?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Montan resin (MR) is a by-product produced during the refinement process of montan wax extracted from lignite and has no usage yet. Chemical modification is an effective method to change the material property for expanding or converting the application area of the material itself. Our previous study found that the high hydrophobicity of MR is the primary limiting factor for its utilization in agriculture. Based on this point, this study attempted to chemically modify MR using the oxidation of peracetic acid, resulting that the MR hydrophilicity was significantly improved, and a water-soluble product (WSP) was obtained. The optimized oxidation conditions of MR, including the reaction temperature (X1), reaction time (X2), weight ratio of oxidant and montan resin (X3), and oxidant concentration (X4), were determined using single-factor experiments and response surface analysis. The modification degree was evaluated using elemental and oil-water partition coefficient analyses, infrared (IR) spectroscopy, and gas chromatography-mass spectrometry (GC-MS), revealing that the oil-water partition coefficient of the modified product decreased and that the number of chemical constituents with oxygen-containing functional groups clearly increased after modification. Furthermore, the WSP was tested its effects on germination and seedling growth of the wheat seed. Compared with the control group, the WSP showed a promoting effect on the growth and germination of wheat. The WSP concentrations of 600 mg·L−1 and 300 mg·L−1 had the most substantial effect on the root and seedling growth of wheat, respectively.
Implications: Montan resin, a useless by-product produced from crude montan wax, was chemically modified via oxidation of peracetic acid. Its hydrophilicity was significantly improved, and a water-soluble product was obtained after the chemical modification. The optimized oxidation conditions of montan resin were determined using single-factor experiments and response surface analysis. The amount of chemical constituents with oxygen-containing functional groups increased in the modified products after modification, as determined by IR and GC-MS analysis, among other methods. The water-soluble modified product showed an obvious effect in promoting growth and germination of wheat at 600 mg·L-1 and 300 mg·L-1, respectively.
Introduction
Lignite is a kind of coal with a low degree of coalification and a high content of moisture (Dong, Yun, and Cao Citation2008). Recently, with the development of many upgrading technologies, including gasification, liquefaction, pyrolysis, and preparation of lignite-based products (Cao Citation2015; Dosodia et al. Citation2009; Gao et al. Citation2019; Mathur, Majumder, and Chatterjee Citation2007; Pande and Sharma Citation2002; Sharma and Dhawan Citation2018; Sharma and Giri Citation1994; Wang et al. Citation2019; Zhao, Wang, and Liu Citation2007), utilization of lignite by a clean and efficient way, instead of its combustion directly as a fuel, has been preferred by many countries and their governments. Montan wax is a chemical product extracted from lignite. Due to the wide application and good performance, it has been the higher valuable product than other lignite-based chemical products, especially refined montan wax. Technically, the crude montan wax was prepared from lignite by direct extraction using organic solvent and contained a high content of montan resin. After removal of montan resin, it could be prepared into deresination montan wax, which could be further oxidized into refined montan wax by bleaching step. Deresination and refined montan wax are two main wax products in the market, which are widely used in aerospace, metallurgy, packaging, printing, and paper-making industries, etc. However, the deresination and refinement processes are accompanied by the production of a large amount of montan resin (MR).
Generally, the MR yield is approximately 200–400 g per kg of crude montan wax (Li et al. Citation2004; Zhang et al. Citation2011). According to some reports we investigated, MR has not been used for anything so far. Our previous studies that primarily used gas chromatography-mass spectrometry (GC-MS), infrared (IR) spectroscopy, and proton nuclear magnetic resonance (1 H-NMR) analysis showed that MR contains steroids, terpenoids, and fatty acids (Li and Zhang Citation1999), and displays the positive effects on wheat seed germination and seedling growth (Liu et al. Citation2016), indicating MR has application potential in the field of agriculture. However, during our research, we found that the high hydrophobicity of MR is the primary limiting factor for its utilization because it must attach to a carrier or filter paper to be absorbed by plants and can not mix with fluid culture medium very well for crops. This issue indicates that direct treatment is not only cumbersome but also inefficient and that the MR hydrophilicity must be improved for dissolving in medium and using by crops better.
Hydrophilicity and hydrophobicity depend on the number of hydrophilic groups in a material, so that a material with a large number of hydrophilic groups is highly hydrophilic. Hydrophilic groups include the carboxyl group, sulfonic acid group, hydroxyl group, and other oxygen-containing groups. Therefore, it is speculated that the hydrophilicity of MR can be improved by the introduction of oxygen-containing groups via oxidation.
Chemical modification is an effective approach to alter the physical and chemical properties of materials, and this approach is widely used in material recycling and reuse (Söğüt and Akgün Citation2007). The chemical modification procedure aims to form the expected products, but in this process, it is critical to avoid causing secondary pollution. Peracetic acid is a green oxidizing agent, and it possesses high oxidation potential (0.1986 V) (Findley, Swern, and Scanlan Citation1945; Long, Ling, and Fang Citation2009; Zhao, Su, and Xing Citation2005; Zhao, Wang, and Liu Citation2007). In order to improve the hydrophilicity of MR for exploring the application potential in agriculture, MR was chemically modified via the oxidation of peracetic acid in the first attempt. To obtain the optimum oxidation conditions, four single factors were studied, including the reaction temperature (X1), reaction time (X2), weight ratio of oxidant and montan resin (X3), and oxidant concentration (X4), and these factors were further optimized using response surface analysis. Under the optimum conditions, the chemical components of MR before and after modification were analyzed using elemental and oil-water partition coefficient analysis, IR, and GC-MS to evaluate the degrees of oxidative modification on MR. The agricultural application of modified products was studied using assays of germination and seedling growth of the wheat seed.
Materials and methods
Materials
The MR was provided by Baoqing Coal Electrochemical Co., Ltd. (China). Peracetic acid (15%, w/w), ethanol, and acetone were produced by Xilong Scientific Co., Ltd. (Sichuan, China). The ether solution of diazomethane was prepared by our group according to the literature method (Lanzhou University Citation2010). Bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) was purchased from Tokyo Chemical Industry (Japan). Elemental analyses for hydrogen, carbon, nitrogen, and sulfur were performed using a Vario EL-III elemental analyzer (Elementar Corporation, Germany), and the content of oxygen was calculated by the subtraction method using the weight percentage. Chemical analyses were performed using a TESOR 27 Fourier transform infrared spectrometer (Bruker Corporation, Germany) and a GC7890B/MS5977A (Agilent, America) fitted with an HP-5 capillary column (30 m × 0.32 mm internal diameter, 0.25-μm film thickness, Agilent). The reagents for the bioassay of the modified products were prepared according to the literature (Liu et al. Citation2016).
Single-factor analysis
Four single factors, including the reaction temperature (X1), reaction time (X2), weight ratio of oxidant and MR (X3), and oxidant concentration (X4), were studied. A 1.00 g sample of MR was placed in a 250 mL three-neck flask and heated to the reaction temperature. Then, electric whisks were set to a speed of 250 r·min−1, and the peracetic acid solution was slowly added using a dropping funnel. Finally, the reacted product was filtered. After the reaction, the solid fraction was extracted by dissolving it in acetone; then, the acetone was evaporated at 85°C for 12 hours, and the product was weighed. The weight of MR before the reaction and the weight of the solid fraction after the reaction were expressed as MR and Ms, respectively. The oxidation rate (OR) was the key index used to evaluate the degree of modification, which was calculated by formula (1).
For this part of the experiment, peracetic acid solution was prepared using equal amounts of A (CH3COOH + H2SO4) and B (H2O2) solutions and allowed to react for 24 hours according to the manufacturer’s instructions. All levels as described next were tested three times. The single factor levels were set as follows: reaction temperatures (X1) of 85°C, 95°C, 105°C, 115°C, and 125°C; reaction times (X2) of 30, 50, 70, 90, 110, and 130 min; weight ratios of oxidant and montan resin (X3) of 20, 25, 30, 35, 40 (w/w); and oxidant concentrations (X4) of 5%, 7.5%, 10%, 12.5%, and 15% (w/w).
Response surface analysis
The optimized oxidation conditions were determined using response surface analysis. According to the Box-Behnken principle, the model was provided by the response surface analysis software (Design Expert 8.0). Three factors, including the reaction temperature (X1), reaction time (X2), and weight ratio of oxidant and montan resin (X3), were selected as independent variables, and the OR (Y) was used as a dependent variable. The ranges of X1, X2, and X3 were set as 85°C–125°C, 30–130 min and 20–40 (w/w), respectively (Tables S1-S3 in supporting information). The method used to measure OR was the same as that used in section 1.2.
Oil-water partition coefficient analysis
The apparent oil-water partition coefficients (Kow) of MR and solid product (SP) in n-octanol-water and n-octanol-buffer systems were determined by the shake-flask method. Three concentration levels at 1.67, 5, and 10 mg/mL of MR and SP were used in this study. The Kow was calculated using formula (2), and Co and Cw were calculated using a standard curve. The absorbance and concentration values were used as analytical values to construct the standard curve in this study, and five concentrations of the MR-octanol and SP-octanol were set at 1, 5, 10, 12, and 15 mg/mL, respectively. The absorbance of the solutions was determined using a spectrophotometer in the visible range at 490 nm.
IR and GC-MS analysis
MR, the modified products included the SP and the water-soluble product (WSP) were dried at 85°C for 12 hours. Then, each sample was weighed to 2 mg for IR analysis.
For GC-MS analysis, the dried MR and modified products, each weighed accurately to 3 mg, were placed in 5-mL bottles and dissolved in 3 mL of acetone, respectively. Then, 1 mL of the ether solution of diazomethane was added to each bottle; the bottles were covered with lids, and the reaction proceeded at 60°C (Yuan et al. Citation2015). Every 10 min, 1 mL of the ether solution of diazomethane was added to each reaction bottle, and this process was performed eight times. Then, 40 μL of BSTFA was added and reacted for 10 min. The solution was filtered through a 0.45-μm membrane filter before GC-MS analysis. The WSP was also dried at 85°C for 12 hours before analysis.
The temperature program of the oven for the initial GC analysis was as follows: 160°C, increased at a rate of 6°C/min to 220°C, then increased at 4°C/min to 300°C and maintained for 5 min. A 1 μL volume of the sample was injected. The temperature of the MS injector was set at 300°C, and splitless mode was used. Nitrogen was used as the carrier gas, with a flow rate of 2.5 mL/min. The temperature of the transfer line was 250°C, and the temperature of the quadrupole was 150°C. The ionization potential of the mass selective detector was 70 eV, and the scanning range was 50–540 amu. SCAN mode was selected in this study. All isolated compounds were estimated using the NIST08 database.
Exploration of agricultural application
In order to exploration of the agricultural application of modified products (including the SP and WSP), their effect on germination and seedling growth of wheat seed were evaluated. The wheat seeds were treated with 10% NaClO for 15 min, washed with double-distilled water and soaked in water for 2 hours; the seeds were then divided into blank control and treatment groups. The blank control group was treated with 10 mL of modified Hoagland’s nutrient solution, and the treatment groups were treated with five concentrations of modified products (each concentration was tested in triplicate). The modified products were dried and dissolved in absolute alcohol to obtain five concentrations of 100, 200, 300, 600, and 800 mg/L. A volume of 10 mL of the prepared solutions was added into the culture dishes of the treated groups. After the absolute alcohol was volatilized, 50 wheat seeds were placed in each dish, and 10 mL of Modified Hoagland’s nutrient solution was added to each culture dish.
All dishes were cultured in an intelligent illumination incubator for 7 days, and all of them were sealed with plastic wrap for the first three days. During the cultivation period, an appropriate amount of double-distilled water was added to each culture dish every day to maintain a constant concentration, as evaluated by the weighing method. The culture conditions are as follows: an illumination time of 12 hours, an intensity of 300 μmol.m−2.s−1, and a temperature of 27°C in the daytime and 17°C in the nighttime. In the blank control group, the modified product solution was replaced by equal amounts of double-distilled water, and the other conditions were the same as those used in the treatment groups. In the treatment groups and the blank control group, the germination rate on the second day and the root length, seedling length, and water content of the seeds on the seventh day were tested.
Results and discussion
The optimized oxidation condition analysis
Single factor analysis and response surface analysis were performed to determine the optimal oxidation conditions for chemical modification. The results of the single factor analysis are shown in . As shown in , the OR increased as the oxidant concentration increased in the range of 5%–15%. The maximum concentration of the commercially available peracetic acid solution is 15%; likewise, the maximum peracetic acid concentration in our study was set to 15%. When the reaction temperature (X1) was higher than 105°C, the OR increased slowly as the temperature increased (). These results revealed that the oxidation ability of peracetic acid could be enhanced by increasing the reaction temperature within a specific range. When the reaction system contained large amounts of water, the influence of the temperature on the reaction decreased gradually once the temperature was beyond a certain value, and the OR tended to gently increase. When the reaction time was longer than 110 min, the OR value stabilized (), indicating that the content of the effective oxidant and the amount of the material that could be modified by oxidation in the reaction system were both reduced as the reaction time increased. As the degree of modification tended to stabilize, the OR did not increase with additional time.
Figure 1. The results of the single-factor experiments (n = 3). (a) reaction temperature (X1); (b) reaction time (X2); (c) weight ratio of oxidant and MR (X3); (d) oxidant concentration (X4).
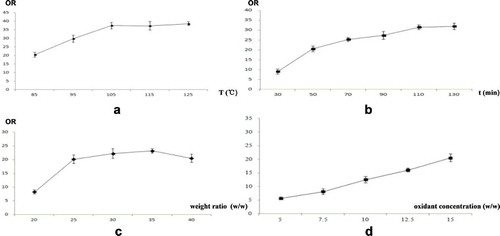
Additionally, when X3 was greater than 35 (w/w), the OR decreased slightly (), which may have resulted from the high content of H2O2 in the reaction system causing a decline in the degree of modification. Peracetic acid is extremely unstable and can decompose over time, generating oxygen at room temperature and causing an explosion risk when the oxygen reaches a high concentration. For safe transportation and storage, only two raw materials are used to commercially prepare peracetic acid; one material contains H2O2, and the highest concentration of the peracetic acid solution is 15%. Based on these facts, peracetic acid was prepared using two solutions in our experiments. When H2O2 was used as an oxidant in some of the experiments, the water solubility of the products might have decreased as the H2O2 concentration in the reaction system increased (Qin et al. Citation2000).
Response surface analysis
In this study, the model was provided by response surface analysis software (Design Expert 8.0). The level of each factor is shown in Table S1 (see in Supporting Information), and the scheme and test results of the response surface design are shown in Table S2 (see in Supporting Information). From the single factor analysis result, the OR increased as the oxidant concentrations increased over a range from 5% to 15%. However, the maximum concentration of the commercially available peracetic acid solution is 15%. Thus, the concentration of peracetic acid in (X4) was not selected in the response surface analysis. The OR data (Y) were subjected to regression analysis, and the regression equation was obtained as follows:
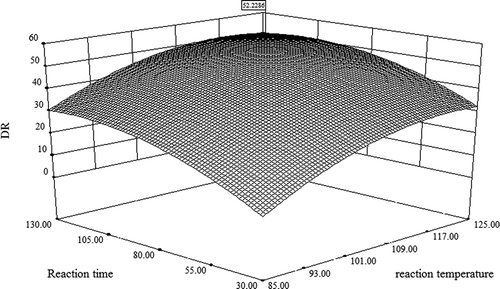
Table S3 (see in Supporting Information) shows the analysis of variance (ANOVA) for the regression equation. The F value of this model was 87.17, and its significance was P < .0001. The regression equation displayed a very high correlation coefficient (R2 = 0.991). The coefficient of variation (CV) was obtained as 4.28%, but the lack of fit was not significant (P = .7043). These results indicate that the regression equation has a good fitting degree and can be used to predict the actual OR. The optimal conditions confirmed by the regression equation are as follows: X1 (reaction temperature) = 118.15°C, X2 (reaction time) = 111.37 min, and X3 (ROM) = 39.6. The predicted OR was 52.2286% (). Furthermore, three confirmatory experiments were performed, with average values of 52.0586% and with a relative error between the confirmatory value and the predictive value of 0.33%. These results showed that the optimized oxidation conditions determined by response surface analysis were feasible.
Elemental analysis
Based on the results of the elemental analysis for MR, SP, and WSP in , the relative content of oxygen in MR was lower than that in the SP and WSP. In the modified products, especially in the WSP, the relative contents of oxygen were significantly improved, indicating that the oxygen-containing groups were richer in the modified products than in MR due to the oxidant modification, which was also supported by the atomic ratios of H/C, O/C, and O/H. Generally, the atomic ratio of H/C was close to 1, meaning that there were more olefinic groups in the sample, while the atomic ratio of H/C was close to 2, meaning that there were more saturated alkyl groups in the sample. Higher atomic ratios of O/C and O/H indicate that the samples are richer in constituents with oxygen-containing groups. A comprehensive analysis of the atomic ratios of H/C, O/C, and O/H in MR, SP, and WSP revealed that olefinic compounds in MR were oxidized to fatty alcohols or fatty acids in the SP and WSP to some extent.
Table 1. The results of the elemental analysis.
Oil-water partition coefficient analysis
displays the results of the apparent oil-water partition coefficient analysis, in which the Kow value of MR was 52.2, which is greater than that of the SP (21.1), indicating that the water solubility of the SP was better than that of MR. Thus, the chemical polarity of the SP was higher than that of MR, confirming that the hydrophilicity of MR could be clearly improved after modification by peracetic acid.
Table 2. The results of the apparent oil-water partition coefficient analysis.
IR and GC-MS analysis
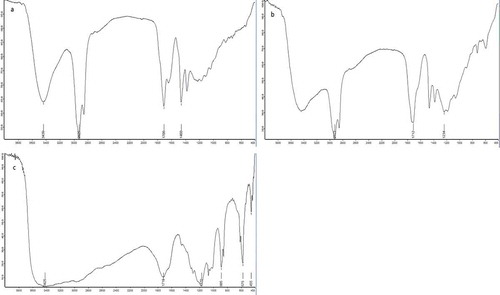
The IR analysis results are displayed in . In the IR spectra, the absorption peaks around 3400 cm−1 of the SP and WSP were wider than those of MR, revealing that more hydroxyl groups were present in the SP and WSP than in MR. Especially in WSP, a wider and stronger absorption peak assigned to hydroxyl groups appeared around 3400 cm−1, suggesting that many intra- and inter-molecular associations occurred among the hydroxyl groups. In addition, the absorption at 1700 cm−1 of the SP and WSP was obviously enhanced, illustrating that the carbonyl contents in the products also increased significantly. These results showed that the content of oxygen-containing functional groups in the modified products clearly increased.
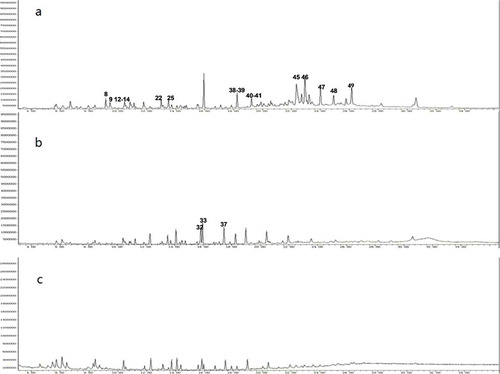
The GC-MS analysis results are shown in and . Based on this information, most of the compounds in MR possess olefinic bonds, but this was not the case in the SP or WSP. It was speculated that the olefinic bonds in MR were more easily oxidized by oxidants. On the other hand, the amount of the compounds containing carboxyl groups in MR accounted for 37.8% of the total number of compounds, while those in the SP and WSP were 65.4% and 53.3%, respectively, revealing that the modified products with oxygen-containing functional groups were richer in the SP and WSP than in MR. This result is in agreement with the results of the IR analysis, thus confirming that MR can be effectively modified by peracetic acid.
Table 3. The results of the GC-MS analysis.
Results of the bioassay for agricultural exploration
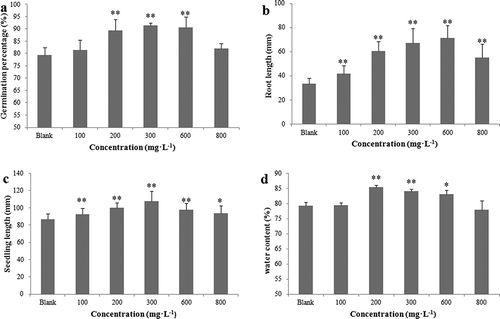
In our study, the germination rate, root length, seedling length, and water content of the seeds were observed to evaluate the effects of the modified products, including the SP and WPS, on the germination and seedling growth of the wheat seed. The WPS showed an obvious activity in promoting germination and seedling growth of the wheat seed, but the SP showed little effect. In , compared with the control group, the root length () and seedling length () in the treated groups greatly increased when the wheat seeds were treated with the WSP at different concentrations (P < .05), with the optimal concentrations being 600 mg·L−1 and 300 mg·L−1, respectively. When the increasing concentrations ranged from 200 mg·L−1 to 600 mg·L−1, the germination rate () and relative water content () also increased obviously (P < .05). These results indicated that the WSP could effectively promote the growth and germination of wheat.
Conclusion
The aims of our study were to chemically modify MR to explore the eco-utilization of the modified products by determining the optimal oxidation conditions of MR. In this study, MR can achieve maximum modification by peracetic acid under the conditions of a reaction temperature of 118.15°C, a reaction time of 111.37 min, and a weight ratio of oxidant and montan resin of 39.6 (w/w); these parameters were optimized using single-factor analysis experiments and response surface analysis. After oxidative modification was performed, more chemicals with oxygen-containing functional groups were found in the modified products than in MR, as determined using elemental and oil-water partition coefficient analysis, IR, and GC-MS. Fortunately, some WSP was obtained after the oxidative modification, and this product showed good effects on the germination and seedling growth of the wheat seed. This study can provide a research basis for the ecological and environmentally friendly recycling and reuse of MR.
Supporting information
Experiment parameters used in response surface analysis (Tables S1-S3) are available free of charge on the Internet.
Supplemental Material
Download MS Word (19.7 KB)Acknowledgment
The authors thank Dr. Zhiwei Wang (Shandong Analysis and Test Center) for assisting with the elemental analysis. The financial support for this research from the National Natural Science Foundation of China (No. 21466018) is gratefully acknowledged.
Disclosure statement
The authors declare no competing financial interest.
Supplementary material
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Hui-Fen Zhang
Hui-Fen Zhang is a Senior Lab Master in Food Security Research Institute of Yunnan Province affiliated to Kunming University of Science and Technology, Kunming, China. She has more than twenty years teaching and research experience in chemistry, function, and application of lignite-based humic acid and montan wax.
Hao Qin
Hao Qin is a Postgraduate Student of Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. He carried out his research in chemical modification and analysis of montan resin. He is interested in modification and analysis of natural products.
Bao-Cai Li
Bao-Cai Li is a Professor in Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. He is a famous expert in the field of comprehensive utilization of lignite by clean and efficient ways in China. He has published a number of research papers in various journals about comprehensive utilization of lignite.
Yi Qin
Yi Qin is a Lab Master in Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. His teaching and researching areas are chemistry, activity, and preparation technology of humic acid and montan wax extracted from lignite and other young coals.
Huan-Hong Wang
Huan-Hong Wang is a Postgraduate Student of Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. She carried out her research in agricultural utilization of modified montan resin. She is interested in biological modification of natural products.
Bing Zhang
Bing Zhang is a Postgraduate Student of Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. He carried out his research on chemical modification of montan resin. He is interested in isolation and identification of natural products.
Mi Zhang
Mi Zhang is a Professor in Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China. She has taught a number of courses on instrumental analysis and natural product chemistry. Her research and publication interests include chemistry and function of natural products from natural resources.
References
- Cao, X. C. 2015. Current status of comprehensive utilization and fundamental research of lignite. Mine Mach. 43:4–7.
- Dong, H., Z. Yun, and Y. Cao. 2008. Comprehensive utilization way and prospect of China lignite. Coal Technol. 27:122–24.
- Dosodia, A., C. Lal, B. P. Singh, R. B. Mathur, and D. K. Sharma. 2009. Development of catalyst free carbon nanotubes from coal and waste plastics. Fuller. Nanotub. Car. N. 17 (5):567–82. doi:10.1080/15363830903133238.
- Findley, T. W., D. Swern, and J. T. Scanlan. 1945. Epoxidation of unsaturated fatty materials with peracetic acid in glacial acetic acid solution. J. Am. Chem. Soc. 67 (3):412–14. doi:10.1021/ja01219a018.
- Gao, H. J., Y. S. Fan, X. Xiong, Z. A. Zheng, L. Z. Jin, and Y. Z. Zhu. 2019. Research progress on in-situ gasification technology of lignite. Pet. Technol. 48:88–93.
- Lanzhou University. 2010. Organic chemistry experiments [M]. Beijing: Higher Education Press.
- Li, B., and H. F. Zhang. 1999. Refinement of brown coal waxes from Yunnan province by oxidation of Na2Cr2O7H2O2 and H2SO4. J. Fuel Chem. Technol. 27:277–81.
- Li, B., J. Zhang, M. C. Zhou, and H. F. Zhang. 2004. Chemical constituents and distributive characteristics of “the alcohols” in brown coal resins separated from montan waxes from Liaohu and Xundian brown coals. J. Kunming Univ. Sci. Technol. 29:82–86.
- Liu, J. X., M. Zhang, B. Li, Y. Qin, C. Xiang, and J. He. 2016. Assessment of montan resin on germination and growth of wheat seed. Agricul. Res. 5 (1):98–103. doi:10.1007/s40003-015-0187-1.
- Long, L. I., L. I. Ling, and J. Fang. 2009. Shrink-proofing of cashmere fibers by peracetic acid treatment. Wool Textile J. 37:18–19.
- Mathur, A. K., C. B. Majumder, and S. Chatterjee. 2007. Combined removal of BTEX in air stream by using mixture of sugar cane bagasse, compost and GAC as biofilter media. J. Hazard. Mater. 148 (1–2):64–74. doi:10.1016/j.jhazmat.2007.02.030.
- Pande, S., and D. K. Sharma. 2002. Ethylenediamine-assisted solvent extraction of coal in N-methyl-2-pyrrolidone: Synergistic effect of ethylenediamine on extraction of coal in N-methyl-2-pyrrolidone. Energy Fuels 16:194–204. doi:10.1021/ef0001742.
- Qin, C. Q., L. Xiao, Y. M. Du, M. Fan, and X. W. Shi. 2000. Prediction and control of extent of depolymerization of chitosan by hydroperoxide. J. Wuhan Univ. 46:195–98.
- Sharma, D. K., and H. Dhawan. 2018. Separative refining of coals through solvolytic extraction under milder conditions: A review. Ind. Eng. Chem. Res. 57 (25):8361–80. doi:10.1021/acs.iecr.8b00345.
- Sharma, D. K., and C. C. Giri. 1994. Solvent deashing of coal to get an environmentally clean and high caloriftc value fuel by batch extractive refining technique under abident condition. Fuel Sci. Technol. Int. 12 (2):191–210. doi:10.1080/08843759408916175.
- Söğüt, O. Ö., and M. Akgün. 2007. Treatment of textile wastewater by SCWO in a tube reactor. J. Supercrit. Fluids 43 (1):106–11. doi:10.1016/j.supflu.2007.04.007.
- Wang, X. H., H. Y. Zhao, Q. Song, Y. H. Li, and X. Q. Shu. 2019. Catalytic upgrading of lignite pyrolysis tar over the different properties of lignite-based catalyst and the analysis of its mechanism. J. Eng. Thermophys. 40:1194–203.
- Yuan, C., Y. Qin, M. Zhang, H. Zhang, S. Jiao, and B. Li. 2015. A new method of testing and evaluating the quality of refined montan wax: Digital Color and GC fingerprint. Chromatographia 78 (19–20):1283–92. doi:10.1007/s10337-015-2937-4.
- Zhang, S., J. Liu, M. Kan, and Y. Jiang. 2011. Lignite wax industrial preparation and purification technology. Chem. Ind. Engineer. 30:504–13.
- Zhao, X., F. Su, and X. Xing. 2005. Oxidation of sodium lignosulphonate by peracetic acid. J. Tsinghua Univ. 45:1244–47.
- Zhao, X. B., L. Wang, and D. H. Liu. 2007. Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 82 (12):1115–21. doi:10.1002/jctb.1775.