?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Due to wet desulfurization system lacks effective control indicators for the oxidation process, so the sulfite oxidation in the slurry and the quality of gypsum become unstable. In this paper actual desulfurization system operation data are used to improve the ORP semi-empirical formula obtained in the laboratory. It was concluded that the ORP was mainly affected by the concentration of sulfite and pH during the operation of the actual desulfurization system, the dissolved oxygen was less affected by the small concentration change during operation. It is verified that the model can correct the ORP measurement value by using other conventional measurement data from the power plant. The results can provide theoretical support for adding ORP as a control index to optimize the oxidation process.
Implications
At present, the main control parameter of wet FGD is pH control, but wet FGD has two main reactions at the same time, one is acid-base reaction, the other is redox, single pH control can not reflect the degree of redox, so it is necessary to introduce another control index to evaluate redox, which is the ORP control proposed in this paper, ORP can reflect the degree of slurry oxidation, avoid the occurrence of excessive oxidation and inadequate oxidation, so that the operation of desulfurization system can be more healthy and stable.
Research background
As of the end of 2018, power generation capacity installed was 1.90 billion kilowatts, and thermal power generation installed capacity accounted for 60%, reaching 1.14 billion kilowatts, of which coal-fired power was 1.01 billion kilowatt in China (Xie et al. Citation2019). During the transformation of the power and energy industry, coal-fired power, as a bridge connecting traditional and future energy, will also play an important role for a long time in the future (Sueyoshi, Li, and Gao Citation2018; Wang et al. Citation2019a, Citation2019b). In order for coal-fired power to continue to complete the power generation task stably and efficiently for a long period of time, the coal–fired power needs to be optimized and improved (Fu et al. Citation2019; Yao et al. Citation2019), and energy conservation and consumption reduction of the desulfurization system can be achieved under the premise of ensuring the emissions standards (Wu et al. Citation2019a).
At present, the most important SO2 removal method is wet limestone-gypsum desulfurization; its most important control index is pH for acid and base reaction (Córdoba Citation2015). Through the real-time monitoring pH of the slurry in the absorption tower, a low pH is beneficial to the dissolution of limestone and will also reduce the dissolution rate of SO2. A high pH will help increase the dissolution rate of SO2 and also inhibit the dissolution of limestone. After a long period of engineering application, it is concluded that when the pH is controlled in the range of 4.8 ~ 5.6, the dissolution rate of limestone and the dissolution rate of SO2 can be well balanced (Zhang et al. Citation2019).
During the maturation and improvement of the desulfurization system in power plants, the SO2 absorption rate should not be regarded as the only regulation index. The whole desulfurization process is a series of chemical reactions. The pH as a control parameter is regulated from the aspect of the acid-base reaction, but the other important reaction of the desulfurization system is not effectively controlled (Wu et al. Citation2019b, Citation2019c). Therefore, it is unreasonable to control the all reactions of the entire absorption tower using a single index of pH. On the premise of ensuring the efficiency of SO2 absorption, there are other shortcomings:
Hysteresis of oxidation control: The existing system judges the oxidation reaction in the absorption tower by analyzing the scale samples and desulfurization by-product gypsum in the absorption tower. The oxidizing airflow is increased when the sulfite content above the standard is found from the absorption tower scale sample and the desulfurization byproduct gypsum. Such a control method has obvious hysteresis, which adversely affects the quality of gypsum, and is not conducive to prevent the scaling problems caused by poor oxidation of the absorption tower and may endanger the normal operation of the desulfurization system.
Waste of electric power inside power plants: The design of the oxidizing fan was based on the design conditions when FGD was built, and the CaSO3 in the slurry can be completely oxidized to CaSO4 while ensuring a certain margin. In consideration of the need to overcome the large pressure of the oxidizing air entering the absorption tower, many fixed-speed Roots fans are used in power plants. The characteristics of the Roots fan constant speed mean that the fan energy consumption has nothing to do with the flue gas flow load and pollutants concentrations. When the SO2 content is lower than the design value and low flue gas flow load, the waste of power consumption cannot be avoided.
In view of the problems of the existing desulfurization system, ORP (oxidation-reduction potential) can be added to the existing control system as a control index to optimize and improve the desulfurization system (Brown, DeVault, and Johnson Citation2013). ORP is an indicator that can reflect the macroscopic redox properties in the liquid system, and has been successfully applied in many aspects, such as wastewater treatment, circulating water treatment, and feed water treatment equipment corrosion protection (Wang et al. Citation2018; Weißbach, Drewes, and Koch Citation2018). By adding the parameter ORP, the oxidation reaction of the desulfurization system can be optimized in a targeted manner, and the desulfurization system can be improved. Mitsubishi Heavy Industries and Córdoba have tried to use the technology, it was concluded that a reasonable control of the slurry ORP range has a significant effect on the control of the sulfur and heavy metal valence in the desulfurization system. In previous research, a semi-empirical formula of ORP and other relevant conditions in the desulfurization system have been obtained under laboratory conditions. Before it can be used in actual engineering, it needs to be evaluated and revised under actual engineering conditions.
ORP semi-empirical formulas
Nernst equation
The classic Nernst equation is an equation that quantitatively describes the diffusion potential of a species between an oxidized form and a reduced form. It is very helpful in determining cell potential, equilibrium constant, etc. It takes into account the values of standard electrode potentials, temperature, activity, and the reaction quotient for the calculation of cell potential.
In the formula, – – Standard oxidation-reduction potential;
[OX] – – Oxidizing substance concentration;
[RED] – – Reducing substance concentration;
K – – Constant, i.e. RT/nF.
In a wet desulfurization system, the redox process that occurs in the slurry is not a specific reaction of an oxidizing substance and a reducing substance in the same phase. In the process, the reaction mainly oxidizes +4 sulfur species to +6 sulfur species under the action of oxygen. The main oxidizing substance in this process is dissolved oxygen. At the same time, there exist the changes in the valence state of other valence -changing elements.
In order to obtain a formula that is more consistent with the ORP change in the wet desulfurization redox reaction process for calculating the concentration in the slurry and ensuring the normal operation of the desulfurization system, a new fitting model based on the classic Nernst equation is needed.
Laboratory model and influencing factors
In the previous laboratory ORP research work, a semi-empirical formula for ORP under laboratory conditions was obtained through simulation experiments on the oxidation process of the wet desulfurization system.
Based on the classic Nernst equation, a new fitting is performed according to the common parameters in the wet desulfurization process, the formula is as follows:
Model parameters include sulfite concentration ( with the unit of mmol/L, dissolved oxygen content (DO) with the unit of mg/L, and pH.
However, it is undeniable that there are differences between the laboratory environment and the actual engineering. To successfully apply the semi-empirical formula to actual engineering and guide the process adjustment, it is necessary to optimize the semi-empirical formula for the engineering environment.
Dissolved oxygen
In the simulation process under laboratory conditions, the dissolved oxygen is from the beginning of the oxidation process to its completion, and the range of the dissolved oxygen in the entire process is large. The actual wet desulfurization and oxidation process is a relatively stable stage at a high level of oxidation rate. Dissolved oxygen is always in the condition of unsaturated dissolved oxygen, and the mass transfer efficiency of oxygen in the slurry is affected by various factors such as temperature, viscosity, and other ion concentrations. During the rise of the oxidizing air bubbles, the surface area will also change, which will change the mass transfer rate of oxygen in the slurry.
The absorption tower slurry can be divided into the upper oxygen-depleted area and the lower oxygen-rich area. Due to the passage of the oxidation fan and the stirring of the slurry circulation pump, the oxygen is fully dissolved in the lower oxygen-rich area, so the sulfite content is very low.
After actual measurement, the concentration of sulfite is basically maintained at about 1 ppm, and the amount of oxygen consumed is very small. The dissolved oxygen will gradually diffuse upward due to buoyancy; the slurry pump simultaneously drives the lower slurry into the spray device through circulation. At this time, the lower dissolved oxygen enters the upper layer. When the oxygen concentration of the lower slurry is too large, the oxygen transfer rate will be too fast, which is not only conducive to the oxidation of the sulfite in the upper slurry, but also causes a large amount of oxygen to overflow from the upper slurry. In summary, there must be an appropriate oxygen flux to ensure that the sulfite is fully oxidized and the oxygen utilization rate reaches a high level. By substituting the parameters into the Nernst equation, it can be obtained whether the oxidation state of the slurry is healthy, which can provide a reference for the subsequent addition of ORP to the oxidation control.
Sulfite concentration
In the wet desulfurization and oxidation process, the insufficiently oxidized product is calcium sulfite. Part of the calcium sulfite exists in the form of ions, the other part exists in the form of calcium sulfite hemihydrate (). Increased calcium sulfite hemihydrate will affect the normal crystallization of raw gypsum. The presence of calcium sulfite hemihydrate and calcium sulfate is that the
in calcium sulfate will be incorporated into the calcium sulfite hemihydrate (
) lattice to form a
solid solution. Occupying the nucleation site of calcium sulfate affects the normal growth and dehydration of calcium sulfate crystals (Fu et al. Citation2017).
pH
During the operation of the wet desulfurization system, the most important control parameter is the pH of the slurry environment. The absorption in the flue gas and the reaction between
and
are both acid-base reactions that affect pH changes. At the same time, both the absorption efficiency of
and the dissociation rate of limestone are affected by pH. ORP could be found to be sensitive to pH during laboratory model building. Because it was difficult to complete the consideration of external factors affecting pH under laboratory conditions, there was a certain difference between the pH active control ranges in engineering applications. Therefore, the effect of pH on ORP value is an aspect that needs to be focused in engineering applications.
Practical tests and applications
Overview of desulfurization system
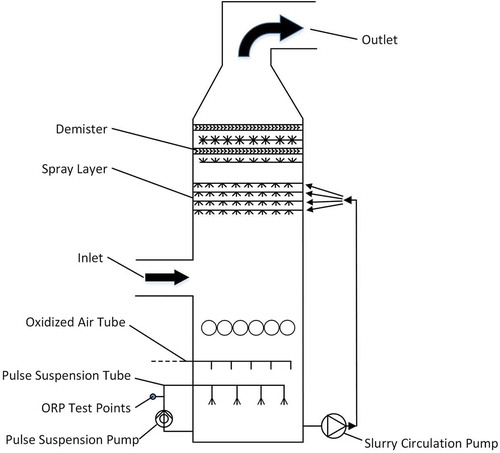
ORP control test was carried out on a 330 MW unit in a power plant in Henan, China. Use the online ORP measuring instrument to monitor the real-time changes of the slurry ORP, and feedback the control signal to the oxidation fan frequency converter controller to control the oxygen fan airflow rate. The desulfurization system of this plant is a double tower series structure (divided into A and B towers) using wet limestone-gypsum method. After the flue gas flows out of the electrostatic precipitator, it passes through the tower A and then the tower B in series. The absorption of SO2 is mainly carried out in the tower A. The structure of the tower A is shown in .
As shown in , after the fly ash is removed by the electrostatic precipitator, the flue gas is sent into the absorption tower A by the induced draft fan and the booster fan. The tower A is a Bischoff structure. The disturbance of the absorption tower is to circulate the slurry downward by a pulse suspension pump. After entering the absorption tower, the flue gas reacts in reverse contact with the sprayed absorption tower slurry to form calcium sulfite, and oxidizes with the oxidizing air entering the absorption tower. Finally, calcium sulfate is generated as a desulfurization byproduct and discharged out of the absorption tower. The actual adjustment method is to ensure that the pH in the absorption tower is stabilized in the optimal range of both absorption and
dissolution by changing the amount of limestone slurry supply (Córdoba et al. Citation2019).
In order to evaluate the idea of adding ORP as a control parameter, the study monitored the ORP of the absorption tower slurry under different boiler loading conditions, measured the sulfite content of the slurry, and sampled and analyzed the absorption tower slurry and gypsum. It was expected that the ORP can be used as a new control parameter in this way.
Reagents and test methods
ORP was monitored by periodic sampling at measurement points located in the pulse suspension pump line of FGD absorber. Because the reaction in the slurry was a continuous process, the measurement and recording of ORP, pH, DO, and sulfite content were performed directly after the sampling completion. The SO2 concentration of flue gas at the inlet of the tower A, flue gas flow rate, oxidized airflow rate, and the SO2 concentration of flue gas at the outlet of the tower A were measured by sensors arranged in the power plant and collected by DSC system.
The reagents and instruments used in the test are shown in .
Table 1. Experimental drugs and instruments.
After the sampling completion, the sulfite in the slurry will continue to undergo an oxidation reaction, so the measurement needs to be completed as soon as possible. The concentration of sulfite ions during the test is measured by titration. In an acidic solution, the sulfite and iodine undergo an oxidation-reduction reaction. The excess iodine is titrated with a sodium thiosulfate standard solution. Its reaction formula is:
Validation of laboratory models
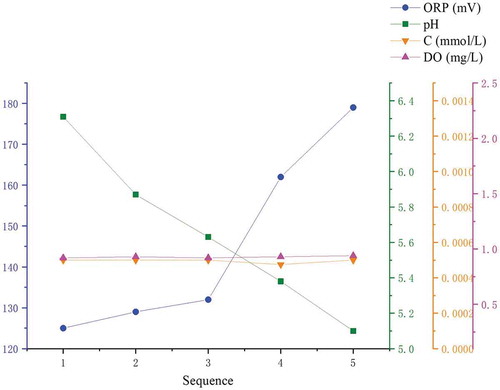
When the power plant measurement data is substituted into the laboratory simulation to obtain a semi-empirical formula, the accuracy was lower than the laboratory simulation conditions. The analysis data is shown in :
It can be found from that the measured sulfite concentration and dissolved oxygen content of the sample are unchanged. In the case of only pH change, the ORP changes greatly, and it is inversely proportional to the pH change. This situation did not occur in the laboratory simulation. Comparing the differences between the operation of the on-site wet desulfurization system and the laboratory simulation can be obtained: The pH change in the laboratory process was mainly due to the hydrolysis of the oxidized substance. The entire experimental environment was relatively stable during the entire oxidation experiment. In actual engineering, in order to ensure high
absorption efficiency, the wet desulfurization system needs to actively control the pH range. The control method is to adjust the supply of limestone slurry. The pH is more related to the relationship between
and
. The method of adjusting the pH by changing the
content interferes with the laboratory’s semi-empirical formula to correctly evaluate the ORP in the oxidation process, and other factors will be masked. In order to correctly evaluate the redox status of the desulfurization system through the ORP value, the impact of pH must be properly handled.
The laboratory semi-empirical formula structure is adjusted as follows:
By fitting 117 data collected during the practical test to the semi-empirical formula fitting, the fitted formula is as follows:
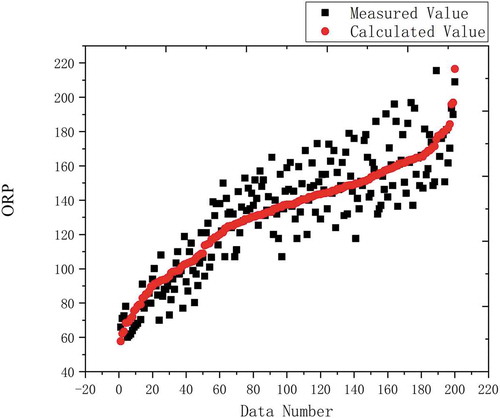
A semi-empirical formula with a relationship of 0.9072 could be obtained by fitting. Compare the 200 actual test data that did not participate in the fitting with the calculation result of the semi-empirical formula. The comparison result is shown in :
In the comparison between the actual measured value and the fitting result, the abscissa is the data number, and the ordinate is the ORP value. The points in the figure are the values calculated by the semi-empirical formula after deformation and the actual measured values on the spot. It can be seen that the data calculated by the semi-empirical formula has a certain range deviation from the actual measured value, but the overall change trend is consistent with the actual measured value. In order to quantitatively evaluate the improved semi-empirical formula, the following evaluation parameters were introduced, including the coefficient of determination (R2), the root mean square error (RMSE), and the normal root mean square error (NRMSE) (Lv et al. Citation2019). Their definitions are:
Among them, n is the sample number, and
are the actual measured ORP value and the calculated ORP value,
is the average value of
,
and
are the maximum and minimum values of
. The evaluation results are shown in :
The fitting group was a comparison of the experimental data points involved in the semi-empirical formula fitting and the results calculated by the improved model. The coefficient determined in the evaluation result was 0.91, and the error was within 0.29 mV, indicating that the experimental data points selected to participate in the establishment of the model have a high degree of fit with the final model. The whole group compares the actual measured data points of all projects with the results calculated by the improved model, its determination coefficient was reduced to 0.76 compared to the fitted group. The root mean square errors of the fitted group and the whole group were 0.29 mV and 15.26 mV.
Table 2. Model analysis.
Effect of ORP regulation on gypsum quality
As a by-product of the wet desulfurization system, gypsum can evaluate the redox condition in the absorption tower. When CaSO4 is precipitated from an aqueous solution, it has three forms: Gypsum (), Plaster (
), Anhydrite (
) (Van Driessche, Stawski, and Kellermeier Citation2019). Under the slurry temperature of 40–60°C, the gypsum is mainly produced in the form of monoclinic gypsum. However, under the condition of poor oxidation status and the presence of calcium sulfite hemihydrate (
), it will affect the normal production of gypsum and produce a solid solution of calcium sulfite and calcium sulfate (
) to increase the difficulty of dehydration (Guan et al. Citation2011; Miao et al. Citation2015). During the formation of raw gypsum, a representative sample of the absorption tower slurry was taken at a low ORP value, the results were compared with normal gypsum after XRD, SEM, and EDS characterization. Under low ORP conditions, gypsum is mainly short-column, with a particle size of less than 50 μm, different sizes, uneven particle size distribution, poor crystallization, and more fine particles. Because the CaSO3 content was higher than the normal gypsum content, it was more difficult to dehydrate the gypsum at this time.
It can be clearly seen from that an excessively low ORP slurry environment can adversely affect the formation and growth of gypsum. The low ORP was due to the poor forced oxidation effect and the increased concentration in the slurry, which affected the quality of gypsum. By comparing the quality of by-products under different ORP conditions, it could be verified that ORP can optimize the oxidation process of the desulfurization system.
Effect of ORP regulation on sulfite
Under the condition of sufficient oxidation, sulfur exists in the form of +6 sulfate. Poor oxidation can lead to increased sulfite concentrations. The concentration of sulfite can directly evaluate the level of oxidation in the desulfurization system. The existence form of pure calcium sulfite is shown in . It was a roughly spherical structure formed by the alternate superposition of needlelike crystal columns, which had a higher specific surface area than gypsum and can absorb a large amount of water. Increasing the sulfite content in the desulfurization system increased the difficulty of gypsum dehydration.
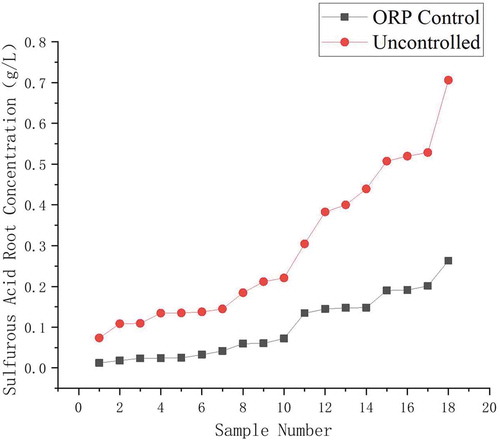
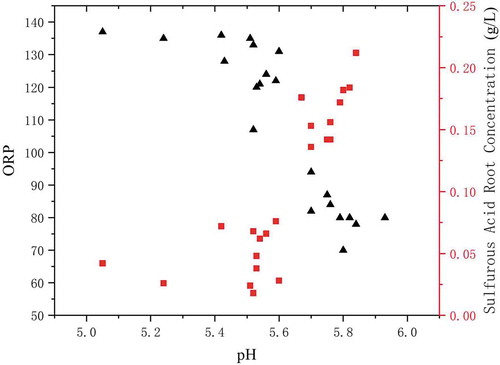
The concentration of sulfite in the absorption tower slurry was compared during the ORP control experiment in the power plant, as shown in .
Under the control of ORP, the sulfite concentration in the slurry significantly reduced due to the sufficient sulfite oxidation. In slurry environment the sulfite concentration and pH changing may produce a mutual effect; the ORP, pH, and sulfite concentration were measured simultaneously, as shown in .
It can be concluded that ORP is affected by both pH and sulfite concentration. The increase in pH and sulfite concentration will reduce ORP. By controlling the ORP, the control of the desulfurization process can be more accurate.
Discussion
In the actual wet desulfurization system operation, the entire oxidation process quickly enters a stable phase after the unit is started. In order to ensure a high level of SO2 absorption in the operating environment, the supply of limestone slurry will be actively adjusted. Under this condition, it will affect the chemical reaction balance in the system to a certain extent. The change of pH will directly cause the changing ORP value. The pH needs to be optimized in the model to obtain a semi-empirical formula more suitable for engineering conditions.
The semi-empirical formula is based on the basic data pH value, DO value, and sulfite concentration that can be obtained by the existing system. The ORP value is calculated by a semi-empirical formula, which can realize the evaluation and correction of the ORP measurement value. The actual operating conditions are more complex and changeable. The accuracy of the semi-empirical formula can continue to improve. At present, the operation reliability after adding the new index of ORP to the system has been verified; it provides basic theoretical support for introducing ORP as a control index in the wet desulfurization system.
During the operation of adding ORP as a control index in the wet desulfurization system. According to the conclusions of basic theoretical research, the frequency change of the oxidizing fan is regulated by comparing the ORP measurement results with the boundary conditions set in the DCS (Distributed Control System). It can optimize the oxidation process of the wet desulfurization system while ensuring the desulfurization efficiency. It effectively avoids the waste of energy consumption under low load conditions and the poor quality of gypsum and scaling of the absorption tower caused by the increase of calcium sulfite concentration under high load.
At the same time, there are some links that need to be improved. Because the ORP electrode was installed in the bypass of the original slurry circulation line, its pipe diameter is small, and blockage may occur also. It can be improved in the future online monitoring design.
Conclusion
ORP is an important parameter for evaluating reaction and environmental redox properties. It has been successfully used in many industries in engineering applications. Based on the ORP semi-empirical formula in the laboratory, this study improves the ORP semi-empirical formula through the ORP data obtained from the slurry in the desulfurization system. The main conclusions are as follows:
Based on the ORP semi-empirical formula established in the laboratory, the ORP semi-empirical formula suitable for the actual wet desulfurization system is obtained through the actual operation data.
The coefficient of determination of the fitting point between the model and the improved model is 0.91, the coefficient of determination between the model and the whole experimental datapoint is 0.76. The model established can be used to correct the ORP measurement.
The ORP of the absorption tower slurry under different load conditions were monitored and used to control the flow rate of oxidation fan. According to the analysis of the sulfite content and gypsum quality of the slurry, the ORP is feasible as a new control parameter for the operation of desulfurization system.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Shuang Chen Ma
Shuang Chen Ma is a research professor studying air pollution control at the department of Environmental Science and Engineering, North China Electric Power University, in Baoding, China.
Fang Xu
Fang Xu is a graduate under Professor Ma's supervision.
Defeng Li
Defeng Li is a production manager from Huadian Qudong Electricity Supply Co., Ltd.
ShuaiJun Fan
ShuaJun Fan is a under-graduate of North China Electric Power University.
Dongsheng Xu
Dongsheng Xu is a production manager from Huadian Qudong Electricity Supply Co., Ltd.
Caini Ma
Caini Ma is a under-graduate of North China Electric Power University.
References
- Brown, S. R., R. F. DeVault, and D. B. Johnson. 2013. ORP as a predictor of WFGD Chemistry and wastewater treatment. Power 157 (7):40–42.
- Córdoba, P. 2015. Status of flue gas desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 144:274–86. doi:10.1016/j.fuel.2014.12.065.
- Córdoba, P., N. R. Lieberman, M. Izquierdo, N. Moreno, and X. Querol. 2019. Understanding the impact of FGD technologies on the emissions of key pollutants in a Co-Firing power plant. J. Energy Inst. 93 (2):518–32. doi:10.1016/j.joei.2019.06.012.
- Fu, B., G. Liu, M. M. Mian, M. Sun, and D. Wu. 2019. Characteristics and speciation of heavy metals in fly ash and FGD gypsum from Chinese coal-fired power plants. Fuel 251:593–602. doi:10.1016/j.fuel.2019.04.055.
- Fu, H., J. Huang, L. Yin, J. Yu, W. Lou, and G. Jiang. 2017. Retarding effect of impurities on the transformation kinetics of FGD gypsum to α-calcium sulfate hemihydrate under atmospheric and hydrothermal conditions. Fuel 203:445–51. doi:10.1016/j.fuel.2017.04.143.
- Guan, B., H. Fu, J. Yu, G. Jiang, B. Kong, and Z. Wu. 2011. Direct transformation of calcium sulfite to α-calcium sulfate hemihydrate in a concentrated Ca–Mg–Mn chloride solution under atmospheric pressure. Fuel 90 (1):36–41. doi:10.1016/j.fuel.2010.08.020.
- Lv, Y., C. E. Romero, J. Charles, K. A. Pauvlinch, and R. W. Watkins. 2019. Developing steady and dynamic ORP models for mercury emissions control in power plants using WFGD operating data. Fuel 235:54–62. doi:10.1016/j.fuel.2018.07.058.
- Miao, M., X. Feng, G. Wang, S. Cao, W. Shi, and L. Shi. 2015. Direct transformation of FGD gypsum to calcium sulfate hemihydrate whiskers: Preparation, simulations, and process analysis. Particuology 19:53–59. doi:10.1016/j.partic.2014.04.010.
- Sueyoshi, T., A. Li, and Y. Gao. 2018. Sector sustainability on fossil fuel power plants across Chinese provinces: Methodological comparison among radial, non-radial and intermediate approaches under group heterogeneity. J. Clean. Prod. 187:819–29. doi:10.1016/j.jclepro.2018.03.216.
- Van Driessche, A. E. S., T. M. Stawski, and M. Kellermeier. 2019. Calcium sulfate precipitation pathways in natural and engineered environments. Chem. Geol. 530:119274. doi:10.1016/j.chemgeo.2019.119274.
- Wang, B., Z. Pan, Z. Du, H. Cheng, and F. Cheng. 2019a. Effect of impure components in flue gas desulfurization (FGD) gypsum on the generation of polymorph CaCO3 during carbonation reaction. J. Hazard. Mater. 369:236–43. doi:10.1016/j.jhazmat.2019.02.002.
- Wang, C., X. Cao, J. Mao, and P. Qin. 2019b. The changes in coal intensity of electricity generation in Chinese coal-fired power plants. Energy Econ. 80:491–501. doi:10.1016/j.eneco.2019.01.032.
- Wang, J., T. Zhang, Y. Mei, and B. Pan. 2018. Treatment of reverse-osmosis concentrate of printing and dyeing wastewater by electro-oxidation process with controlled oxidation-reduction potential (ORP). Chemosphere 201:621–26. doi:10.1016/j.chemosphere.2018.03.051.
- Weißbach, M., J. E. Drewes, and K. Koch. 2018. Application of the oxidation reduction potential (ORP) for process control and monitoring nitrite in a coupled aerobic-anoxic nitrous decomposition operation (CANDO). Chem. Eng. J. 343:484–91. doi:10.1016/j.cej.2018.03.038.
- Wu, B., H. Tian, Y. Hao, S. Liu, Y. Sun, X. Bai, W. Liu, S. Lin, C. Zhu, J. Hao, et al. 2019a. Refined assessment of size-fractioned particulate matter (PM2.5/PM10/PMtotal) emissions from coal-fired power plants in China. Sci. Total Environ. 706:135735. doi:10.1016/j.scitotenv.2019.135735.
- Wu, Q., M. Gu, Y. Du, and H. Zeng. 2019b. Chemical composition and morphology of particles emitted from a wet flue gas desulfurization (WFGD) system. Process Saf. Environ. 124:196–203. doi:10.1016/j.psep.2019.02.013.
- Wu, Q., H. Ma, Q. Chen, Z. Huang, C. Zhang, and T. Yang. 2019c. Preparation of waterproof block by silicate clinker modified FGD gypsum. Constr. Build. Mater. 214:318–25. doi:10.1016/j.conbuildmat.2019.04.053.
- Xie, J., Z. Liang, X. Zhang, and L. Zhu. 2019. Efficiency evaluation of thermal power plants in China based on the weighted Russell directional distance method. J. Clean. Prod. 222:573–83. doi:10.1016/j.jclepro.2019.03.078.
- Yao, S., S. Cheng, J. Li, H. Zhang, J. Jia, and X. Sun. 2019. Effect of wet flue gas desulfurization (WFGD) on fine particle (PM2.5) emission from coal-fired boilers. J. Environ. Sci. 77:32–42. doi:10.1016/j.jes.2018.05.005.
- Zhang, X., X. Wang, B. Jin, X. Liu, and L. Yang. 2019. Crystal structure formation of hemihydrate calcium sulfate whiskers (HH-CSWs) prepared using FGD gypsum. Polyhedron 173:114140. doi:10.1016/j.poly.2019.114140.