?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, granular activated carbon (GAC) was used as an adsorbent for biogas desulfurization. Biogas containing 932–2,350 ppm of H2S was collected from an anaerobic digester to treat the wastewater from a dairy farm with about 200 cows. An adsorption test was performed by introducing the biogas to a column that was packed with approximately 50 L of commercial GAC. The operation ceased if the effluent gas had an H2S concentration of over 100 ppm. The GAC was replaced by a given weight of new GAC in a subsequent test. According to the results, for H2S concentrations in the range of 932–1,560 ppm (average±SD = 1,260 ± 256 ppm), 1 kg of the GAC yielded biogas treatment capacities of 568 ± 112 m3 and H2S adsorption capacities of 979 ± 235 g. For the higher influent H2S concentrations of 2,110 ± 219 ppm, the biogas treatment and H2S-adsorption capacities decreased to 229 ± 18 m3 and 668 ± 47 g, respectively. An estimation indicated a requisite cost of US$16.5 for the purification of 1,000 m3 of biogas containing 2,110 ppm of H2S. This cost is approximately 5% of US$330, the value of 1,000 m3 of biogas.
Implications
Biogas generated from anaerobic digesters of animal manure and municipal wastewater sludge contains hydrogen sulfide which must be removed before it can be combusted in electricity-generation engines. This study demonstrated that commercial activated carbon adsorption can be an economical and effective approach for removing hydrogen sulfide from biogas. In this study, granular activated carbon (GAC) was used as an adsorbent for biogas desulfurization. The biogas containing 932–2,350 ppm of H2S was collected from an anaerobic digester for treating wastewater collected from a 200 dairy farm. The adsorption test was performed by introducing the biogas to a PVC column packed with a commercial GAC of around 50 L. Operation ceased if the effluent gas had an H2S concentration of over 100 ppm. A given weight of the new GAC was replaced for a successive test.
Introduction
Biogas is produced through the anaerobic digestion of various types of feedstock, such as animal manure, food waste, municipal wastewater sludge, and crop residue. The gas is composed of 40–70% methane, 60–30% carbon dioxide, and 100–3000 ppm of hydrogen sulfide (H2S). In general, H2S must be removed from the biogas before the biogas can be used in engines because H2S causes equipment corrosion and the formation of sulfur oxides when combusted (Ho Citation2012; Smith and Ndegwa Citation2012; Solcia et al. Citation2014). The removal of H2S has been identified as a technological barrier in the production and use of biogas.
The techniques for the removal of H2S from biogas or industrial waste gases include physical, chemical, and biological methods. Physical methods involve water scrubbing or activated carbon adsorption (Lien, Lin, and Tin Citation2014; Pipatmanomai, Kaewluan, and Vitidsant Citation2009). Chemical methods, such as the oxidative absorption of H2S into an aqueous solution of ferric sulfate, involve expensive chemicals and necessitate the management of the sulfur slurry that is formed from H2S. The removal of H2S from gases through microbiological methods has been intensively studied. Most relevant studies have focused on fixed-film processes such as the use of bioscrubbing towers (BSTs) and biofilters (BFs) or biotrickling filters (BTFs) (Liang and Liang Citation2013; Siró and Plackett Citation2010; Solcia et al. Citation2014; Su et al. Citation2013; Su, Chen, and Chang Citation2014). BFs and BTs are not suitable for the removal of H2S from biogases because the limited oxygen contents in most biogases are not enough to oxidize the absorbed H2S to sulfate.
Although H2S removal through the use of activated carbon adsorption requires the replacement of the adsorbent after a certain operation time to restore its performance, this method is potentially effective and economical for small- to medium-sized cattle or pig farms. As noted in Ho (Citation2012), molecular sieves have high capacities for H2S removal under dry conditions (Tanada and Boki Citation1974), but their effectiveness is decreased for wet streams; by contrast, activated carbon has strong adsorption capacity for both dry and wet streams (Meeyoo, Trimm, and Cant Citation1997). Nano/micro fibrillated cellulose materials or polymeric adsorbents may be served as adsorbents for H2S or reduced sulfur compounds from aqueous solutions, hydrocarbon streams, and diesels (Hokkanen et al. Citation2014; Nazir et al. Citation2018; Ramirez-Corredores et al. Citation2008; Siró and Plackett Citation2010; Skrzypski et al. Citation2011; Xia et al. Citation2016). These materials are promising for present and future applications to the H2S removal from biogases.
Activated carbon enables the direct oxidation of H2S through the following reaction (Ghosh and Tollefson Citation1986).
Shen et al. (Citation2018) demonstrated that adsorption of H2S leads to the formations of C-S or C-SH on activated carbon surface, followed by their evolution into C-S-C. These sulfur species including C-S, C-S-C and C-SH are stable on activated carbon. The chemical adsorption behavior implies that higher adsorption capacities of H2S can be attributed to the formation of the above-cited species as compared with the physical adsorption one. Shen et al. (Citation2019) also investigated the roles of oxygen functional groups in hydrogen sulfide adsorption on the activated carbon surface. Results indicate that pyrone, carbonyl, ester, and carboxyl groups are active sites for H2S adsorption, except for hydroxyl group. H2S can react directly with a carbonyl oxygen atom in these oxygen groups leading to the formation of C–S, C–OH, and C–SH species.
Roddaeng, Promvonge, and Anuwattana (Citation2018) conducted an experimental study on H2S adsorption behaviors using amine-impregnated solid adsorbents (granular activated carbon [GAC] and polyethyleneimine [PEI]) for gaseous H2S concentrations in the range of 200–400 ppm in N2 gas. Their results indicated that the adsorbent (GAC impregnated with 2.0 g L−1 PEI solution) exhibited an adsorption capacity of approximately 110 g H2S (kg adsorbent)−1 for the influent gas containing 200 ppm of H2S. An adsorption capacity of approximately 230 g H2S (kg adsorbent) −1 was obtained for the non-impregnated GAC and for the influent gas containing 400 ppm of H2 S. For capacity estimation, the breakthrough concentration of H2S was defined as 25% of the influent concentration. In addition, a NaOH-impregnated activated carbon sample having a hydrogen sulfide adsorption capacity of 15 g H2S (kg adsorbent) −1 from a biogas with 100 ppm of H2S was developed (Menezes et al. Citation2018). Melina et al. (Citation2016) studied the adsorption of CO2 and H2S on commercial activated carbon Desorex K43 impregnated with K2CO3, NaOH, or Fe2O3. Results showed high adsorption capacities of 156 g H2S (kg adsorbent) −1 for H2S under dry conditions and 1 bar.
Activated carbon has been used as a catalyst or absorbent in the removal of H2S from air, natural gas, and other gas streams. Their use in the catalytic partial oxidation of H2S has also been identified as a highly promising approach for removing sulfur from fuel cell feedstocks (Gardner et al. Citation2002). Types of activated carbon that are derived from various precursors have been studied, and different performance levels have been determined. The results reported by various researchers have often been inconsistent. Experiments have demonstrated that carbon is an effective catalyst for H2S oxidation, even though impurities present in some commercial variants of active carbon also serve as catalysts (Cariaso and Walker Citation1975).
The presence of water, O2, and lower gas hourly space velocity (GHSV) has beneficial effects on activated carbon performance. CO2 decreases adsorption capacity due to its acidity. The highest adsorption capacity, for an influent H2S of 50 ppm and a breakthrough H2 S of 1 ppm, was obtained as 13 wt% in KOH/CaO-impregnated activated carbon in a CH4 (60%)/CO2 (38%)/O2 (2%) gas atmosphere at ambient temperature (RH 90%, and 5000 h−1 GHSV). Sulfur species formation was verified in the exhausted samples (Isik-Gulsac Citation2016).
Although there are many researches on the removal of H2S from biogas or industrial gases, to the authors’ knowledge, there are no field data on the removal of H2S from biogas by activated carbon. In this study, a long-term evaluation was conducted of the data on activated carbon as an adsorbent for biogas desulfurization. The study focuses on activated carbon’s potential to develop into an economical and easy-to-use unit for the removal of H2S in biogas generated by small- to medium-sized cattle farms. Biogas containing approximately 900–2100 ppm of H2S was collected from an anaerobic digester to treat the wastewater from a 200 dairy cows farm. In this study, non-impregnated commercially available GAC was used as an adsorbent for the biogas desulfurization.
Materials and methods
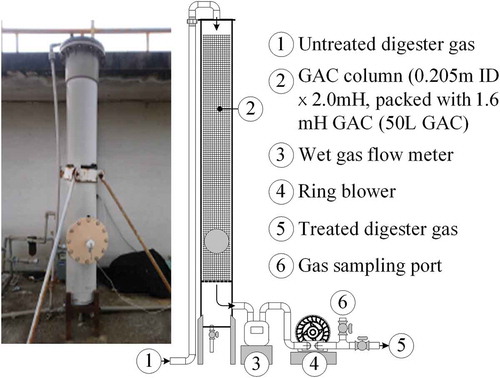
The biogas, containing CH4 65 ± 3.0%, CO2 28 ± 1.5%, and O2 0.54 ± 0.22%, was collected from an anaerobic digester to treat wastewater from 200 dairy farms (Ou, Chou, and Chang Citation2020). As illustrated in , the adsorption test was performed by introducing the biogas through a ring blower into a PVC (poly vinyl chloride) column (2.0 m height, 0.205 m inner diameter) packed with a brand of commercial GAC (approximately 50 L) to a maximum height of 1.60 m. lists some characteristics of the experimental GAC.
Table 1. Some characteristics of the experimental GAC as received.
The column was initially filled with the GAC to a certain height. Subsequently, the biogas flow rate to the GAC column was adjusted to a preset value. During the test, the effluent biogas flow rate, temperature, and H2S concentrations in the biogas influent to and effluent from the column were measured. In addition, the concentrations of methane, carbon dioxide, and oxygen in the effluent biogas were measured. The operation ceased if the effluent gas had an H2S concentration of over 100 ppm. A given weight of the virgin GAC was replaced in a subsequent test. For the test runs, the biogas flow rates were approximately 22.3 m3 h−1 at 20–25°C, and the calculated space velocity (GHSV) for the biogas flowing through the GAC-packed space was approximately 450 h−1 [22,300 Lh−1 (50 L)−1]. The calculated superficial velocity for the biogas flowing through the cross-section of the packed space was 0.19 m s−1.
H2S concentrations in the gas samples were measured using a portable multiple gas analyzer (Dräger X-am 7000, Dräger Safety AG & Co. KGaA, Germany). Data on gaseous CH4, CO2, and O2 could also be obtained through the gas analyzer.
Results and discussion
provides an example of the breakthrough curves for the adsorption of H2 S by the GAC from the biogas. The test was conducted from November to December 2013 under the following operating conditions: influent H2 S 1,500 ± 100 ppm, effluent CH4 57.3 ± 2.0%, CO2 24.7 ± 3.3%, O2 1.57 ± 0.29%, flow rate 22.3 ± 1.9 m3 h−1, and temperature 20–25°C. illustrates the curves for another example obtained from a test conducted from January to February, 2014, under the following operating conditions: influent H2 S 1,500 ± 100 ppm, effluent CH4 58.0 ± 4.6%, CO2 26.5 ± 3.9%, O2 1.55 ± 0.99%, flow rate 22.4 ± 3.7 m3 h−1, and temperature 20–25°C. Methane and CO2 contents in the effluent biogas were lower than those in the influent one might be attributed to that some methane and CO2 were adsorbed by the activated carbon (De-Oliveira et al., Citation2019; Guo, Chang, and Xie Citation2006). Both curves in and indicate that at the beginning of both adsorption runs with the virgin GAC in the column, H2 S removal could reach almost 100%. H2 S in the effluent biogas appeared near the end of the run within 10 days, which was approximately a fifth of the breakthrough time of 50 days. In other words, at the end of the breakthrough time, around 1.28 m or 4/5 depth of the packed carbon was not able to adsorb H2S from the influent biogas. The mass-transfer zone for the adsorption of biogas H2S from about 1,500 ppm to 100 ppm could be estimated to be 0.32 m with the biogas flowing through the bed cross section at a superficial velocity of 0.19 m/s.
Figure 2. Breakthrough curve for adsorption of H2 S from the biogas by the GAC in Nov. to Dec., 2013. (Run 2: Influent H2 S 1,500 ± 100 ppm; effluent CH4 57.3 ± 2.0%, CO2 24.7 ± 3.3%, O2 1.57 ± 0.29%, Flow rate 22.3 ± 1.9 m3 h−1, and Temp. 20–25°C).
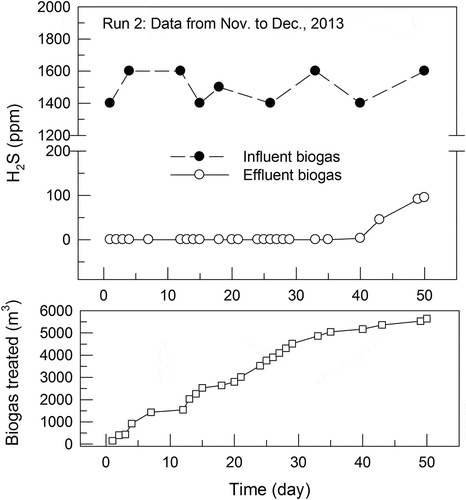
Figure 3. Breakthrough curve for adsorption of H2 S from the biogas by the GAC in Jan. to Feb., 2014. (Run 3: Influent H2 S 1,500 ± 100 ppm; effluent CH4 58.0 ± 4.6%, CO2 26.5 ± 3.9%, O2 1.55 ± 0.99%, Flow rate 22.4 ± 3.7 m3 h−1, and Temp. 20–25°C).
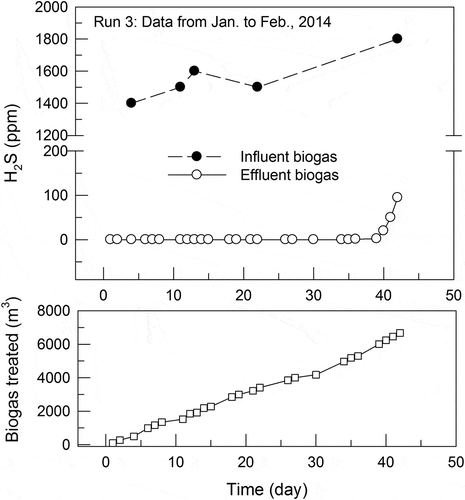
and list the experimental data and calculated data of K (average treatment capacity of the biogas, in m3 of biogas per kg of GAC) and S (H2S adsorption capacity, in g of H2S per kg of GAC) for a total of nine test runs. The data in indicate that for H2S concentrations in the range of 932–1,560 ppm (average±SD = 1,260 ± 256 ppm), 1 kg of the GAC had biogas treatment capacities of 568 ± 112 m3 and H2S adsorption capacities of 0.979 ± 0.235 kg. For the higher influent H2S concentrations of 2,110 ± 219 ppm, as depicted in , the biogas treatment and H2S adsorption capacities decreased to 229 ± 18 m3 and 668 ± 47 g, respectively.
Table 2. Experimental data and calculated data of K and S for Co = 932–1,560 ppm.
Table 3. Experimental data and calculated data of K and S for Co = 1,920–2,350 ppm [Note].
The average H2S adsorption capacities of 980 and 670 g (kg GAC) −1 for the influent H2S concentrations of 1,260 and 2,110 ppm, respectively, are comparable with those cited in Roddaeng, Promvonge, and Anuwattana (Citation2018). The study obtained a capacity of 230 g of H2S per kg of a non-impregnated GAC with influent gas of 400 ppm H2S in N2 gas. The capacity could reach an absorbent value of approximately 725 g H2S (kg GAC)−1 (230 × 1,260/400) with H2S in the influent biogas increasing to 1,260 ppm, assuming that the capacity is linearly proportional to the influent H2S concentration in the cited range. The capacity in the present study is higher than the value estimated from the data of Roddaeng, Promvonge, and Anuwattana (Citation2018). The higher value in the present study may be attributed to the presence of water and O2, which have beneficial effects on activated carbon performance (Isik-Gulsac Citation2016). The H2S adsorption capacity of 980 g (kg GAC)−1 was much higher than the value of 190 g (kg GAC) −1 reported in Menezes et al. (Citation2018) if corrected to an influent H2S concentration of 1,260 ppm.
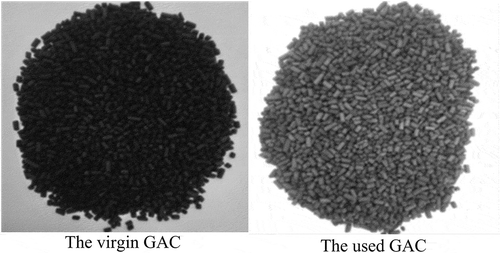
In the present study, the volumetric biogas treatment and H2S adsorption capacities decreased with higher H2S concentrations in the tested biogas. This is attributable to the formation of elementary sulfur that rapidly clogged the pores and covered the surface area used for H2S adsorption as well as its subsequent conversion to elementary sulfur. displays the appearances of the virgin and used GAC obtained from run No. 9. The used GAC was covered with sulfur powder and produced additional H2S fumes.
To ensure practicability, an evaluation of the process’ attendant cost of treating the biogas was required. indicates that on average, 1 kg of the GAC was able to purify approximately 568 m3 of biogas containing 1,260 ppm of H2S. The average cost for the treatment of 1,000 m3 of biogas was 5 USD.28, at a GAC cost of 3 USD.0 kg−1. In addition, the 1-HP gas pump operated for 4.5 h per day, and the electricity cost for the purification of 1,000 m3 of biogas was 3 USD.36, at an electricity cost of 0 USD.10 kWh−1. The total daily operation cost (GAC and electricity) was 8 USD.64 for the purification of 1,000 m3 of biogas, as illustrated in . The table also lists an operation cost of 16 USD.5 for the purification of 1,000 m3 of biogas containing 2,110 ppm of H2S. This cost of 16 USD.5 is approximately 5% of 330 USD, the value of 1,000 m3 of biogas. A total mass of 173 kg of the activated carbon was consumed in the 3 test years. Because of the small amount of the activated carbon and low desulfurization cost for the biogas, no attempt was made to regenerate the spent carbon during the test time. However, it may be worthy to regenerate it for the application of the technology to high biogas flows.
Table 4. Operation cost estimation.
Conclusion
In this study, GAC was used as an adsorbent for biogas desulfurization. Experimental results revealed that for H2S concentrations in the range of 932–1,560 ppm (average±SD = 1,260 ± 256 ppm), 1 kg of GAC had biogas treatment capacities of 568 ± 112 m3 and H2S adsorption capacities of 979 ± 235 g. For the higher influent H2S concentrations of 2,110 ± 219 ppm, the biogas treatment and H2 S adsorption capacities decreased to 229 ± 18 m3 and 668 ± 47 g, respectively. In total, 16 USD.5 is required to purify 1,000 m3 of biogas containing 2,110 ppm of H2 S. This cost is approximately 5% of 330 USD, the value of 1,000 m3 of biogas.
Acknowledgment
The authors thank the co-author participants for their research and support. Financial support by Livestock Research Institute, Council of Agriculture, Executive Yuan of Taiwan, R.O.C. is also appreciated.
Disclosure statement
The authors declare they have no competing interests.
Additional information
Funding
Notes on contributors
H.W. Ou
H.W. Ou is a Ph.D. candidate of the Institute of Environmental Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, ROC. Also, he is an Assistant Researcher of the Animal Industry Division, Livestock Research Institute, Council of Agriculture, Executive Yuan, Tainan, Taiwan, ROC.
M.L. Fang
M.L. Fang is a postdoctoral research fellow of the Center for Environmental Toxin and Emerging-Contaminant Research, Cheng Shiu University, Kaohsiung 83347, Taiwan, ROC. Also,She is a postdoctoral research fellow of the Super Micro Research and Technology Center, Cheng Shiu University.
M.S. Chou
M.S. Chou is an emeritus professor of the Institute of Environmental Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, ROC.
H.Y. Chang
H.Y. Chang is a postdoctoral research fellow of the Institute of Environmental Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, ROC.
T.F. Shiao
T.F. Shiao is a Researcher of the Animal Industry Division, Livestock Research Institute, Council of Agriculture, Executive Yuan, Tainan, Taiwan, ROC.
References
- Cariaso, O. C., and P. L. Walker Jr. 1975. Oxidation of hydrogen sulfide over microporous carbons. Carbon 13 (3):233–39. doi:10.1016/0008-6223(75)90237-7.
- de Oliveira, L. H., J. G. Meneguin, M. V. Pereira, J. F. Do Nascimento, and P. A. Arroyo. 2019. Adsorption of hydrogen sulfide, carbon dioxide, methane, and their mixtures on activated carbon. Chem. Eng. Commun. 206 (11):1533–53. doi:10.1080/00986445.2019.1601627.
- Gardner, T. H., D. A. Berry, K. D. Lyons, S. K. Beer, and A. D. Freed. 2002. Fuel processor integrated H2S catalytic partial oxidation technology for sulfur removal in fuel cell power plants. Fuel 81 (17):2157–66. doi:10.1016/S0016-2361(02)00161-8.
- Ghosh, T. K., and E. L. Tollefson. 1986. A continuous process for recovery of sulfur from natural gas containing low concentrations of hydrogen sulfide. Can. J. Chem. Eng. 64 (6):960–68. doi:10.1002/cjce.5450640612.
- Guo, B., L. Chang, and K. Xie. 2006. Adsorption of carbon dioxide on activated carbon. J. Nat. Gas Chem. 15 (3):223–29. doi:10.1016/S1003-9953(06)60030-3.
- Ho, N. 2012. Modeling hydrogen sulfide adsorption by activated carbon made from anaerobic digestion by-product. Master Thesis, Department of Chemical Engineering and Applied Chemistry, University of Toronto, Canada.
- Hokkanen, S., E. Repo, A. Bhatnagar, W. Z. Tang, and M. Sillanpää. 2014. Adsorption of hydrogen sulphide from aqueous solutions using modified nano/micro fibrillated cellulose. Environ. Technol. 35 (18):2334–46. doi:10.1080/09593330.2014.903300.
- Isik-Gulsac, I. G. 2016. Investigation of impregnated activated carbon properties used in hydrogen sulfide fine removal. Braz. J. Chem. Eng. 33 (4):1021–30. doi:10.1590/0104-6632.20160334s20150164.
- Liang, M. S., and Y. Liang. 2013. Biological removal of H2S from the livestock manure using a biofilter. Biotechnol. Bioproc. Eng. 18 (5):1008–15. doi:10.1007/s12257-013-0025-x.
- Lien, C. C., J. L. Lin, and C. H. Tin. 2014. Water scrubbing for removal of hydrogen sulfide [H2S] in biogas from hog farms. J. Agri. Chem. Environ. 3 (2):1–6.
- Meeyoo, V., D. L. Trimm, and N. W. Cant. 1997. Adsorption-reaction processes for the removal of hydrogen sulphide from gas streams. J. Chem. Technol. Biotech. 68 (4):441–416.
- Melina, C., K. O. M. Castrillon, A. A. Caiuã, M. Bastos-Neto, C. S. A. Diana, J. Hofmann, J. Möllmer, W.-D. Einicke, and R. Gläser. 2016. CO2 and H2S removal from CH4-rich streams by adsorption on activated carbons modified with K2CO3, NaOH, or Fe2O3. Energy Fuels 30 (11):9596–604.
- Menezes, R. L. C. B., K. O. Moura, S. M. P. de Lucena, D. C. S. Azevedo, and M. Bastos-Neto. 2018. Insights on the mechanisms of H2S retention at low concentration on impregnated carbons. Ind. Eng. Chem. Res. 57 (6):2248–57. doi:10.1021/acs.iecr.7b03402.
- Nazir, M. S., H. Ajab, M. R. Raza, and M. A. Abdullah. 2018. Surface modification of cellulose fibers from oil palm empty fruit bunches for heavy metal ion sorption and diesel desulphurization. Desalin. Water Treat. 107:241–56.
- Ou, H. W., M. S. Chou, and H. Y. Chang. 2020. Removal of hydrogen sulfide from biogas using a bubbling tank fed with aerated wastewater. Aerosol Air Qual. Res. 20:643–53.
- Pipatmanomai, S., S. Kaewluan, and T. Vitidsant. 2009. Economic assessment of biogas-to-electricity generation system with H2S removal by activated carbon in small pig farm. Appl. Energy 86 (5):669–74.
- Ramirez-Corredores, M. M., Z. Hernandez, J. Guerra, and R. V. Navarro. 2008. Selective sulfur removal from hydrocarbon streams by absorption. Patent No.: US 7,446,077 B2.
- Roddaeng, S., P. Promvonge, and R. Anuwattana. 2018. Behaviors of hydrogen sulfide removal using granular activated carbon and modified granular activated carbon. MATEC Web of Conferences, Warsaw, Poland, vol. 192, 03037.
- Shen, F., J. Liu, C. Gu, and D. Wu. 2019. Roles of oxygen functional groups in hydrogen sulfide adsorption on activated carbon surface: A density functional study. Ind. Eng. Chem. Res. 58 (14):5526–32.
- Shen, F., J. Liu, Z. Zhang, Y. Yuchen Dong, and C. Gu. 2018. Density functional study of hydrogen sulfide adsorption mechanism on activated carbon. Fuel Process. Technol. 171:258–64.
- Siró, I., and D. Plackett. 2010. Micro fibrillated cellulose and new nano composite materials: A review. Cellulose 17:459–94.
- Skrzypski, I. J., I. Bezverkhyy, O. Heintz, and J. P. Bellat. 2011. Low temperature H2S removal with metal-doped nanostructure ZnO sorbents: Study of the origin of enhanced reactivity in Cu-containing materials. Ind. Eng. Chem. Res. 50 (9):5714–22.
- Smith, S. A., and P. M. Ndegwa. 2012. Hydrogen sulfide concentrations in biogas from diary manure digesters, in diary manure anaerobic digesters. State of Washington, USA: Department of Ecology Report to the Legislature, Department of Ecology.
- Solcia, R. B., M. Ramīez, M. Fernāndez, D. Cantero, and D. Bevilaqua. 2014. Hydrogen sulphide removal from air by biotrickling filter using open-pore polyurethane foam as a carrier. Biochem. Eng. J. 84:1–8.
- Su, J. J., Y. C. Chang, Y. J. Chen, K. C. Chang, and S. Y. Lee. 2013. Hydrogen sulfide removal from livestock biogas by a farm-scale bio-filter desulfurization system. Water Sci. Technol. 67 (6):1288–93.
- Su, J. J., Y. J. Chen, and Y. C. Chang. 2014. A study of a pilot-scale biogas bio-filter system for utilization on pig farms. J. Agricul. Sci. 152 (2):217–24.
- Tanada, S., and K. Boki. 1974. Properties of various adsorbents for removal of hydrogen sulfide gas. Chem. Pharma. 22 (11):2703–09.
- Xia, Y., Y. Li, Y. Gu, T. Jin, Q. Yang, J. Hu, H. Liu, and H. Wang. 2016. Adsorption desulfurization by hierarchical porous organic polymer of poly-methylbenzene with metal impregnation. Fuel 170:100–06.