?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This article presents the results of an industrial-scale study (on 400 MWe lignite fired unit) of simultaneous NOx, SO2, and HgT removal in FGD absorber with oxidant injection (NaClO2) into flue gas. It was confirmed that the injection of sodium chlorite upstream the FGD (Flue Gas Desulfurization) absorber oxidize NO to NO2, Hg0 to Hg2+, and enhancing NOx and HgT removal efficiency from exhaust gas in FGD absorber. Mercury removal efficiency grows with the rise of degree of oxidation NO to NO2 and was limited by the phenomenon of re-emission. For NOx removal the most critical parameters is slurry pH and temperature. There was no negative effect on sulfur dioxide removal efficiency caused by oxidant injection in tested FGD absorber. Based on the data provided, NOx and HgT emissions can be reduced by adjusting the FGD absorber operating parameters combined with oxidant injection.
Implications
The emissions of nitrogen oxides and mercury from coal combustion have great influence of environment and human health. The injection of sodium chlorite upstream the FGD absorber oxidize NO to NO2, Hg0 to Hg2+ and enhancing NOx and HgT removal efficiency from flue gas.
Introduction
The most onerous atmospheric gas pollutants include nitrogen oxides NOx (NO and NO2), sulfur dioxide (SO2), particulate matter (PM 10 and 2.5), and heavy metals including mercury (Carpenter, Citation2013). The pollutants are mostly the products of the combustion processes of solid fuels, and the energy, heating, and transport industries are their primary emission sources (Tavoulareas and Józewicz Citation2005). All developed countries regulate the emissions of these pollutants (EU Commission, Citation2017; US EPA, Citation2018a, Citation2018b). The restrictive emission standards for these pollutants open the way for the application of high-efficiency methods of simultaneous removal of many pollutants within one flue gas cleaning system (Carpenter, Citation2013). Until now, their use has been limited due to the availability of methods aimed at one pollution; e.g., SCR is used for nitrogen oxides; semi-dry and wet installations of flue gas desulfurization are used for acid gases (SO2, HF, and HCl) (Tavoulareas and Józewicz Citation2005); and activated carbon addition to exhaust is used for mercury control (Srivastava et al. Citation2006). The pursuit of a “zero-emission” economy model means that the existing flue gas cleaning systems may be insufficient in the near future. Therefore, new methods should be sought that will allow achieving minimal concentrations of contaminants in the stack both for large (> 50 MWt) and smaller (<50 MWt) power facilities.
The methods of simultaneous removal of nitrogen oxides, sulfur dioxide, and mercury from flue gas can be divided into dry or wet methods, methods using low-temperature plasma, and oxidation methods (Kuropka Citation2012). Many flue gas cleaning methods use existing flue gas cleaning systems (e.g., an electrostatic precipitator or flue gas desulfurization system), expanding the scope of their application (Carpenter, Citation2013). The problem with the simultaneous removal of several pollutants within the flue gas desulfurization system is the difference in solubility between SO2, NOx, and Hg0 (Głomba et al. Citation2016). The solution to this problem could be the oxidation of NO to NO2 and Hg0 to Hg2+ to enable their absorption together with SO2 in the FGD absorber (Carpenter, Citation2013).
Chlorine compounds, hydrogen peroxide, ozone, and combinations of these oxidants are used to oxidize insoluble flue gas components (Ding, Zhong, and Zhang Citation2014; Hutson, Krzyżyńska, and Srivastava Citation2008; Skalska et al. Citation2016). The use of hydrogen peroxide is limited due to its low efficiency in relation to the oxidation of NO, while ozone, despite its remarkably high efficiency of pollutants oxidation, is an unstable compound at temperatures significantly higher than 1000C (Batakliev et al. Citation2014). Based on literature review, it has been found that many researchers use sodium chlorite for oxidation of pollutants, and laboratory scale results confirmed its high efficiency in relation to oxidation and simultaneous removal of NOx, SO2, and Hg0 (Hutson, Krzyżyńska, and Srivastava Citation2008; Lee, Deshwal, and Yoo Citation2005; Sada et al. Citation1978). Some tests were carried out on a pilot scale (Krzyżyńska, Zhao, and Hutson Citation2010), and no descriptions of experiments carried out under industrial conditions were found.
Sodium chlorite was tested as additive for FGD slurry where in acidic conditions (pH < 5.0) the chlorine dioxide (ClO2) is formed, which can oxidize metallic mercury and nitrogen oxide (Krzyżyńska, Zhao, and Hutson Citation2010; Lee, Deshwal, and Yoo Citation2005; Sada et al. Citation1978). Because of the possible reaction of sodium chlorite with calcium sulfite and other slurry compound, this method of introducing sodium chlorite in industrial scale is not favorable. Krzyżyńska and Hutson (Citation2012a) tested in laboratory conditions the influence of different location of injection of sodium chlorite on efficiency of simultaneous SO2, NO, and Hg0 removal. The highest simultaneous contaminations removal were achieved for injection before FGD absorber, the most favorable for additive consumption was injection after FGD absorber. Other tests were performed for addition of NaClO2 before wet electrostatic precipitator; for these tests, the largest release of ClO2 gas occured when the initial pH of NaClO2 were strong acidic (pH = 2.0) with resulted 94.4% of NO removal and 100% of SO2 removal (Park H-W and Park Citation2015). The oxidation efficiency of NO and Hg0 by sodium chlorite solution is dependent on combination of solution pH and temperature, because these parameters affect release of chlorine dioxide (Hao et al. Citation2017).
The presented work shows the results of tests on an industrial scale on the simultaneous removal of nitrogen oxides, sulfur dioxide, and mercury from flue gas using a pilot installation of sodium chlorite (NaClO2) injection to the flue gas upstream the FGD absorber.
Experimental setup
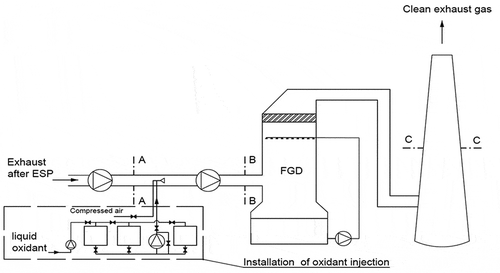
Tests were carried out using flue gas from a pulverized lignite coal-fired boiler (~ 400 MWe) equipped with an selective non-catalytic reduction of NOx (SNCR), electrostatic precipitator (ESP), a wet flue gas desulfurization system (WFGD) fed by CaCO3 (technical grade, purity < 97%) slurry, and an experimental mercury oxidation system upstream the FGD absorber. The absorber was equipped with 4 levels of spraying, and adipic acid was added to the slurry to increase the efficiency of the flue gas desulfurization. The experimental oxidant injection system (fed by technical grade NaClO2 25% w/w solution by Brenntag) was located between the flue gas fan and the auxiliary fan and before the flue gas desulfurization system (). The selection of the injection site guaranteed a uniform mixing of the oxidant with the flue gas.
Figure 1. Technological scheme of test installation, location of measurement cross-sections during tests. (a) Measurement cross-section before oxidant injection system. (b) Measurement cross-section behind oxidant injection system. (c) Measurement cross-section located in the stack.
The basic parameters of flue gas, CaCO3 slurry, and coal for performed research (oxidation system switched off) are summarized in .
Table 1. Basic parameters of flue gas, CaCO3 slurry, and coal for performed research (in parenthesis minimal and maximal values).
Test methods
In cross-sections located upstream the absorber (A) and in stack (C) – continuous measurements of total mercury concentration (CMM system with CVAF mercury analyzer of Gasmet, accuracy < 2% of measurement range) were carried out in the flue gas. In cross-section (A, B, and C) – continuous measurements of NO, NO2, NOx and SO2 concentration (CEMS system with DX 4000 FTIR gas analyzer of Gasmet, accuracy < 2% of measurement range) were carried out in the flue gas. Additionally, in all 3 cross-sections (A, B, and C) the measurements of mercury speciation (Hg2+ and Hg0) in flue gas were carried out using the Ontario-Hydro method (ASTM, Citation2019). Based on the results of these measurements, the oxidation degree and removal efficiency of pollutants in the FGD absorber were determined. The FGD absorber operating parameters were continuously measured during testing based on monitoring system of power plant (slurry pH was measured by electrode Memosens CPS171D by Endress + Hausner, ORP was measured by electrode CPS42 ORP by Endress + Hausner).
NO to NO2 oxidation degree
To determine the NO to NO2 oxidation degree in a given measurement cross-section, the volumetric share of NO2 in the flue gas in relation to the sum of nitric oxide and nitrogen dioxide (NOx) was determined. The NO to NO2 oxidation degree was calculated by means of the following relations:
where
NO2B – NO2 concentration in the flue gas in the measurement cross-section (B), ppm;
NO2C – NO2 concentration in the stack (C), ppm;
NOxB – NOx concentration in the flue gas in the measurement cross-section (B), ppm;
NOxC – NOx concentration in the stack (C), ppm.
NOx removal efficiency in FGD absorber
The effectiveness of NOx removal from the flue gas in the FGD absorber was determined based on the measurement of NOx concentration (sum of NO and NO2 calculated as NO2 (PN, Citation1993)) in the cross-section located in the stack (C) and upstream the FGD absorber (A). The NOx removal efficiency was determined by means of the following relation:
where
NOxA – average NOx concentration in the flue gas upstream the absorber (A), mg/m3USR;
NOxC – average NOx concentration in flue gas in the stack (C), mg/m3USR.
SO2 removal efficiency in FGD absorber
The SO2 removal efficiency from the flue gas in the FGD absorber was determined on the basis of the measurement of SO2 concentration in the stack (C) and upstream the FGD absorber (A):
where
SO2A – averaged SO2 concentration in the flue gas upstream the absorber (A), mg/m3USR;
SO2C – averaged SO2 concentration in the stack (C), mg/m3USR.
HgT removal efficiency in FGD absorber
The measurement of mercury concentration in the flue gas was carried out downstream a high-efficiency dust collector (electrostatic precipitator); therefore, particle-bound mercury (HgP) was not taken into account by calculating the total mercury concentration (HgT) in the exhaust gas. The total mercury (HgT = Hg0 + Hg2+) removal efficiency in the FGD absorber was determined on the basis of continuous measurements in cross-sections located in the stack (C) and upstream the injection (A) according to the following formula:
where
HgTA – average total mercury concentration in measurement cross-section (A), μg/m3USR;
HgTC – average total mercury concentration in the stack (C), μg/m3USR.
Molar ratio X
To specify the number of moles of the oxidant to be applied in relation to the moles of nitrogen oxide in the flue gas, a molar ratio was introduced, as follows:
Calculation of the molar ratio X was made for the concentration of NO in the flue gas measured in the stack (C) in the period immediately prior to the oxidant injection.
Pollutants oxidation by sodium chlorite and removal in FGD absorber
The behavior of pollutants during the tests with the injection of the oxidant into the flue gas varied depending on the measurement cross-section. Exemplary course of changes in concentration of sulfur dioxide, nitric oxides (NO, NO2, and NOx), and total mercury in the measurement cross-sections A, B, and C is shown in . The oxidizer for flue gases was injected in variable quantities as follows: Series I: X = 0.65 (1.8 m3/h of 25% w/w NaClO2 solution; injection starts at 11:30 a.m. and stops at 01:30 p. m.); Series II: X = 0.92 (2.25 m3/h of 25% w/w NaClO2 solution; injection time from 01:30 p. m. to 02:32 p. m.). For the presented results, power of the unit was constant at 380 MW, and flue gas volumetric flow was 2,110,000 m3USR/h in stack (C).
Figure 2. Changes in NO, NO2, NOx, SO2, and HgT concentrations during oxidant injection (X = 0.65 and 0.92) at measurement cross-sections (a, b, and c).
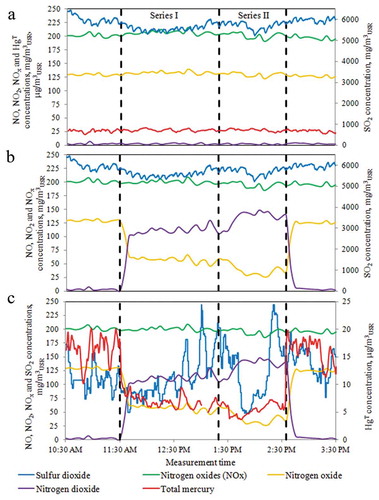
When the oxidant was injected into the flue gas, it led to the production of nitrogen dioxide, while the total amount of nitric oxides (NOx) in the flue gas in the cross-sections (A) and (B) remained unchanged. Increasing the amount of the injected oxidant resulted in an increase in the share of NO2 in the flue gas. The effect of the oxidizer injection on the change in the concentration of sulfur dioxide in the flue gas upstream the FGD absorber (B) was not observed, whereas the composition of mercury forms appearing in the flue gas has changed, as illustrated by the data in .
Table 2. Comparison of shares of individual mercury forms in the flue gas with and without the oxidant injection (determined by the Ontario-Hydro method, in cross-section (B)).
The addition of an oxidant to the flue gas resulted in the increase in the concentration of Hg2+ in the flue gas upstream the absorber (B). There was a clear decrease in the total mercury concentration in the stack (C) when the oxidizer was injected. The increase in the amount of the injected oxidant resulted in an improvement in HgT removal efficiency from the flue gas in the FGD absorber (Series I: 74%; Series II: 79.9%), which was directly related to the increase in the amount of Hg2+ in the flue gas.
No effect was noticed of the oxidant injection on the SO2 concentration in flue gas in the stack (C), and the sulfur dioxide removal efficiency in the FGD absorber was, respectively: Series I: 98%; Series II: 97.9%. Such a high SO2 removal efficiency was mainly a result of the addition of adipic acid to the slurry, which improves the solubility of CaCO3 (Frandsen, Kiil, and Johnsson Citation2001).
A comparison of the NO to NO2 oxidation degree was performed for the cross-sections located downstream the oxidizer injection site (B) and in the stack (C). Calculation of NO to NO2 oxidation degree was done using EquationEquations 1(1)
(1) and Equation2
(2)
(2) , and the results are summarized in .
Table 3. A comparison of the NO to NO2 oxidation degree for the cross-sections located in the stack (C) and downstream the oxidizer injection site (B).
Based on the analysis of the presented data, it was noticed that the degree of NO to NO2 oxidation increased with the amount of the injected oxidant and was at the same level in the cross-section downstream the injection site (B) and in the stack (C). This means that the efficiency of NO2 absorption from flue gas for the presented example is very low (for Series I, ηNOx = 7.4%; and for Series II, ηNOx = 5.4%).
When the aqueous solution of sodium chlorite is sprayed in the flue gas upstream the absorber, first it evaporates (the temperature of the flue gas during the 280tests at the oxidant injection site (A) varies from 165 to 170oC). As a result of the reaction of gaseous sodium chlorite with nitric oxide (initial pH of sodium chlorite solution was 11.5) nitrogen dioxide and sodium chloride being formed (Lee, Deshwal, and Yoo Citation2005):
Due to the significant share of moisture in the flue gas (from 28 to 29%), there were very good conditions for the formation of nitric and nitrous acids (Sun et al. Citation2017):
The nitric acid formed in the flue gas reacted with the metallic mercury and oxidized it to the form Hg2+ (mercury (II) nitrate), which explains the relationship between the increase in the amount of oxidant and the HgT removal efficiency from flue gas (Krzyżyńska, Zhao, and Hutson Citation2010; Lee, Deshwal, and Yoo Citation2005):
The nitrogen dioxide produced in this way may further contribute to the formation of nitric acid, which results in the observed high efficiency of HgT removal from boiler flue gas for the tested FGD absorber. Because flue gas contains acidic gases such as SO2, HCl, and HF, they can be absorbed by oxidant droplets and can drop its pH before evaporation, which causes the release of ClO2 (Hao et al. Citation2017). Chlorine dioxide can directly oxidize NO and Hg0; additionally, emission of chlorine radical is possible, which can enhance Hg0 oxidation (Hao et al. Citation2017; Krzyżyńska, Zhao, and Hutson Citation2010):
In such a complicated gas mixture as flue gases from lignite combustion, the presented mechanism can occur simultaneously. As the concentration of NOx in cross-section (A) is at the same level as in cross-section (B), there was no reaction of forming higher nitric oxides N2O3 and N2O4 in the gas phase, which could affect the NOx absorption efficiency in the CaCO3 slurry (Paiva and Kachan Citation2004).
Influence of FGD absorber operation parameters on NOx removal efficiency
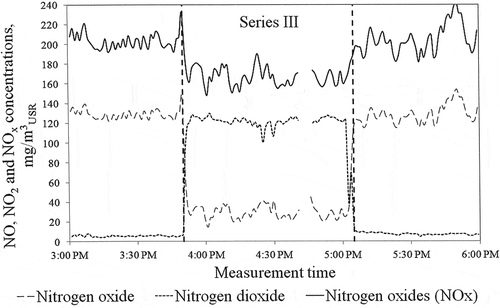
Nitric oxide (NO) is practically insoluble in water, and a change in the NOx concentration in the flue gas downstream the absorber is mainly the result of the NO2 absorption in the slurry or in the water washing off the flue gas mist separator. For the results presented in , corresponding to the average slurry parameters: pH = 6.08, ORP = 109 mV, T = 61.6°C, the NOx removal efficiency from the flue gas was low. For the measurement series III for the average slurry parameters: pH = 5.80, ORP = 135 mV, T = 60.3°C (), the NOx removal efficiency from flue gas was 17.8% at a similar excess of the oxidant as for Series I (Series III: X = 0.6, 1.45 m3/h of 25% w/w NaClO2 solution, injection time from 03:43 p.m. to 04:58 p.m.). For presented results, the power of the unit was constant at 368 MW and flue gas volumetric flow was 1,814,507 m3USR/h in stack (C).
The obtained results indicate that the nitric oxide removal efficiency from flue gas is dependent not only on the NO to NO2 oxidation degree but also on the operating parameters of the FGD absorber. The analysis of the impact of absorber operating parameters (pH, ORP, and temperature of the slurry) was performed with the oxidant injection system in operation. The measurement results were taken into account where the oxidizer was injected into the flue gas in such an amount that X was in the range of 0.50.6. The change in the oxidation degree of NO to NO2 ranged from 43 to 64%. The influence of average slurry parameters on the NOx removal efficiency from flue gas is shown in .
Figure 4. NOx removal efficiency in function of average slurry parameters (pH (a), temperature (b), and ORP (c)).
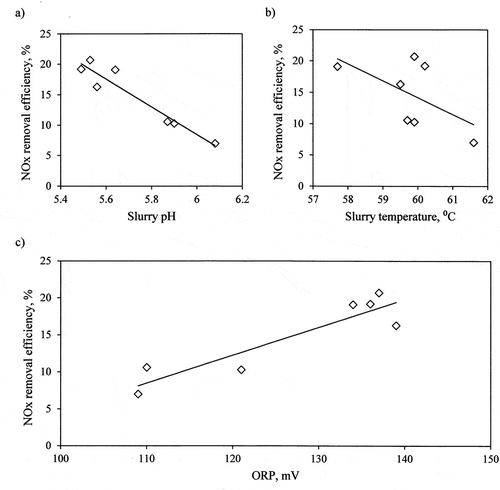
The decrease in the pH of the slurry in the absorber improves the NOx removal efficiency from flue gas as well as the lowering of the temperature of the slurry. The maximum NOx removal efficiency from flue gas at 20.7% was obtained for pH = 5.64, ORP = 137 mV, and the temperature of the slurry in the absorber at 59.9°C.
The obtained data confirms the results obtained on the laboratory scale (Heidel, Hilber, and Scheffknecht Citation2014; Krzyżynska and Hutson Citation2012b), where low pH < 7.0 of the slurry promoted the absorption of NO2 from the carrier gas in the calcium carbonate slurry. It is because nitrogen dioxide reacts with and oxidizes HSO3− and SO32- ions present in the slurry (Wang et al. Citation2012). For pH between 5.3 and 6.1, sulfite, sulfate, and hydrogen sulfate ions coexist with each other in the slurry (Glamser, Eikmeier, and Petzel Citation1989), the lower pH of the slurry effected the higher share of HSO3− (for pH around 4.0, there are mainly HSO3− ions):
Nitrogen dioxide present in the flue gas can react with sulfite and hydrogen sulfate ions and oxidize them (Littlejohn, Wang, and Chang Citation1993; Shen and Gary Citation1998; Turšič, Grgic´, and Bizjak Citation2001):
The formed nitrite ions may be oxidized in the tank under the absorber with oxygen dissolved in the slurry (air is supplied to oxidize CaSO3 to CaSO4) (Glamser, Eikmeier, and Petzel Citation1989):
Thus, the main products of the flue gas denitrification process should be nitrate and nitrite ions. shows the results of the NO3− and NO2− ion content determination, in the sample of slurry from the absorber.
Table 4. The content of nitrite and nitrate ions in the slurry sample from the absorber.
In the slurry samples taken before and after the oxidant injection, mainly the increase in the concentration of nitrate ions and the slight increase in the concentration of nitrite ions were observed. Interestingly, for Series III, where the NOx removal efficiency was at a higher level, the NO3− and NO2− concentration increase was low (no wastewater was removed from the tested FGD absorber). Some researchers indicate that at low pH values of the slurry, it is possible for the nitrous acid to react with the hydrogen sulfate, which leads to formation of hydroxylamine-disulfonate (HADS) (Siddiqi, Petersen, and Lucas Citation2001), which is the precursor to the formation of many N-S compounds (e.g., (HO3 S)2NOH, HO3 SNO, and (HO3S)3 N) (MacNeil et al. Citation1998):
However, because the results of the slurry composition analysis show that the main product of the method are nitrate ions (), the amount of potentially formed N-S compounds is small. Another phenomenon that could potentially occur in the tested FGD absorber is the NO2 reduction to molecular nitrogen:
This effect is particularly visible at high concentrations of SO32- or HSO3− ions (Łuszkiewicz Citation2017). The available results of the reduction process are focused on the role of SO32- ions (Chirona and Alshuter Citation1999); however, as the present results show, a similar effect can be obtained with a high concentration of HSO3− ions. Since high concentrations of HSO3− ions are present at low pH in the slurry, reactions of reduction of nitric oxide to molecular nitrogen and the formation of N-S compounds may take place simultaneously. The illustration of this process is different molar balance of nitrogen compounds calculated on the basis of gas measurements and slurry analysis, for Series III and I + II.
Influence of FGD absorber operation parameters on HgT removal efficiency
The addition of sodium chlorite to flue gas resulted in an increase of oxidized mercury content before FGD absorber (), that should have enhanced a mercury removal in the tested FGD absorber. The influence of average parameters of the desulphurization process (pH, temperature, and ORP) on the efficiency of HgT removal from flue gases was analyzed. The measurement results were taken into account where the oxidizer was injected into the flue gas in such an amount that X was in the range of 0.60.7 ().
Figure 5. HgT removal efficiency in function of average slurry parameters (pH (a), temperature (b) and ORP (c)).
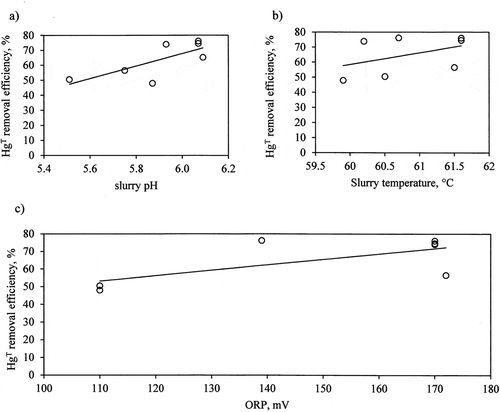
The phenomenon that limits the mercury removal efficiency from flue gas by means of the oxidizer injection is its re-emission from the FGD absorber. This phenomenon is affected by the absorber’s operating parameters, SO32- ions concentration, Hg2+ concentration in flue gases upstream the absorber, and the addition of adipic acid to the slurry (Chang et al. Citation2017; Chen et al. Citation2014; Heidel, Hilber, and Scheffknecht Citation2014; Heidel and Klein Citation2017; Omine et al. Citation2012;). Studies on the re-emission phenomenon for the tested FGD absorber were presented in the publication (Jędrusik et al. Citation2019). In presented research, HgT removal efficiency decreased with a pH drop in slurry tank, when pH = 5.5 HgT removal efficiency was 50%; when slurry pH at 6.05 efficiency reached 73%. The observed effect of pH can be explained by interaction of absorbed Hg2+ with SO32- in slurry (Heidel, Hilber, and Scheffknecht Citation2014; Heidel and Klein Citation2017):
For presented data, the increasing value of slurry temperature and ORP enhanced HgT removal efficiency, the observed effect is similar as in the literature (Heidel, Hilber, and Scheffknecht Citation2014; Heidel and Klein Citation2017).
Influence of molar ratio X on HgT and NOx removal efficiency
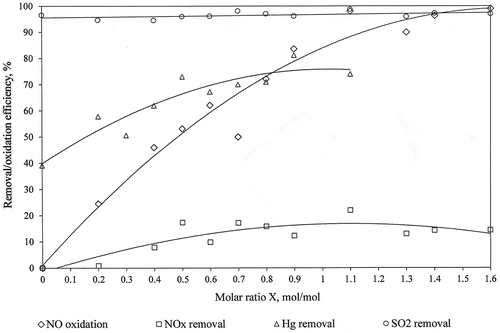
The collective summary of results of measurements of NO to NO2 oxidation efficiency, NOx, SO2, and HgT removal efficiency in FGD absorber as a function of the molar ratio X (mol NaClO2/mol NO) is shown in (averaged values from all performed experiments).
Both the NO to NO2 oxidation degree and the NOx and HgT removal efficiency increased with the amount of the oxidizer injected into the flue gas duct upstream of the tested FGD absorber. The NO to NO2 oxidation for X > 1 ranged between 90 and 100%, which means that optimal conditions for mixing the oxidizer with the flue gas were ensured. The efficiency of removing nitrogen oxides from the flue gas increased slightly with the amount of injected oxidant, reaching a maximum of 22%, while the absorption efficiency depended mainly on the slurry parameters () and not on the amount of the injected oxidant.
The total mercury (HgT) is removed in the tested absorber with an efficiency of up to 80% and increases with the amount of the injected oxidant, which is responsible for increasing the share of Hg2+ in the flue gas upstream the absorber (). Efficiency of mercury removal in FGD absorber is limited by mercury re-emission. Studies on this phenomenon for the tested FGD absorber are presented in the publication (Jędrusik et al. Citation2019), where it was shown that for high excess of oxidant X > 0.9 there was a possible phenomenon of rapid re-emission.
The sulfur dioxide removal efficiency in the absorber was very high and resulted from the addition of adipic acid to the slurry. The amount of oxidant to be injected had no evident impact on the removal of sulfur dioxide, despite the fact that sodium chlorite is a source of Na+ ions in the slurry, which can contribute to improving desulfurization efficiency (Koech et al. Citation2014):
Operational costs of sodium chlorite injection to flue gas for 400 MW lignite-fired block is in the range of 2.1 – 3 $ per MWh of produced electric power. Calculation were made for operation time of the unit: 8,000 h per year, concentration of nitrogen oxides in flue gas: 200 mg/m3USR, the price of NaClO2 solution at 500 $ per ton, and for molar ratio X in the range of 0.5–1.0. The investment costs of installation of sodium chlorine injection to flue gas depends on specific conditions that occur on the object. For research object, the investment costs of industrial installation were estimated at 300 000 $.
Conclusion
Based on the presented results of tests carried out under full scale conditions, the following conclusions can be shown:
Injection of the oxidizer (sodium chlorite) into the flue gas channel upstream the FGD absorber allows for simultaneous reduction of NOx and HgT emissions from flue gas.
Injection of the oxidizer causes the increase of NO2 concentration in the flue gas upstream the absorber, which creates nitric acid in the flue gas, which allows for Hg0 oxidation, which improves the removal of HgT from the flue gas in the FGD absorber.
The oxidizer injection does not have a negative effect on the SO2 removal efficiency from the flue gas.
For the tested absorber, using the oxidant injection, the removal efficiency of 99% for sulfur dioxide, 80% for total mercury, and 22% for nitric oxides under the industrial conditions was obtained.
Based on the data provided, NOx and HgT emissions can be reduced by adjusting the FGD absorber operating parameters combined with oxidant injection.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Maria Jędrusik
Maria Jędrusik is associate professor at Wrocław University of Science and Technology, Wrocław, Poland. She can be contacted at [email protected].
Dariusz Łuszkiewicz
Dariusz Łuszkiewicz is assistant professor at Wrocław University of Science and Technology, Wrocław, Poland. He can be contacted at [email protected].
Arkadiusz Świerczok
Arkadiusz Świerczok is associate professor at Wrocław University of Science and Technology, Wrocław, Poland. He can be contacted at [email protected].
Mieczysław Adam Gostomczyk
Mieczysław Adam Gostomczyk is professor at Wrocław University of Science and Technology, Wrocław, Poland. He can be contacted at [email protected].
Mariola Kobylańska-Pawlisz
Mariola Kobylańska-Pawlisz is head of innovation in flue gas purification department at Rafako S.A., Racibórz, Poland. She can be contacted at [email protected].
References
- American Society for Testing and Materials, USA. 2019. Standard test method for elemental, oxidized, particle-bound and total mercury in flue gas gened from coal-fired stationary sources (ASTM D6784). Accessed January 18, 2019. https://compass.astm.org/EDIT/html_annot.cgi?D6784+16.
- Batakliev, T., V. Georgiev, M. Anachkov, S. Rakovsky, and G. E. Zaikov. 2014. Ozone decomposition. Interdiscip. Toxicol. 7 (2):47–59. doi:10.2478/intox-2014-0008.
- Carpenter, A. M. 2013. Advances in multi-pollutant control; CCC/227; IEA clean coal centre, November; Accessed July 26, 2018. https://www.usea.org/sites/default/files/112013_Advances%20in%20multi-pollutant%20control_ccc227.pdf.
- Chang, L., Y. Zhao, H. Li, C. Tian, Y. Zhang, and X. Yu. 2017. Effect of sulfide on divalent mercury reduction and re-emission in a simulated desulfurization aqueous solution. Fuel Process. Technol. 165:138–44. doi:10.1016/j.fuproc.2017.05.016.
- Chen, C., S. Liu, Y. Gao, and Y. Liu. 2014. Investigation on mercury reemission from limestone-gypsum wet flue gas desulfurization slurry. Sci. World J. ID:581724. doi:10.1155/2014/581724.
- Chirona, R. J., and B. Alshuter. 1999. Chemical aspects of NOx scrubbing. Pollut. Eng. 31:33–36.
- Ding, J., Q. Zhong, and S. L. Zhang. 2014. Catalytic efficiency of iron oxides in decomposition of H2O2 for simultaneous NOx and SO2 removal: Effect of calcination temperature. J. Mol. Catal. A: Chem. 393:222–31. doi:10.1016/j.molcata.2014.06.018.
- EU COMMISSION IMPLEMENTING DECISION 2017/1442 of 31 July 2017 establishing the Best Available Techniques (BAT) Conclusions for large combustion plants in accordance with Directive 2010/75/EU of the European Parliament and of the Council (L 212/1).
- Frandsen, J. B. W., S. Kiil, and J. E. Johnsson. 2001. Optimisation of a wet FGD pilot plant using fine limestone and organic acids. Chem. Eng. Sci. 56:3275–87. doi:10.1016/S0009-2509(01)00010-0.
- Glamser, J., M. Eikmeier, and H.-K. Petzel. 1989. Advanced concepts in FGD technology: The SHU process with cooling tower discharge. JAPCA 39 (9):1262–67. doi:10.1080/08940630.1989.10466618.
- Głomba, M., A. Hałat, W. Kordylewski, and D. Łuszkiewicz. 2016. Research on products of simultaneous removal of SO2 and NOx from flue gas by ozonation and alkaline absorption. Environ. Prot. Eng. 42 (2):125–36. doi:10.5277/epe160208.
- Hao, R., X. Wang, Y. Liang, Y. Lu, Y. Cai, X. Mao, B. Yuan, and Y. Zhao. 2017. Reactivity of NaClO2 and HA-Na in air pollutants removal: Active species identification and cooperative effect revelation. Chem. Eng. Jour. 330:1279–88. doi:10.1016/j.cej.2017.08.085.
- Heidel, B., M. Hilber, and G. Scheffknecht. 2014. Impact of additives for enhanced sulfur dioxide removal on re-emissions of mercury in wet flue gas desulfurization. Appl. Energy. 114:485–91. doi:10.1016/j.apenergy.2013.09.059.
- Heidel, B., and B. Klein. 2017. Reemission of elemental mercury and mercury halides in wet flue gas desulfurization. Int. J. Coal Geol. 170:28–34. doi:10.1016/j.coal.2016.09.003.
- Hutson, N. D., R. Krzyżyńska, and R. K. Srivastava. 2008. Simultaneous removal of SO2, NOx and Hg from coal flue gas using a NaClO2-enhanced wet scrubber. Ind. Eng. Chem. Res. 47:5825–31. doi:10.1021/ie800339p.
- Jędrusik, M., M. A. Gostomczyk, A. Świerczok, D. Łuszkiewicz, and M. Kobylańska. 2019. Mercury re-emission from adipic acid enhanced FGD absorber – Full scale investigations on ~ 400 MWe boiler (lignite) with oxidant injection to flue gas. Fuel 238:507–31. doi:10.1016/j.fuel.2018.10.131.
- Koech, L., R. Everson, H. Neomagus, and H. Rutto. 2014. Dissolution kinetics of sorbents and effect of additives in wet flue gas desulfurization. Rev. Chem. Eng. 30 (6):553–65. doi:10.1515/revce-2014-0024.
- Krzyżynska, R., and N. D. Hutson. 2012b. Effect of solution pH on SO2, NOx, and Hg removals from simulated coal combustion flue gas in an oxidant-enhanced wet scrubber. J. Air Waste Manage. Assoc. 62:212–20. doi:10.1080/10473289.2011.642951.
- Krzyżyńska, R., and N. D. Hutson. 2012a. The importance of the location of sodium chlorite application in multipollutant flue gas cleaning system. J. Air Waste Manage. Assoc. 62 (6):707–16. doi:10.1080/10962247.2012.668158.
- Krzyżyńska, R., Y. Zhao, and N. D. Hutson. 2010. Absorption of NOx, SO2 and mercury in a simulated additive-enhanced wet flue gas desulphurization scrubber. Polish J. Of Environ. Stud. 19 (9):1255–62.
- Kuropka, J. 2012. Technologies for purification of gases from sulphur dioxide and nitrogen oxides. Wrocław: Oficyna Wydawnicza Politechniki Wrocławskiej, (in Polish).
- Lee, H. K., B. R. Deshwal, and K. S. Yoo. 2005. Simultaneous removal of SO2 and NO by sodium chlorite solution in wetted-wall column. Korean J. Chem. Eng. 22:208–13. doi:10.1007/BF02701486.
- Littlejohn, D., T. Wang, and S. G. Chang. 1993. Oxidation of aqueous sulfite ion by nitrogen dioxide. Environ Sci. Technol. 27 (10):2162–67. doi:10.1021/es00047a024.
- Łuszkiewicz, D. 2017. Removal of pollutants from the exhaust gas with ozone - characteristics of the product (dissertation in polish). Wroclaw University of Science and Technology. https://fbc.pionier.net.pl/details/oai:www.dbc.wroc.pl:42256
- MacNeil, J. H., P. A. Berseth, G. Westwood, and W. C. Trogler. 1998. Aqueous catalytic disproportionation and oxidation of nitric oxide. Environ Sci. Technol. 32 (7):876–81. doi:10.1021/es970743t.
- Omine, N., C. E. Romero, H. Kikkawa, S. Wu, and S. Eswaran. 2012. Study of elemental mercury re-emission in simulated wet scrubber. Fuel 91:93–101. doi:10.1016/j.fuel.2011.06.018.
- Paiva, J. L., and G. C. Kachan. 2004. Absorption of nitrogen oxides in aqueous solutions in a structured packing pilot column. Chem. Eng. And Process. 43:941–48. doi:10.1016/j.cep.2003.08.005.
- Park H-W, C. S., and D.-W. Park. 2015. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with plasma electrostatic precipitator. J. Of Hazard. Mater. 285:117–26. doi:10.1016/j.jhazmat.2014.11.040.
- Polish Norma: Air purity protection. 1993. Examination of the content of nitrogen and its compounds, PN93/Z-04009/06 (in Polish).
- Sada, E., H. Kumazawa, Y. Yamanaka, I. Kudo, and T. Kondo. 1978. Absorption of sulfur dioxide and nitric oxide in aqueous mixed solutions of sodium chlorite and sodium hydroxide. J. Chem. Eng. Jpn. 11:276–82. doi:10.1252/jcej.11.276.
- Shen, C. H., and T. Gary. 1998. Nitrogen dioxide absorption and sulfite oxidation in aqueous sulfite. Environ Sci. Technol. 32 (13):1994–2003. doi:10.1021/es970466q.
- Siddiqi, M. A., J. Petersen, and K. Lucas. 2001. A study of the effect of nitrogen dioxide on the absorption of sulfur dioxide in wet flue gas cleaning processes. Ind. Eng. Chem. Res. 40 (9):2116–27. doi:10.1021/ie000815g.
- Skalska, K., S. Ledakowicz, R. Louwe, and R. Szymczak. 2016. Nitrogen oxides pre-ozonation in flue gases from phosphate rock digestion. Chem. Eng. J. 318:181–88. doi:10.1016/j.cej.2016.06.048.
- Srivastava, R. K., N. Hutson, B. Martin, F. Princiotta, and J. Staudt. 2006. Control of mercury emissions from coal-fired electric utility boilers. Environ. Sci. Technol. 40 (5):1385–93. doi:10.1021/es062639u.
- Sun, Y., X. Hong, T. Zhu, X. Guo, and D. Xie. 2017. The chemical behaviors of nitrogen dioxide absorption in sulfite solution. Appl. Sci. 7:377. doi:10.3390/app7040377.
- Tavoulareas, E. S., and W. Józewicz 2005. Multi pollutant emission control technology options for coal-fired power plants. EPA-600/R-05/034. Accessed July 26, 2018. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NRMRL&dirEntryId=118703.
- Turšič, J., I. Grgic´, and M. Bizjak. 2001. Influence of NO2 and dissolved iron on the S(IV) oxidation in synthetic aqueous solution. Atmos. Environ. 35 (1):97–104. doi:10.1016/S1352-2310(00)00283-1.
- U.S. Environmental Protection Agency. 2018a. Mercury and Air Toxics Standards (MATS) for power plants. Accessed July 26, 2018. http://www.epa.gov/mats/.
- U.S. Environmental Protection Agency. 2018b. Emission standards regulations. Accessed July 29, 2018. https://www.epa.gov/emission-standards-reference-guide/epa-emission-standards-regulations
- Wang, Z., X. Zhang, Z. Zhou, W.-Y. Chen, J. Zhou, and K. Cen. 2012. Effect of additive agents on the simultaneous absorption of NO2 and SO2 in the calcium sulfite slurry. Energy & Fuels 26:5583−5589. doi:10.1021/ef3007504.