ABSTRACT
Lignin obtained from renewable biomass is a potential feedstock for the synthesis of various value-added chemicals through efficient biocatalytic routes. The ligninolytic enzymes-assisted depolymerization of lignin to vanillin constitutes the most commercially attractive and promising approach in green chemistry as vanillin constitutes the second most prevalent flavoring agent. Thus, in the present work, immobilized laccase and versatile peroxidase, and further, a co-immobilized laccase and versatile peroxidase system on magnetic silica microspheres (MSMS) were developed to generate a robust biocatalytic system that mediates the depolymerization of lignin obtained from Casuarina equisetifolia biomass. The depolymerization of lignin by free and immobilized laccase showed a vanillin yield of 24.8% and 23%, respectively, at pH 4.0 in 6 h at 30°C against a vanillin yield of 20% and 21.7% with the free and immobilized versatile peroxidase, respectively, at pH 5.0°C and 50°C. Comparatively, the system with the co-immobilized laccase and versatile peroxidase exhibited a 1-fold and 1.2-fold higher vanillin yield than the free and immobilized laccase system, respectively. On comparing with the versatile peroxidase system, the co-immobilized biocatalytic system displayed 1.3-fold and 1.2-fold increased vanillin yield than the free and immobilized versatile peroxidase system, respectively, at a pH of 6.0 in 6 h at 30°C with an enzyme concentration of 1 U/ml. The reusability studies of the co-immobilized biocatalytic system exhibited that both the enzymes retained up to 40% of its activity till sixth cycle.
Implications: The waste biomass of Casuarina equisetifolia is widely available around the coastal regions of India which does not have any agricultural or industrial applications. The present work exploits the lignocellulosic content of the Casuarina biomass to extract the lignin, which provides a renewable alternative for the production of the commercially high-valued compound, vanillin. This work also integrates a co-immobilized biocatalytic process comprising of laccase and versatile peroxidase leading to an environmentally benign enzymatic process for the depolymerization of lignin to vanillin.
Introduction
The transition from the petrochemical-based industry, due to the dwindling of the crude oil reservoirs, to a sustainable bioeconomy is a major challenge of the twenty-first century. One achievable approach toward sustainable development is the accession of the renewable biomass, and lignocellulosic biomass constitutes a relatively cheaper alternative for the production of fuels and chemicals. Casuarina equisetifolia or Australian pine tree, found abundantly in Oceania, Southeast Asia, and the Indo-Australian Archipelago, institutes as a preeminent source of lignocellulosic biomass as it is not grown as fodder or for industrial applications (Nicodemus Citation2009; Saikia et al. Citation2020). It mainly comprises of 48.7% cellulose, 27.13% hemicellulose, and 26.64% lignin (Senthilkumar et al. Citation2015), and thus, provide an essential platform for the sustainable development of the bio-based refinery.
In the recent years, several works have been published on the depolymerization of lignin to produce various aromatic compounds like guaiacol, ringol, eugenol, vanillin, anisole, veratrole to name a few (Camarero, Martínez, and Martínez Citation2014). Out of these compounds, vanillin constitutes a greater interest as it has been widely used in food industries and as a fragrant ingredient in perfumes (Wang et al. Citation2018). Along with these applications, it can also be used as an antifoaming agent in herbicides production and as a chemical precursor in pharmaceutical industries (Kumar, Sharma, and Mishra Citation2012).
At a commercial scale, depolymerization of lignin is commonly attained by chemical treatment, which is not environment friendly and has high energy demand. To address these disadvantages, various microbial and enzymatic treatments of lignin were established (Yang et al. Citation2019). Amongst these biological treatments, the depolymerization of lignin by a cocktail of ligninolytic enzymes belonging to the group of lignin-modifying enzymes, specifically laccase and versatile peroxidase, have been extensively studied (Hämäläinen et al. Citation2018). The initial oxidative attack on the phenolic moiety of lignin by laccase results in the release of phenolic residues along with oxidized side chains, which then act as natural mediators in the oxidation of more recalcitrant non-phenolic components of lignin, leading to the complete lignin depolymerization (Christopher, Yao, and Ji Citation2014). On the other hand, versatile peroxidase depolymerizes lignin by forming lignin phenoxy radicals by utilizing low molecular-weight oxidants like Mn2+, veratryl alcohol unsaturated lipids, which act as redox mediators during the depolymerization process (Kamimura et al. Citation2019). Previous studies suggested that a cumulative laccase-peroxidase complex maximizes the proximity effect between these enzymes and thus, the degradative synergistic effect has the potential of establishing an oxidative enzyme complex with higher potential for lignin depolymerization (Crestini, Melone, and Saladino Citation2011; Shin et al. Citation2019). However, the application of free enzymes possesses implications for large-scale industrial applications as it limits the long-term operational stability, reusability, and recovery of the enzymes, thereby affecting the operational cost of the process (Ba and Kumar Citation2017; Crestini, Melone, and Saladino Citation2011; Vishnu et al. Citation2017). In this front, the convergence of magnetic nanotechnology with enzymatic catalysis provides a facile and effective method for the immobilization of enzymes which can be utilized in continuous or batch production of vanillin.
The magnetic biocatalysts confer remarkable physical properties that provide long-term stability of the biocatalysts and easy separation from the reaction mixture by using a magnetic field (Ba and Kumar Citation2017). In our previous study, we developed magnetically separable immobilized laccase, immobilized versatile peroxidase and co-immobilized laccase and versatile peroxidase for environment applications (Vishnu et al. Citation2017), which were exploited in the present study for green route synthesis (Gasser et al. Citation2012). Thus, utilizing the advantages of magnetic-assisted immobilization, the aim of the present work was to utilize the developed co-immobilized biocatalysts in a strategic depolymerization process for the production of vanillin from Casuarina waste biomass-derived lignin. Subsequently, various operating process parameters were optimized for maximizing the vanillin production.
Materials and methods
Chemicals and enzymes
All the chemicals and substrates used in the study were of HPLC grade (≥98% purity) and were procured from Sigma Aldrich (MO, USA). The enzyme laccase from Trametes versicolor (0.3 U/mg) was procured from Sigma Aldrich (MO, USA) and the enzyme, versatile peroxidase from Bjerkandera adusta (~3.8 U/mg), was purchased from Jena Bioscience (Jena, Germany). The PLN 257 MSMS were provided by Materium Innovations Inc. (Granby, Quebec, Canada).
Extraction of lignin from Casuarina equisetifolia
Lignin was extracted from Casuarina biomass by acid pre-treatment using formic acid/acetic acid (70:30, v/v) according to a published report (Watkins et al. Citation2015). The lignin dissolved in the acid mixture was precipitated by adding distilled water and thereafter, the precipitate was filtered. The obtained precipitate was then washed with distilled water and vacuum dried before identification.
Synthesis of immobilized biocatalysts
The co-immobilization of laccase and versatile peroxidase on multi-functionalized magnetic silica microspheres (MSMS) was achieved according to our previous work (Vishnu et al. Citation2017). The laccase activity was determined spectrophotometrically at 420 nm using ABTS as the substrate, and the versatile peroxidase activity was measured spectrophotometrically at 310 nm using veratryl alcohol as the substrate according to established protocols (Kumar, Sivanesan, and Cabana Citation2014; Vishnu et al. Citation2017). The amount of protein in the samples was quantified by Lowry’s method.
Production of vanillin by lignin depolymerization
The enzymatic depolymerization of lignin to the highly functionalized molecule, vanillin, was achieved by using free laccase and versatile peroxidase, immobilized laccase, and versatile peroxidase and further, co-immobilized laccase and versatile peroxidase. In an air-tight glass vial, 5% lignin was incubated with 1.0 U/ml of the respective biocatalysts for a period of (0–24) h at a temperature range of (20–40) °C. The effect of pH on lignin depolymerization was established by preparing 5% lignin in buffers of varying pH (pH 3.0 to pH 7.0) and the optimum enzyme concentration was validated by varying the concentration as (0.5–2.0) U/ml. To enhance the reaction in the system containing laccase, CuSO4 (0.5 mM) was added to these systems. Further, since versatile peroxidase has the dual effect of manganese peroxidase (MnP) and lignin peroxidase (LiP), the substrates MnSO4 (0.4 mM, for MnP) and H2O2 (1 mM, for LiP) were introduced into the reaction systems containing versatile peroxidase. After incubation, the fractionated sample was centrifuged at 10,000 rpm for 15 min. The residue was extracted using chloroform and after evaporating the organic phase, the residue was resuspended in a solution of methanol/water (60:40, v/v) for RP-HPLC and GC-MS analysis (Min et al. Citation2017). The detailed reaction conditions for the individual reaction is explained in Table S2.
GC-MS analysis
After 6 h of lignin depolymerization, the products obtained were identified by GC-MS. The analytes were extracted with chloroform (three times) and the organic phase was analyzed by GC-MS (Agilent 6000 series, Santa Clara, CA, USA), equipped with a HP-5 MS column (30 m × 0.25 mm internal diameter; 0.25 μm film thickness). The capillary column fused with silica (30 m x 0.25 mm i.d.) was used with helium (99.99%) as the column and carrier gas at a constant flow rate (1.2 mL min−1). The chromatographic peaks were identified by the retention time and the mass spectra library (NIST02) (Min et al. Citation2017).
HPLC analysis
Vanillin produced from lignin was analyzed by HPLC (SP 8000 Spectra Physics, Santa Clara, CA, USA) at 280 nm using a variable wavelength UV – detector. During analysis, the fractions were injected by a reverse phase C-18 column (Macherey, Nagel & Co., Dtiren, FRG, 250 × 4.6 mm i.d). The gradient separation was performed from distilled water (solvent A) to methanol (solvent B) using the following conditions: flow rate 0.8 mL min−1, column temperature 25°C, time 0 min – 5% B, time 5 min – 25% B, time 10 min – 40% B, time 30 min-50% B, time 35 min-100% B (Min et al. Citation2017).
Reusability of the immobilized biocatalysts during vanillin production
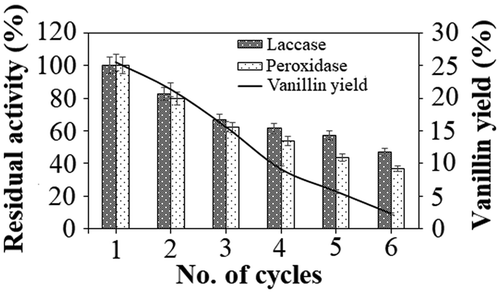
Reusability of immobilized biocatalysts specifies the operational viability of an industrial process as it reduces the operating cost. The reusability of the co-immobilized laccase and versatile peroxidase during the production of vanillin was studied in a repeated batch system. To assess the reusability of the co-immobilized biocatalysts, 5% lignin in pH 6.0 buffer was incubated with the immobilized biocatalysts for 6 h at 30°C for one cycle of vanillin production. At the end of the cycle, the immobilized biocatalysts were recovered from the reaction mixture by magnetic decantation and washed three times with 10 ml pH 6.0 buffer. The same procedure was followed for the subsequent cycles starting with 5% lignin (in pH 6.0 buffer) and the co-immobilized biocatalysts were recycled up to 6 cycles. As a control for the study, inactivated co-immobilized laccase and versatile peroxidase, obtained by autoclaving the co-immobilized biocatalytic system at 120°C for 20 min, was introduced into the reaction mixture containing 5% lignin in pH 6 buffer for 6 h at 30°C. The inactivation of both the enzymes was confirmed by the absence of laccase and versatile peroxidase activities (Kumar, Sivanesan, and Cabana Citation2014; Vishnu et al. Citation2017).
Results and discussion
Production of vanillin by lignin depolymerization
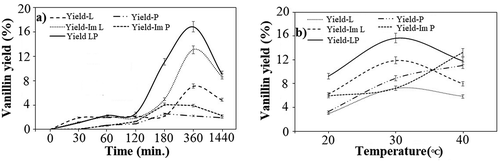
The extraction of lignin attained by mild acid treatment led to a lignin yield of around 24.3% (obtained by gravimetrical analysis) and is relatable to the yield of lignin obtained from various biomass (Shin et al. Citation2019; Watkins et al. Citation2015). The enzymatic depolymerization of lignin to vanillin was achieved by using the free and co-immobilized laccase and versatile peroxidase and the production of vanillin was confirmed by GC-MS analysis (Figure S1-S3) and quantified using HPLC (Figure S4). Since the reaction time greatly influences the extent of lignin depolymerization (Bourbonnais et al. Citation1997; Christopher, Yao, and Ji Citation2014) and further, the production of vanillin, the reaction was examined for a time interval of (0–24) h. It was observed that in the reaction mixture containing only laccase, the yield of vanillin increased over the period of 0.5 h to 6 h from nearly 2% in 0.5 h to 7.1% in 6 h with free laccase, and from 1.63% in 0.5 h to 13% in 6 h with the immobilized laccase. Further increasing the reaction time to 24 h led to a decrease in vanillin by 1.47-fold and 1.5-fold with the free and immobilized laccase, respectively. The initial oxidation of the phenolic components followed by Cα-Cβ cleavage of the non-phenolic sites of lignin by laccase mediated the increased lignin depolymerization (Christopher, Yao, and Ji Citation2014; Ibarra et al. Citation2006). Moreover, fungal laccases have a high redox potential difference between the T1 copper site and the substrate, which accelerates phenol oxidation (Munk et al. Citation2015). However, an increase in incubation time correlates to the inactivation of laccase due to oxidation of phenolic substances which explained the reduction in vanillin yield over a period of 24 h (Kim and Nicell Citation2006). When the reaction was performed with versatile peroxidase, a similar trend in the vanillin yield was observed where the maximum yield of 2.57% and 4.13% was obtained after 3 h for the free and immobilized peroxidase, respectively. Versatile peroxidase represents a hybrid of MnP and LiP and thus, oxidizes the non-phenolic rings present in lignin via long-range electron transfer which produces unstable cationic radicals that cleaves the Cα-Cβ and C4-ether linkages (Gasser et al. Citation2012). Further, the oxidation of Mn2+ to Mn3+ leads to the chelation of Mn3+ which acts as a redox mediator in the oxidation of the phenolic structures.
Versatile peroxidase is also known to initiate lipid peroxidation, which further aids in extended lignin depolymerization (Gasser et al. Citation2012). However, on increasing the reaction time beyond 3 h yielded a lower vanillin yield and a 1.3-fold and 1.8-fold reduction in vanillin yield was obtained with free and immobilized versatile peroxidase, respectively, at 24 h. This reduction in vanillin yield can be attributed to the saturation of versatile peroxidase activity after a prolonged incubation time (Li et al. Citation2019). In the final reaction system comprising the co-immobilized laccase and versatile peroxidase, the vanillin yield increased for the time duration of 0.5 h to 6 h and the maximum vanillin yield of 16.86% was obtained after 6 h. This yield was 2.3-fold and 6.5-fold higher than that obtained with individual systems with free laccase and peroxidase, respectively. On the other hand, the yield with the co-immobilized system was 1.3-fold and 4.1-fold higher than the immobilized laccase and versatile peroxidase systems, respectively. Similar to the other strategies, with the increase in time, a gradual reduction in the yield by 1.6-fold was observed in the co-immobilized laccase and versatile peroxidase system at 24 h (). To understand the effect of temperature on the depolymerization process, the reaction was examined at a temperature range of (20–40)°C (). In the reactions containing only laccase, the vanillin yield increased from 2.97% at 20°C to 7.2%°C at 30°C and 6.2% at 20°C to 11.9% at 30°C using the free and immobilized laccase, respectively. However, at a temperature of 40°C, the vanillin yield reduced by 1.3-fold and 1.5-fold using the free and immobilized laccase, respectively, which could be attributed to the lowered thermal stability of lignin at 40°C over prolonged incubation (Ibarra et al. Citation2006). Similarly, in the reactions consisting of only peroxidase, an equivalent increase in the vanillin yield was observed from temperature 20°C to 40°C and the maximum yield of 11% and 13.3% was achieved at 40°C using the free and immobilized peroxidase, respectively, which was 3.3-fold and 1.2-fold higher than the respective systems at 20°C. Meanwhile, in the co-immobilized laccase and peroxidase system, the maximum vanillin yield of 15.6% was obtained at 30°C. This yield was 2.2-fold and 1.4-fold higher than that achieved in the free laccase and peroxidase systems, respectively, and further, 1.3-fold and 1.1-fold higher than the immobilized laccase and peroxidase systems, respectively. This shift in optimum temperature to 30°C was probably due to the synergistic effects of laccase and peroxidase which lowered the energy requirement for the reaction (Vishnu et al. Citation2017).
Figure 1. Effect of, a) Time (min.) and b) Temperature (°C) on vanillin production using free laccase (L), free peroxidase (P), immobilized laccase (Im L), immobilized peroxidase (Im P) and co-immobilized laccase and peroxidase (LP). The experiments were performed in triplicates and expressed as mean average with an error
Subsequently, pH plays the most prominent role in the enzyme-assisted production of vanillin, and therefore, to increase the yield of vanillin, the reaction was performed at a pH range of 3.0–7.0. It was observed that at pH 3.0, there was no nearly production of vanillin in the reaction mixtures consisting of only free laccase, free versatile peroxidase, and the co-immobilized laccase and versatile peroxidase. The low solubility of lignin at acidic pH, attributing to its limited oxidation, could be contributed to no vanillin yield at the acidic pH (Yang et al. Citation2019). However, with the increase in pH, the system consisting of only versatile peroxidase led to the maximum vanillin yield of 18.9% and 20% with free and immobilized peroxidase, respectively, at pH 5.0, after which the yield decreased by 2.3-fold and 1.8-fold correspondingly at pH 6.0 for both the systems. Further, increasing the pH to 7.0 showed no production of vanillin in the versatile peroxidase-containing reaction system, which is due to the lowered versatile peroxidase activity at neutral and alkaline pH (Falade et al. Citation2019). On the other hand, on increasing the pH from 3.0 to 6.0, the maximum vanillin yield 11.6% and 16.5% in the free and immobilized laccase-mediated reactions, respectively, was achieved at a pH of 6.0, which was correspondingly 3-fold and 2.2-fold higher than the yield obtained at pH 7.0. At neutral and alkaline pH, binding of a hydroxide anion to the trinuclear coppers of laccase interrupts the internal electron transfer from T1 to the trinuclear center along with the ionization of critical amino acids, which could have contributed to the reduced yield of vanillin at pH 7.0 (Munoz et al. Citation1997). Further, in the co-immobilized laccase and versatile peroxidase system, on increasing the pH from 3.0 to 6.0, 1-fold increase in the yield of vanillin was achieved at a pH of 6.0 when compared to the yield at pH 5.0. The vanillin yield obtained at pH 6.0 in the co-immobilized system was found to be comparatively an average of 3.5-fold higher than the free enzyme systems, and it was an average of 2.4-fold higher than the immobilized enzyme systems. However, with the increase of pH to 7.0, no production of vanillin was observed (), which could be due to the lowered activities of laccase and versatile peroxidase at higher pH due to inactivation (Kim and Nicell Citation2006; Mizobutsi et al. Citation2010).
Figure 2. Effect of, a) pH and b) Enzyme concentration (U/mL) on vanillin production using free laccase (L), free peroxidase (P), immobilized laccase (Im L), immobilized peroxidase (Im P) and co-immobilized laccase and peroxidase (LP). The experiments were performed in triplicates and expressed as mean average with error
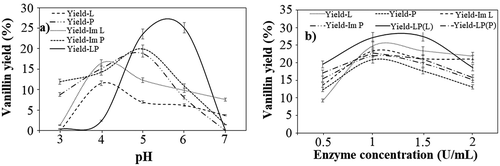
Further, the effect of enzyme concentration was studied by varying the enzyme concentration as (0.5–2) U/ml (). For the systems containing only free and immobilized laccase, the maximum vanillin yield of 24.8% and 23.2%, respectively, was achieved at an enzyme concentration of 1 U/ml. However, increasing the enzyme concentration to 2 U/ml led to a 1-fold decrease in the vanillin yield in both the free and immobilized laccase systems. Similarly, in the systems containing only free and immobilized peroxidase, an enzyme concentration of 1 U/ml showed a maximum vanillin yield of 20.9% and 21.6% in the free and immobilized peroxidase systems, respectively. Further, a 1.5-fold and 1.3-fold decrease in the vanillin yield was observed in the free and immobilized systems, respectively, when the enzyme concentration was increased to 2 U/ml. Consecutively, the co-immobilized laccase and peroxidase system followed a similar trend to its individual counterpart where the maximum vanillin yield of 27.21% and 23% was obtained when the laccase and peroxidase concentration in the co-immobilized system were, respectively, 1 U/ml. At the optimized enzyme concentration, the vanillin yield in the co-immobilized system was 1.1-fold and 1.2-fold higher than that obtained in the free and immobilized laccase systems, respectively. Similarly, on comparing the yields with the free and immobilized versatile peroxidase systems, the vanillin yield was 1.1-fold and 1-fold, respectively, higher in the co-immobilized system. However, on increasing the enzyme concentration to 2 U/ml, a drastic reduction in the vanillin yield was observed. The reduced yield at an enzyme concentration of more than 1 U/ml in all the systems could be due to the saturation of the enzymatic reaction and further the deactivation of the enzyme with prolonged incubation (Balakshin et al. Citation2001).
Further, on comparing the yield of vanillin with free laccase and free peroxidase under the same reaction condition of the co-immobilized biocatalytic system, it was observed that the vanillin yield was, respectively, 1.1-fold and 1-fold lower than the co-immobilized system. Although laccase has the ability for depolymerization of phenolic compounds, in the presence of a radical mediator, it cannot act on non-phenolic compounds alone. On the other hand, versatile peroxidase oxidizes lignin at phenolic and non-phenolic aryl-ether positions (Crestini, Melone, and Saladino Citation2011; Vishnu et al. Citation2017). This explains the lowered vanillin yield in the individual laccase and peroxidase system when compared to the co-immobilized biocatalytic system. Another advantage of the co-immobilized system is that laccase and versatile peroxidase can constitute oxidative processes over a wide range of pH and have low substrate specificity (Crestini, Melone, and Saladino Citation2011). Subsequently, on analyzing the individual immobilized laccase and peroxidase system under the same reaction conditions of the co-immobilized system, there was a 1.16-fold and 1.08-fold reduction in vanillin yield in the immobilized laccase and peroxidase system, respectively, when compared to the co-immobilized system. This increase in the vanillin yield in the co-immobilized biocatalytic system can be attributed to the synergistic effect of laccase and versatile peroxidase which induced the depolymerization of phenolic and non-phenolic compounds of lignin for the enhanced production of vanillin (Vishnu et al. Citation2017).
Reusability of the immobilized biocatalysts during vanillin production
Reusability of an immobilized biocatalytic system determines the operational efficiency and feasibility of the system for a large-scale application (Bilal et al. Citation2018). In this study, to determine the operational stability of the co-immobilized laccase and versatile peroxidase during vanillin production, the co-immobilized biocatalytic system was used up to six consecutive cycles of lignin depolymerization. It was observed that both the enzymes, laccase, and versatile peroxidase, maintained more than 80% of their activities in the second cycle, which gradually decreased to nearly 60% in the fourth cycle for both the enzymes. Meanwhile, the vanillin production reduced to nearly 21% in the second cycle from 26% in the first cycle, which further reduced to approximately 10% in the fourth cycle. Further reusing the immobilized biocatalysts led to a decrease in their activities to nearly 40% with a reduction in the vanillin production to only 3%. The reduction in the residual activities of laccase and versatile peroxidase was due to the repeated washing of the immobilized system after every depolymerization cycle, which led to the gradual leaching out of the enzymes, thus reducing the overall enzymatic potential (Vishnu et al. Citation2017). Further, the reduction in the biocatalytic activities of the immobilized system led to reduced depolymerization rate of lignin which, in turn, drastically reduced the production of vanillin by the sixth cycle ().
Conclusion
Sustainable development encompasses safe raw materials resources for industrial applications and the efficient depolymerization of Casuarina biomass-derived lignin using a co-immobilized biocatalytic system constitutes an attractive and effective route for vanillin production. The enzymatic depolymerization of lignin led to the vanillin yield of 27%, which represents an effective route for the management of the large quantities of lignocellulosic wastes produced worldwide and to address the ever-increasing demand of the flavoring agent, vanillin. The reusability of the immobilized biocatalytic system up to six cycles of lignin depolymerization constitute a cost-effective utilization of the biocatalysts in repeated batch or continuous operations in bioprocessing applications.
Supplemental Material
Download Zip (267.2 KB)Supplemental Material
Download MS Word (146.2 KB)Acknowledgment
This work was done as a part of the ‘Indo-Mexican Bilateral Project (No. DST/INT/MEXICO/P-13/2016)’ funded by the Department of Science and Technology India and supported by Natural Sciences and Engineering Research Council of Canada. The authors would also like to extend their gratitude to Sri Ramaswamy Memorial Institute of Science and Technology, Tamil Nadu, India for their help and for aiding in facilitating the research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
Notes on contributors
Kongkona Saikia
Kongkona Saikia is a Ph.D. student in the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Chennai, India.
Dhanya Vishnu
Dhanya Vishnu is a Ph.D. student in the Department of Chemical Engineering, SSN College of Engineering, Chennai, India.
Abiram Karanam Rathankumar
Abiram Karanam Rathankumar is a Ph.D. student in the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Chennai, India.
Balakumaran Palanisamy Athiyaman
Balakumaran Palanisamy Athiyaman is a scientist in CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, Kerala, India.
Ramón Alberto Batista-García
Ramón Alberto Batista-García is a Professor in the Centro de Investigaciones en Dinámica Celular, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico.
Jorge Luis Folch-Mallol
Jorge Luis Folch-Mallol is a Professor in the Centro de Investigaciones en Dinámica Celular, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico.
Hubert Cabana
Hubert Cabana is a Professor in Faculté de génie, Université de Sherbrooke, 2500 boul. de l’Université, Sherbrooke, Québec, Canada.
Vaidyanathan Vinoth Kumar
Vaidyanathan Vinoth Kumar is an Associate Professor in the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Chennai, India.
References
- Ba, S., and V. V. Kumar. 2017. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 37:819–32. doi:10.1080/07388551.2016.1261081.
- Balakshin, M. Y., D. Evtuguin, C. P. Neto, and A. Cavaco-Paulo. 2001. Polyoxometalates as mediators in the laccase catalyzed delignification. J. Mol. Catal. B: Enzym. 16:131–40. doi:10.1016/S1381-1177(01)00054-6.
- Bilal, M., Y. Zhao, T. Rasheed, and H. M. Iqbal. 2018. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 120:2530–44. doi:10.1016/j.ijbiomac.2018.09.025.
- Bourbonnais, R., M. Paice, B. Freiermuth, E. Bodie, and S. Borneman. 1997. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 63:4627–32. doi:10.1128/AEM.63.12.4627-4632.1997.
- Camarero, S., M. J. Martínez, and A. T. Martínez. 2014. Understanding lignin biodegradation for the improved utilization of plant biomass in modern biorefineries. Biofuel Bioprod. Biorefin. 8:615–25. doi:10.1002/bbb.1467.
- Christopher, L. P., B. Yao, and Y. Ji. 2014. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2:12–20. doi:10.3389/fenrg.2014.00012.
- Crestini, C., F. Melone, and R. Saladino. 2011. Novel multienzyme oxidative biocatalyst for lignin bioprocessing. Bioorgan. Med. Chem. 19:5071–78. doi:10.1016/j.bmc.2011.05.058.
- Falade, A. O., L. V. Mabinya, A. I. Okoh, and U. U. Nwodo. 2019. Agrowastes utilization by Raoultella ornithinolytica for optimal extracellular peroxidase activity. Biotechnol. Appl. Biochem. 66:60–67. doi:10.1002/bab.1696.
- Gasser, C. A., G. Hommes, A. Schäffer, and P. F.-X. Corvini. 2012. Multi-catalysis reactions: New prospects and challenges of biotechnology to valorize lignin. Appl. Microbiol. Biotechnol. 95:1115–34. doi:10.1007/s00253-012-4178-x.
- Hämäläinen, V., T. Grönroos, A. Suonpää, M. W. Heikkilä, B. Romein, P. Ihalainen, S. Malandra, and K. R. Birikh. 2018. Enzymatic processes to unlock the lignin value. Front. Bioeng. Biotechnol. 6:20–26. doi:10.3389/fbioe.2018.00020.
- Ibarra, D., J. Romero, M. J. Martínez, A. T. Martínez, and S. Camarero. 2006. Exploring the enzymatic parameters for optimal delignification of eucalypt pulp by laccase-mediator. Enzyme Microb. Technol. 39:1319–27. doi:10.1016/j.enzmictec.2006.03.019.
- Kamimura, N., S. Sakamoto, N. Mitsuda, E. Masai, and S. Kajita. 2019. Advances in microbial lignin degradation and its applications. Curr. Opin. Biotechnol. 56:179–86. doi:10.1016/j.copbio.2018.11.011.
- Kim, Y.-J., and J. A. Nicell. 2006. Impact of reaction conditions on the laccase-catalyzed conversion of bisphenol A. Bioresour. Technol. 97:1431–42. doi:10.1016/j.biortech.2005.06.017.
- Kumar, R., P. Sharma, and P. Mishra. 2012. A review on the vanillin derivatives showing various biological activities. Int. J. Pharmtech. Res. 4:266–79.
- Kumar, V. V., S. Sivanesan, and H. Cabana. 2014. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 487:830–39. doi:10.1016/j.scitotenv.2014.04.009.
- Li, C., C. Chen, X. Wu, C.-W. Tsang, J. Mou, J. Yan, Y. Liu, and C. S. K. Lin. 2019. Recent advancement in lignin biorefinery: With special focus on enzymatic degradation and valorization. Bioresour. Technol. 291:121898. doi:10.1016/j.biortech.2019.121898.
- Min, K., T. Yum, J. Kim, H. M. Woo, Y. Kim, B.-I. Sang, Y. J. Yoo, Y. H. Kim, and Y. Um. 2017. Perspectives for biocatalytic lignin utilization: Cleaving 4-O-5 and C α–C β bonds in dimeric lignin model compounds catalyzed by a promiscuous activity of tyrosinase. Biotechnol. Biofuel. 10:212–21. doi:10.1186/s13068-017-0900-3.
- Mizobutsi, G. P., F. L. Finger, R. A. Ribeiro, R. Puschmann, L. L. D. M. Neves, and W. F. D. Mota. 2010. Effect of pH and temperature on peroxidase and polyphenoloxidase activities of litchi pericarp. Scientia Agricola. 67:213–17. doi:10.1590/S0103-90162010000200013.
- Munk, L., A. K. Sitarz, D. C. Kalyani, J. D. Mikkelsen, and A. S. Meyer. 2015. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 33:13–24. doi:10.1016/j.biotechadv.2014.12.008.
- Munoz, C., F. Guillen, A. Martinez, and M. Martinez. 1997. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr. Microbiol. 34:1–5. doi:10.1007/s002849900134.
- Nicodemus, A. 2009. Casuarina–A guide for cultivation. Coimbatore, India: Institute of Forest Genetics and Tree Breeding (Indian Council of Forestry Research and Education).
- Saikia, K., A. K. kumar, K. Ramachandran, H. Sridharan, P. Bohra, N. Bharadwaj, A. Vyas, and V. K. Vaidyanathan. 2020. A comparative study on the chemo-enzymatic upgrading of renewable biomass to 5-Hydroxymethylfurfural. J. Air Waste Manage. (just-accepted). doi:10.1080/10962247.2020.1723739.
- Senthilkumar, N., S. Murugesan, R. Sumathi, and D. Babu. 2015. Compositional analysis of lignocellulosic biomass from certain fast growing tree species in India. Der. Chemica. Sinica. 6:25–28.
- Shin, S. K., Y. J. Ko, J. E. Hyeon, and S. O. Han. 2019. Studies of advanced lignin valorization based on various types of lignolytic enzymes and microbes. Bioresour. Technol. 291:121728. doi:10.1016/j.biortech.2019.121728.
- Vishnu, D., G. Neeraj, R. Swaroopini, R. Shobana, V. V. Kumar, and H. Cabana. 2017. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ. Sci. Pollut. Res. 24:17993–8009. doi:10.1007/s11356-017-9318-5.
- Wang, Y., S. Sun, F. Li, X. Cao, and R. Sun. 2018. Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield. Ind. Crops Prod. 116:116–21. doi:10.1016/j.indcrop.2018.02.043.
- Watkins, D., M. Nuruddin, M. Hosur, A. Tcherbi-Narteh, and S. Jeelani. 2015. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 4:26–32. doi:10.1016/j.jmrt.2014.10.009.
- Yang, Y., W.-Y. Song, H.-G. Hur, T.-Y. Kim, and S. Ghatge. 2019. Thermoalkaliphilic laccase treatment for enhanced production of high-value benzaldehyde chemicals from lignin. Int. J. Biol. Macromol. 124:200–08. doi:10.1016/j.ijbiomac.2018.11.144.