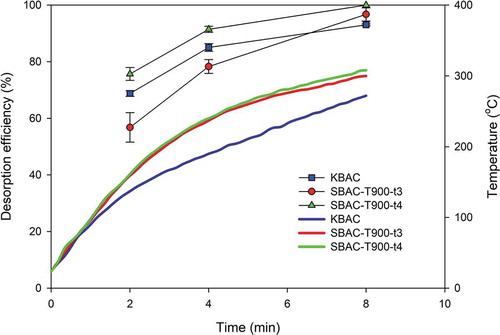
Desorption efficiencies were evaluated for different durations (2, 4, or 8 min) of microwave application under a constant flow rate of purging nitrogen gas (2.0 SLPM) and power output (600 W). The experimental results are shown in . It was found that the desorption efficiencies were greater in SBACs than those in KBAC, mainly due to the higher temperatures achieved under microwave irradiation (the measured highest temperatures were 272°C, 300°C, and 308°C for KBAC, SBAC-T900-t3, and SBAC-T900-t4, respectively). This result indicates that AC with the same precursor could generate similar temperature raising pattern, probably resulted from the similar chemical composition and dielectric characteristics (Pan et al. Citation2016). Within 8 min of microwave irradiation, the desorption efficiencies reached 96.73 ± 2.32, 100 ± 0.00, and 93.03 ± 0.35% for SBAC-T900-t3, SBAC-T900-t4, and KBAC, respectively. Kim and Ahn (Citation2010) regenerated AC loaded with MEK using conventional heating at 300°C with a desorption time of 1 hr, and the desorption efficiency was 99.1%. In the results of Shah, Pre, and Alappat (Citation2014), 2 hr of conductive heating at 160°C was required for regenerating MEK-saturated AC to obtain a desorption efficiency of 98% while successive adsorption-desorption cycle resulted in the continuous degradation of MEK adsorption capacity. While cyclic regeneration is not shown in the current study, it is clear that comparing to the conventional thermal regeneration, the microwave regeneration is more time-saving and can easily achieve high desorption efficiencies. When using microwave heating, the electromagnetic energy is converted to thermal energy inside the adsorbent, thus creating a flow of heat from the hotter area (the core of the solid) to the cooler area (the surface of the solid). This phenomenon added a beneficial effect to further drive the desorbed adsorbate out of the adsorbent. In contrast, when regenerated in a traditional furnace, the temperature gradient around an adsorbent is opposite to that of the microwave-heated adsorbent, creating a barrier for the desorbed adsorbate to diffuse out, hampering the desorption process (Cherbanski et al. Citation2011).
Conclusion
Bamboo-tar-derived BACs were successfully prepared through suspension polymerization and physical (CO2) activation. Comparing to the commercial BAC, the bamboo-tar-derived BAC possessed higher total surface area when activated at 900°C for >3 hr. The specific surface area was up to 1364 m2 g−1, and are mostly contributed by micropores. Adsorption tests and isotherm fitting showed that VOCs adsorption on the derived adsorbent is mainly physisorption while increasing VOCs loading increases the amount of the heat released due to the micropore filling mechanism. Microwave heating technique was demonstrated to be effective in regenerating the spent BAC in this study. However, two limitations of this new adsorbent were found, including insufficient mechanical strength and lower adsorption capacity under low concentration ranges. Future studies to address these disadvantages will focus on optimizing the carbonization temperatures and durations to improve the mechanical strength, and developing pore structures with suitable size distributions by different activation methods.
Supplemental Material
Download MS Word (136.2 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Yu-Ting Chen
Yu-Ting Chen is a graduated master degree student from Graduate Institute of Environmental Engineering, National Taiwan University, in Taipei, Taiwan.
Ying-Pin Huang
Ying-Pin Huang is a researcher at Industrial Technology Research Institute (ITRI) southern region campus, in Tainan, Taiwan.
Can Wang
Can Wang is a professor at School of Environmental Science and Engineering, Tianjin University, and Tianjin Key Lab of Indoor Air Environmental Quality Control, in Tianjin, China
Ji-Guang Deng
Ji-Guang Deng is a researcher at College of Environmental and Energy Engineering, Beijing University of Technology, in Beijing, China.
Hsing-Cheng Hsi
Hsing-Cheng Hsi is a professor at Graduate Institute of Environmental Engineering, National Taiwan University, in Taipei, Taiwan.
References
- Cal, M. P., M. J. Rood, and S. M. Larson. 1997. Gas phase adsorption of volatile organic compounds and water vapor on activated carbon cloth. Energy Fuels 11 (2):311–15. doi:10.1021/ef960200p.
- Chen, Y. T., Y. P. Huang, and H. C. Hsi. 2019. Valorizing waste bamboo tar to novel bead carbonaceous adsorbent for volatile organic compound removal. J. Environ. Eng.-ASCE 145 (12):04019088. doi:10.1061/(ASCE)EE.1943-7870.0001609.
- Cheng, S., Q. Chen, H. Y. Xia, L. B. Zhang, J. H. Peng, G. Lin, X. F. Liao, X. Jiang, and Q. Zhang. 2018. Microwave one-pot production of ZnO/Fe3O4/activated carbon composite for organic dye removal and the pyrolysis exhaust recycle. J. Clean Prod. 188:900–10. doi:10.1016/j.jclepro.2018.03.308.
- Cheng, S., L. B. Zhang, H. Y. Xia, J. H. Peng, J. H. Shu, C. Y. Li, X. Jiang, and Q. Zhang. 2017. Adsorption behavior of methylene blue onto waste-derived adsorbent and exhaust gases recycling. RSC Adv. 7 (44):27331–41. doi:10.1039/C7RA01482A.
- Cherbanski, R., M. Komorowska-Durka, G. D. Stefanidis, and A. I. Stankiewicz. 2011. Microwave swing regeneration vs temperature swing regeneration-comparison of desorption kinetics. Ind. Eng. Chem. Res. 50 (14):8632–44. doi:10.1021/ie102490v.
- Cherbanski, R., and E. Molga. 2009. Intensification of desorption processes by use of microwaves-An overview of possible applications and industrial perspectives. Chem. Eng. Process 48 (1):48–58. doi:10.1016/j.cep.2008.01.004.
- Chiang, Y. C., P. C. Chiang, and C. P. Huang. 2001. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 39 (4):523–34. doi:10.1016/S0008-6223(00)00161-5.
- Do, D. D. 1998. Adsorption analysis: Equilibria and kinetics. London, UK: Imperial College Press.
- Dubinin, M. M. 1989. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 27 (3):457–67. doi:10.1016/0008-6223(89)90078-X.
- Foo, K. Y., and B. H. Hameed. 2012. Microwave-assisted regeneration of activated carbon. Bioresour. Technol. 119:234–40. doi:10.1016/j.biortech.2012.05.061.
- Foo, K. Y., L. K. Lee, and B. H. Hameed. 2013. Preparation of banana frond activated carbon by microwave induced activation for the removal of boron and total iron from landfill leachate. Chem. Eng. J. 223:604–10. doi:10.1016/j.cej.2013.03.009.
- Ghafari, M., and J. D. Atkinson. 2018. Impact of styrenic polymer one-step hyper-cross-linking on volatile organic compound adsorption and desorption performance. J. Hazard. Mater. 351:117–23. doi:10.1016/j.jhazmat.2018.02.051.
- Ghoshal, A. K., and S. D. Manjare. 2002. Selection of appropriate adsorption technique for recovery of VOCs: An analysis. J. Loss Prev. Process Ind. 15 (6):413–21. doi:10.1016/S0950-4230(02)00042-6.
- Hashisho, Z., M. J. Rood, and L. Botich. 2005. Microwave-swing adsorption to capture and recover vapors from air streams with activated carbon fiber cloth. Environ. Sci. Technol. 39 (17):6851–59. doi:10.1021/es050338z.
- Hsi, H. C., R. S. Horng, T. A. Pan, and S. K. Lee. 2011. Preparation of activated carbons from raw and biotreated agricultural residues for removal of volatile organic compounds. J. Air Waste Manag. Assoc. 61 (5):543–51. doi:10.3155/1047-3289.61.5.543.
- Huang, Y. P., H. C. Hsi, and S. C. Liu. 2013. Preparation of spherical activated phenol-formaldehyde beads from bamboo tar for adsorption of toluene. J. Air Waste Manag. Assoc. 63 (8):977–83. doi:10.1080/10962247.2013.804011.
- Johnsen, D. L., H. Emamipour, J. S. Guest, and M. J. Rood. 2016. Environmental and economic assessment of electrothermal swing adsorption of air emissions from sheet-foam production compared to conventional abatement techniques. Environ. Sci. Technol. 50 (3):1465–72. doi:10.1021/acs.est.5b05004.
- Kamal, M. S., S. A. Razzak, and M. M. Hossain. 2016. Catalytic oxidation of volatile organic compounds (VOCs) - A review. Atmos. Environ. 140:117–34. doi:10.1016/j.atmosenv.2016.05.031.
- Kamravaei, S., P. Shariaty, M. J. Lashaki, J. D. Atkinson, Z. Hashisho, J. H. Phillips, J. E. Anderson, and M. Nichols. 2017. Effect of beaded activated carbon fluidization on adsorption of volatile organic compounds. Ind. Eng. Chem. Res. 56 (5):1297–305. doi:10.1021/acs.iecr.6b04165.
- Kim, K. J., and H. G. Ahn. 2010. The adsorption and desorption characteristics of a binary component system of toluene and methylethylketone on activated carbon modified with phosphoric acid. Carbon 48 (8):2198–202. doi:10.1016/j.carbon.2010.02.021.
- Kim, M. I., C. H. Yun, Y. J. Kim, C. R. Park, and M. Inagaki. 2002. Changes in pore properties of phenol formaldehyde-based carbon with carbonization and oxidation conditions. Carbon 40 (11):2003–12. doi:10.1016/S0008-6223(02)00058-1.
- Kim, Y. J., M. I. I. Kim, C. H. Yun, J. Y. Chang, C. R. Park, and M. Inagaki. 2004. Comparative study of carbon dioxide and nitrogen atmospheric effects on the chemical structure changes during pyrolysis of phenol-formaldehyde spheres. J. Colloid Interface Sci. 274 (2):555–62. doi:10.1016/j.jcis.2003.12.029.
- Lashaki, M. J., M. Fayaz, S. Niknaddaf, and Z. Hashisho. 2012. Effect of the adsorbate kinetic diameter on the accuracy of the Dubinin-Radushkevich equation for modeling adsorption of organic vapors on activated carbon. J. Hazard. Mater. 241-242:154–63. doi:10.1016/j.jhazmat.2012.09.024.
- Lee, W. J., C. Y. Yu, K. C. Chang, Y. P. Huang, C. H. Chang, and C. T. Liu. 2011. Spherical PF resin beads prepared from phenol-liquefied Bambusa dolichoclada with suspension polymerization. Holzforsch 65 (2):163–69. doi:10.1515/hf.2010.120.
- Lelieveld, J., J. S. Evans, M. Fnais, D. Giannadaki, and A. Pozzer. 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525 (7569):367. doi:10.1038/nature15371.
- Li, L. Q., Z. Sun, H. L. Li, and T. C. Keener. 2012. Effects of activated carbon surface properties on the adsorption of volatile organic compounds. J. Air Waste Manag. Assoc. 62 (10):1196–202. doi:10.1080/10962247.2012.700633.
- Liang, Q., Y. Wang, F. Lin, M. Jiang, P. Li, and B. Huang. 2017. A facile microwave-hydrothermal synthesis of fluorescent carbon quantum dots from bamboo tar and their application. Anal. Methods 9 (24):3675–81. doi:10.1039/C7AY01069A.
- Lillo-Ródenas, M. A., D. Cazorla-Amoros, and A. Linares-Solano. 2005. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 43 (8):1758–67. doi:10.1016/j.carbon.2005.02.023.
- Long, C., Y. Li, W. H. Yu, and A. M. Li. 2012. Removal of benzene and methyl ethyl ketone vapor: Comparison of hypercrosslinked polymeric adsorbent with activated carbon. J. Hazard. Mater. 203:251–56. doi:10.1016/j.jhazmat.2011.12.010.
- Luo, L., D. Ramirez, M. J. Rood, G. Grevillot, K. J. Hay, and D. L. Thurston. 2006. Adsorption and electrothermal desorption of organic vapors using activated carbon adsorbents with novel morphologies. Carbon 44 (13):2715–23. doi:10.1016/j.carbon.2006.04.007.
- Mao, H. Y., D. G. Zhou, Z. Hashisho, S. G. Wang, H. Chen, H. Y. Wang, and M. J. Lashaki. 2015b. Microporous activated carbon from pinewood and wheat straw by microwave-assisted KOH treatment for the adsorption of toluene and acetone vapors. RSC Adv. 5 (45):36051–58. doi:10.1039/C5RA01320H.
- Mao, H. Y., D. G. Zhou, Z. Hashisho, S. U. Wang, H. Chen, and H. Y. Wang. 2015a. Constant power and constant temperature microwave regeneration of toluene and acetone loaded on microporous activated carbon from agricultural residue. J. Ind. Eng. Chem. 21:516–25. doi:10.1016/j.jiec.2014.03.014.
- Mosher, K., J. J. He, Y. Y. Liu, E. Rupp, and J. Wilcox. 2013. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 109:36–44. doi:10.1016/j.coal.2013.01.001.
- Mudliar, S., B. Giri, K. Padoley, D. Satpute, R. Dixit, P. Bhatt, R. Pandey, A. Juwarkar, and A. Vaidya. 2010. Bioreactors for treatment of VOCs and odours - A review. J. Environ. Manage 91 (5):1039–54. doi:10.1016/j.jenvman.2010.01.006.
- Nguyen, C., and D. D. Do. 2001. The Dubinin-Radushkevich equation and the underlying microscopic adsorption description. Carbon 39 (9):1327–36. doi:10.1016/S0008-6223(00)00265-7.
- Ouzzine, M., A. J. Romero-Anaya, M. A. Lillo-Ródenas, and A. Linares-Solano. 2019. Spherical activated carbons for the adsorption of a real multicomponent VOC mixture. Carbon 148:214–23. doi:10.1016/j.carbon.2019.03.075.
- Ozturk, B., and D. Yilmaz. 2006. Absorptive removal of volatile organic compounds from flue gas streams. Process Saf. Environ. Prot. 84 (5):391–98. doi:10.1205/psep05003.
- Pan, R. R., F. L. Fan, Y. Li, and X. J. Jin. 2016. Microwave regeneration of phenol-loaded activated carbons obtained from Arundo donax and waste fiberboard. RSC Adv. 6 (39):32960–66. doi:10.1039/C6RA01642A.
- Pei, J., and J. S. S. Zhang. 2012. Determination of adsorption isotherm and diffusion coefficient of toluene on activated carbon at low concentrations. Build. Environ. 48:66–76. doi:10.1016/j.buildenv.2011.08.005.
- Price, D. W., and P. S. Schmidt. 1998. VOC recovery through microwave regeneration of adsorbents: Comparative economic feasibility studies. J. Air Waste Manag. Assoc. 48 (12):1146–55. doi:10.1080/10473289.1998.10463759.
- Qi, J. W., J. S. Li, Y. Li, X. F. Fang, X. Y. Sun, J. Y. Shen, W. Q. Han, and L. J. Wang. 2017. Synthesis of porous carbon beads with controllable pore structure for volatile organic compounds removal. Chem. Eng. J. 307:989–98. doi:10.1016/j.cej.2016.09.022.
- Qian, Q., C. Gong, Z. Zhang, and G. Yuan. 2015. Removal of VOCs by activated carbon microspheres derived from polymer: A comparative study. Adsorption 21 (4):333–41. doi:10.1007/s10450-015-9673-9.
- Qiao, W. M., Y. Song, M. Huda, X. Zhang, S. H. Yoon, I. Mochida, O. Katou, H. Hayashi, and K. Kawamoto. 2005. Development of carbon precursor from bamboo tar. Carbon 43 (14):3021–25. doi:10.1016/j.carbon.2005.06.023.
- Ramirez, D., S. Y. Qi, and M. J. Rood. 2005. Equilibrium and heat of adsorption for organic vapors and activated carbons. Environ. Sci. Technol. 39 (15):5864–71. doi:10.1021/es048144r.
- Romero-Anaya, A. J., M. A. Lillo-Ródenas, and A. Linares-Solano. 2010. Spherical activated carbons for low concentration toluene adsorption. Carbon 48 (9):2625–33. doi:10.1016/j.carbon.2010.03.067.
- Romero-Anaya, A. J., M. A. Lillo-Ródenas, and A. Linares-Solano. 2014. Activation of a spherical carbon for toluene adsorption at low concentration. Carbon 77:616–26. doi:10.1016/j.carbon.2014.05.066.
- Romero-Anaya, A. J., M. A. Lillo-Ródenas, and A. Linares-Solano. 2015. Factors governing the adsorption of ethanol on spherical activated carbons. Carbon 83:240–49. doi:10.1016/j.carbon.2014.10.092.
- Shah, I. K., P. Pre, and B. J. Alappat. 2014. Effect of thermal regeneration of spent activated carbon on volatile organic compound adsorption performances. J. Taiwan Inst. Chem. Eng. 45 (4):1733–38. doi:10.1016/j.jtice.2014.01.006.
- Singh, A., and D. Lal. 2008. Microporous activated carbon spheres prepared from resole-type crosslinked phenolic beads by physical activation. J. Appl. Polym. Sci. 110 (5):3283–91. doi:10.1002/app.v110:5.
- Singh, A., and D. Lal. 2010. Preparation and characterization of activated carbon spheres from polystyrene sulphonate beads by steam and carbon dioxide activation. J. Appl. Polym. Sci. 115 (4):2409–15. doi:10.1002/app.31340.
- Song, Y., W. R. Qiao, S. H. Yoon, and I. Mochida. 2005. Toluene adsorption on various activated carbons with different pore structures. New Carbon Mater. 20 (4):294–98.
- Thommes, M., K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, and K. S. W. Sing. 2015. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 87 (9–10):1051–69. doi:10.1515/pac-2014-1117.
- Wang, H., M. J. Lashaki, M. Fayaz, Z. Hashisho, J. H. Philips, J. E. Anderson, and M. Nichols. 2012. Adsorption and desorption of mixtures of organic vapors on beaded activated carbon. Environ. Sci. Technol. 46 (15):8341–50. doi:10.1021/es3013062.
- Wang, Q., X. Liang, W. Qiao, C. Liu, X. Liu, L. Zhan, and L. Ling. 2009. Preparation of polystyrene-based activated carbon spheres with high surface area and their adsorption to dibenzothiophene. Fuel Process Technol. 90 (3):381–87. doi:10.1016/j.fuproc.2008.10.008.
- Yu, X. N., S. J. Liu, G. X. Lin, X. C. Zhu, S. Zhang, R. Y. Qu, C. H. Zheng, and X. Gao. 2018. Insight into the significant roles of microstructures and functional groups on carbonaceous surfaces for acetone adsorption. RSC Adv. 8 (38):21541–50. doi:10.1039/C8RA03099E.
- Zhang, C., W. Song, X. Zhang, R. Li, S. Zhao, and C. Fan. 2018. Synthesis, characterization and evaluation of resin-based carbon spheres modified by oxygen functional groups for gaseous elemental mercury capture. J. Mater. Sci. 53 (13):9429–48. doi:10.1007/s10853-018-2231-6.
- Zhang, X. Y., B. Gao, A. E. Creamer, C. C. Cao, and Y. C. Li. 2017. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 338:102–23. doi:10.1016/j.jhazmat.2017.05.013.
- Zheng, Y. N., Q. Z. Li, C. C. Yuan, Q. L. Tao, Y. Zhao, G. Y. Zhang, J. F. Liu, and G. Qi. 2018. Thermodynamic analysis of high-pressure methane adsorption on coal-based activated carbon. Fuel 230:172–84. doi:10.1016/j.fuel.2018.05.056.
- Zhu, Z., A. Li, S. Zhong, F. Liu, and Q. Zhang. 2008. Preparation and characterization of polymer-based spherical activated carbons with tailored pore structure. J. Appl. Polym. Sci. 109 (3):1692–98. doi:10.1002/app.28304.