?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
When the inevitable generation of waste is considered as hazardous to health, damaging ecosystem to our environment, it is important to develop an innovative technologies to remediate pollutant sources for the safety and environmental protection. The development of adsorption technique for the reduction of extremely effective pollutants in this regard. Green mussel and zeolite mixing media were investigated for the reduction of the concentration of organic constituents (COD) and ammoniacal nitrogen from leachate. The leachate treatability was analyzed under various stages of treatment parameter, namely mixing ratio, shaking speed, contact time, and pH. Both adsorbent were sieve values in between 2.00–3.35 mm particle size. The optimum pH, shaking speed, contact time, and mixing ratio were determined. Leachate samples were collected from influent untreated detention pond at Simpang Renggam landfill site in Johor, Malaysia. The result of leachate characterization properties revealed that non-biodegradability leachate with higher concentrations of COD (1829 mg/L), ammoniacal nitrogen (406.68 mg/L) and biodegradability value (0.08) respectively. The optimal reduction condition of COD and ammoniacal nitrogen was obtained at 200 rpm shaken speed, 120 minute shaken time, optimum green mussel and zeolite mix ratio was 2.0:2.0, and pH 7. The isothermic study of adsorption shows that Langmuir is best suited for experimental results in terms of Freundlich model. The mixing media also provided promising results to treating leachate. This would be greatly applicable in conventionally minimizing zeolite use and thereby lowering the operating cost of leachate treatment.
Implications: The concentration of organic constituents (COD) and ammoniacal nitrogen in stabilized landfill leachate have significant strong influences of human health and environmental. The combination of mixing media green mussel and zeolite adsorbent COD and ammoniacal nitrogen reduction efficiency from leachate. This would be greatly applicable in future research era as well as conventionally minimizing high cost materials like zeolite use and thereby lowering the operating cost of leachate treatment.
Introduction
In Malaysia, municipal solid waste (MSW) generation has increased significantly in recent years as a result of capital development, rapid urbanization, and socioeconomics improvements (Johari et al. Citation2014). Currently, more than 23,000 tons of waste is generated on a daily basis all over Malaysia. However, the number is estimated to exceed 30,000 tonnes by the year 2020. The amount of generation rate of waste continues to increase due to development and populations growth year after year; only < 5% of waste is being recycled (Sreenivasan et al. Citation2012). Traditionally, the Malaysia has followed the conventional method of landfilling to dispose of . In Malaysia, there are in total 296 landfills across the country, of which 166 landfills are still in operating conditions, while 130 are closed. The current situation in Malaysia reveals that around 94.5% of municipal solid waste is disposed of on the landfills while the rest are diverted into resource recovery and composting activities because of lower operational cost, easiest setup, and simple monitoring requirements of landfill site (Budhiarta, Siwar, and Basri Citation2012; Kamaruddin et al. Citation2017).
Consequently, the leachate is a byproduct derived from MSW, a major problematic concerning issue everywhere around the globe as a result of its changes in their physically, chemically, and biologically. Recently, inadequate treatment method strategies have become a major concern issue considering that the generation rate of waste increasing due to the growth rate of human populations. Furthermore, high toxic pollutants are directly discharged into the water bodies (river or stream) or may percolate through soils and pollute receiving waters (Kamaruddin et al. Citation2017).
Several technique approaches are proposed for addressing these environmental problems; these technique approaches include adsorption as an efficient and environmental friendly method for resolving the problem of leachate (Daud et al. Citation2020a). A major problem with landfills is the higher concentration of ammoniacal nitrogen; it prevents microorganism growth and activity due to eutrophication; it accelerates and dissolves oxygen reduction increases and adverse effect on marine organism. Conventional technique methods are difficult and challenging, particularly for stabilized leachate with ammoniacal nitrogen level (Kurniawan et al. Citation2010; Yu et al. Citation2015; Zulfikar et al. Citation2013).
Organic matters is used to quantify by the degree of Biological Oxygen Demand (BOD) and the concentration of organic constituents (COD) in leachate. Given their feasibility, economics, and environment, the biological treatment technique is considered an effective method for the treatment of leachate. Oxidation, microfiltration, photo catalysis, and electrocoagulation are widely used to reduce the higher concentration of COD and ammoniacal nitrogen from leachate wastewater.
However, all these techniques are relatively expensive and main drawbacks caused secondary pollutants in a few cases. Therefore, a new technique should be developed for the leachate treatment. Several techniques are used for the reduction of COD and ammoniacal nitrogen including reverse osmosis (Renou et al. Citation2008), up-flow anaerobic sludge blanket associated with aerobic reactor (Liu et al. Citation2015), adsorption (Aziz et al. Citation2004), membrane bioreactor (Bohdziewicz, Neczaj, and Kwarciak Citation2008), and nanofiltration (Agenson and Urase Citation2007).
In addition, conventional technique methods for leachate treatment involve high technology processes; such methods contribute to a higher pre/post-operational costs, waste residues generation, and less adaptation to wider varieties of pollutants (Latiff et al. Citation2010; Qi et al. Citation2011). Many research studies have been carried out on adsorption by utilizing conventional materials, i.e., zeolite and particularly stabilized leachate treatment.
In the present research study, an effort is made to add to the achievement of the usage of waste materials in wastewater treatment, and the article is focused on the optimization process to determine optimum conditions using granular mixing media as adsorbents in the adsorptive treatment of COD and ammoniacal nitrogen from a stabilized leachate by partially replacing zeolite with green mussel. The effect of adsorbent dosage, shaking speed, contact time, and pH solution on the reduction of contaminants was investigated using granular mixing media.
Materials and methods
Sampling
The leachate samples were collected manually from SimpangRenggam landfill sited at latitude (1°53′41.64″N, 103°22′34.68″E) in Kluang District, Johor, Malaysia, using high density polyethylene (HDPE) clean plastic container and conveyed to research laboratories and kept in a chiller at 4°C. Leachate had been characterized within 24 hours in compliance with Standard for Examination of Water and Wastewater (Federation WE Citation2012). All chemical analyses for leachate characterization were of analytical grade.
A total area of the Simpang Renggam Landfill (SRL) site covers approximately 14.82 acres and for the process of leachate treatment the aerated lagoon system is used. The landfill was managed for many years. The SRL site is now more than 12 years old. The initial size of the area was only 6 hectares and cannot contain the high volume of waste being transported; therefore, the government established a new sanitary landfill beside the existing one to cater for the waste brought from the surrounding districts and the SRL site receives about 250 tons of solid waste per day covering three regions (i.e., Simpang Ringgam region, Batu Pahat region and Kluang district).
Media
The granular mixing media was procured from PT. Anugerah Alam Sdn Bhd Malaysia at about (RM4000 and RM400 per ton), respectively. The green mussel was collected from the Ceria Maju Restaurant located in Parit Raja, Johor. Both adsorbents green mussel and zeolite were pulverized using mortar grinder model Fritsch and then sieved to 150 μm particle sizes.
The chemical compositions of green mussel (GM) and zeolite (ZE) was perform X-ray fluoresces spectroscopic (XRF) (S4 poineer Bruker Model). The density of the media was determined conventionally (dry weight/volume). The chemical compositions of GM and ZE was illustrated in . In the beginning of the experimental setup, each one parameter analyzed separately whereas all others parameters held constant.
Table 1. Chemical composition of green mussel (GM) and zeolite (ZE)
The experiment was performed by agitating a series of 250 mL Erlenmeyer flask containing 100 mL of fresh leachate and varying media amounts were added to the flask based on shaken at predetermined speed, time, and pH at ambient temperature using orbital shaker. The samples were taken then filtered using 0.45 µm filter paper after the agitation process and analyzed for residual COD and ammoniacal nitrogen, which are the notable pollutants in the leachate. The COD and ammoniacal nitrogen was determined using the UV VIS Spectrophotometer HACH DR6000 Model. Experimental results were calculated as percentage removal of COD and ammoniacal nitrogen according to EquationEquation (1)(1)
(1) .
Optimum mixing ratio
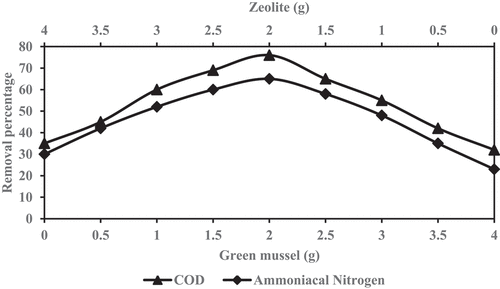
The experiment was performed to determine the optimal mixing ratio for the reduction of COD and ammoniacal nitrogen. The optimum shaking speed that was obtained in previous experiments was used for the further experiment. A total volume of mixing media of 4 g in each Erlenmeyer flask was thoroughly shaken to ensure complete mixing media. The order of mixing media ratio between (GM:ZE) were used 0.0:4.0, 0.5:3.5, 1.0:3.0, 1.5:2.5, 2.0:2.0, 2.5:1.5, 3.0:1.0, 3.5:0.5, 4.0:0.0, respectively. The optimum mixing ratio of two combined-media shows the maximum reduction of COD and ammoniacal nitrogen were consider as optimum ratio and used for further experiment.
Optimum shaking speed
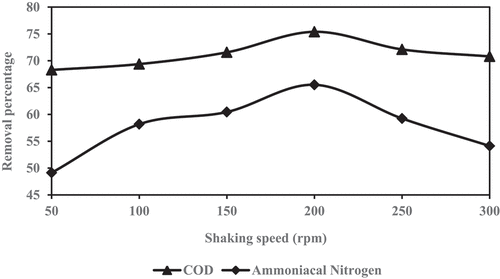
The static batch experiment was performed by agitating a series of 250 mL Erlenmeyer flask containing 100 mL of fresh leachate and 2.0:2.0 mixing media were added to the flask. The flask was shaken at fixed shaken time of 120 minutes and pH 7 at ambient temperature by using orbital shaker. The optimal shaken speed was calculated by changing the shaken speeds from 50 rpm to 300 rpm. The sample was then filtered by passing through 0.45 µm filter after the agitation process and analyzed for residual COD and ammoniacal nitrogen, which are the notable pollutants in the leachate. The COD and ammoniacal nitrogen was investigated by using UV VIS Spectrophotometer HACH DR6000 Model. The optimum rotational speed of the leachate samples show the maximum reduction of COD and ammoniacal nitrogen were considered as optimum speed and used for the further experiment.
Optimum contact time
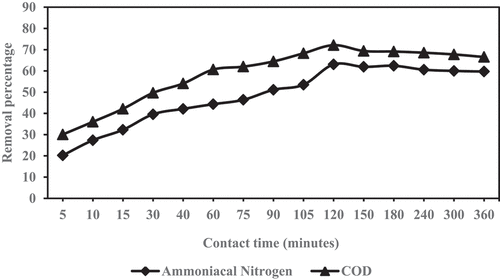
The experiment was performed to investigate the optimum contact time for reduction of COD and ammoniacal nitrogen by adjusting time interval value of from 5 to 360 minutes. The order of adjusting contact time, the experiment was carried out at predetermined optimal mixing ratio and shaken speed. The maximum reduction of COD and ammoniacal nitrogen was considered as optimum contact time.
Optimum pH solution
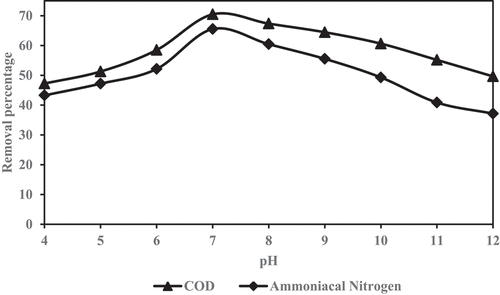
The experiment were performed to investigate the optimal pH for reduction of COD and ammoniacal nitrogen by adjusting pH value of leachate from 4 to 12. The pH adjustment was conducted using 97% H2SO4 and 1M NaOH. The order of adjusting pH, the experiment was carried out at predetermined optimal mixing ratio, shaken speed, and contact time. The maximum reduction of COD and ammoniacal nitrogen was considered as optimum pH.
Adsorption study
The adsorption isotherm study experiment were conducted by containing 4.0 g of green mussel and zeolite adsorbent with 100 mL of leachate concentration (100 to 10-degree dilution) in 250 mL volumetric glass, allowing sufficient time, 120 minutes appropriate time for adsorption equilibrium. The amounts of green mussel and zeolite (mg/g) adsorbent was determined based on mass bxalance EquationEquation (2)(2)
(2) :
The optimal batch study was conducted at room temperatures for reduction of COD and ammoniacal nitrogen was obtained at 200 rpm rotational speed, 120 minutes shaking time, and pH 7 with 2.0:2.0 g optimum dosage of green mussel and zeolite respectively.
The collected specimen sample was examined under the same condition in triplicates; an average result was opted. The dosage of adsorbents green mussel and zeolite was calculated by the range of 0.0–4.0 g/L of adsorbent dosage mixing with 100 mL of leachate sample. The leachate sample was tested for COD and ammoniacal nitrogen, one of the most severe wastewater contaminants. The amount of COD and ammoniacal nitrogen adsorbed in the green mussel and zeolite per unit was estimated using the EquationEquation (3)(3)
(3) :
Results and discussion
Comparative study of COD and ammoniacal nitrogen with other adsorbent
Mainly, in this research, the two different adsorbent materials, i.e., green mussel (perna viridis) and zeolite are analyzed and compared with the other researchers. From the analysis, green mussel and zeolite has shown better performance as compared to other adsorbent material. Additionally, the overall reduction percentage comparison of green mussel and zeolite adsorbent is also included as listed in the . From the table, it can be concluded that green mussel and zeolite show better reduction percentage in terms of COD and ammoniacal nitrogen as compared to others.
Table 2. Performance comparison wit h various other adsorbent
Chemical analysis of leachate
In general, many previously published research studies have been investigated that the characterization of leachate quality varies due to different leachate sampling landfill sites (Barakat Citation2011). The obtained characterization result revealed that the concentration of leachate values (mg/L) of COD, NH3-N, BOD, pH, suspended solid, color and BOD5/COD were 1829, 406.68, 163, 8.27, 367, 4788, and 0.08, respectively. The numbers of COD and BOD5 indicate that it is a stabilized leachate as data illustrated in . Past research studies findings also revealed that the value of pH > 7.5 was considered as stabilized leachate (Daud et al. Citation2016).
Table 3. Simpang Renggam landfill site leachate characteristic
Determination of optimum mixing media
The reduction percentage of COD and ammoniacal nitrogen are illustrated in , respectively, to achieve the optimum mixing ratio in the samples after combining GM with ZE and shaken at different rotational speeds. The most favorable initial condition for the reduction of two parameters (COD and NH3-N) were achieved at 2.5:1.5 and 2.0:2.0. Based on the ratio 2.5:1.5, it consumes more amount of conventional adsorbent (zeolite) and it is more expensive than the 2.0:2.0 mixing ratio. The optimal reduction of COD and ammoniacal nitrogen are acceptable at the mixing ratio of 2.0:2.0 (GM:ZE) at 200 rpm speed are 76% and 65%, respectively. The optimal reduction result was used for the further experiment. Considerably, the ratio achieves a significant replacement of the conventional media without detriment to pollutant removal.
Determination of optimum shaking speed
The reduction percentage of COD and ammoniacal nitrogen are illustrated in , respectively, to achieve the optimum shaking rotational speed in the samples after combining GM with ZE and shaken at different rotational speeds (50 rpm to 300 rpm). The most favorable initial condition for the reduction of two parameters (COD and NH3-N) were achieved at 200 rpm are 75% and 65%, respectively. The optimal reduction of COD and ammoniacal nitrogen are acceptable at the mixing ratio of 2.0:2.0 (GM:ZE) at 200 rpm speed. The optimal reduction result was used for the further experiment.
Determination of optimum contact time
The reduction percentage of COD and ammoniacal nitrogen are illustrated in , respectively, to achieve the optimum shaking time in the samples after combining GM with ZE and shaken at different time intervals (5 minutes to 360 minutes). The most favorable initial conditions for the reduction of two parameters (COD and NH3-N) were achieved at 120 minutes, at 72% and 63%, respectively. The optimal reduction of COD and ammoniacal nitrogen are acceptable at the mixing ratio of 2.0:2.0 (GM:ZE), 200 rpm speed at 120 minutes shaken time. The optimal reduction result was used for the further experiment.
Determination of optimum pH
The reduction percentage of COD and ammoniacal nitrogen are illustrated in , respectively, to achieve the optimum pH level in the samples after combining GM with ZE and shaken at different pH intervals (4 to 12). The most favorable initial condition for the reduction of two parameters (COD and NH3-N) were achieved at pH 7 of 70% and 65%, respectively. The optimal reduction of COD and ammoniacal nitrogen are acceptable at the mixing ratio of 2.0:2.0 (GM:ZE), 200 rpm speed, 120 minutes shaken time, and pH 7.
Adsorption isotherm
Langmuir and Freundlich isotherm models are usually applied to describe the adsorption processes between the sorbate and sorbent molecules in an aqueous solution. In this work, isotherm models such as Langmuir and Freundlich were applied to evaluate the sorption process. According to the Langmuir model, all the accessible sorption sites are homogeneous and morphologically uniform (Demirbas Citation2008). Langmuir model equation is represented by the following equation:
The Freundlich model defines adsorption as a reversible multilayer process over heterogeneous surface (Foo and Hameed Citation2010). The model equation is represented by the following equation:
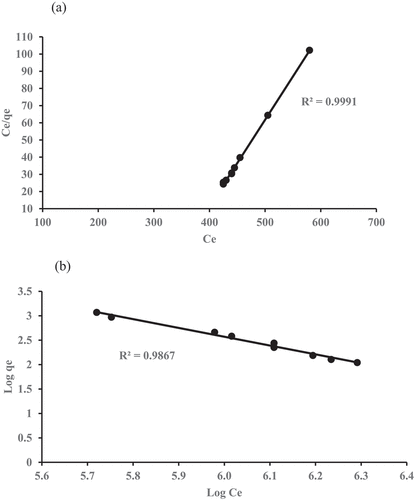
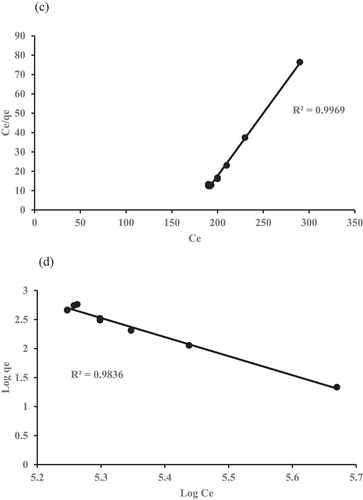
represent the COD and ammoniacal nitrogen plots onto GM-ZE for Langmuir and Freundlich isotherm.
shows that Langmuir data is exhibited better fitted than Freundlich data. It is confirmed from the result the correlation coefficient R-square value is higher for Langmuir for COD (0.9991) and ammoniacal nitrogen (0.9867) compared with Freundlich for COD (0.9969) and ammoniacal nitrogen (0.9836), and this shows adsorption on both parameters (COD and NH3-N) onto GM:ZE occurs as monolayer of adsorption (i.e., the surface is homogeneous) (Adeleke et al. Citation2016; Aziz et al. Citation2010).
The values of isotherm models parameters for the removal of COD and ammoniacal nitrogen are presented in and . The variations in model parameter directly relation to properties of the system. The finding results indicates that the Langmuir model better fit and related parameters to determine the removal of COD and ammoniacal nitrogen by the adsorbent. The table also shows that the Langmuir model was in accordance with experimental data for the removal of contaminants investigated with R2 value of 0.9798 and 0.9920 for COD and ammoniacal nitrogen, respectively. Therefore, the Langmuir model was more appropriate to be used to describe the process than the Freundlich model.
Table 4. Isotherm data for COD and ammoniacal nitrogen reduction by adsorbent
Conclusion
In the present research, the results revealed that the combination of green mussel and zeolite shown the potential for reduction of COD and ammoniacal nitrogen from stabilized leachate. The best combination ratio of GM:ZE for reduction of COD and ammoniacal nitrogen was achieved at 2.0:2.0, 200 rpm rotational speed, 120 minutes contact time, and at pH 7. The obtained results indicate that adsorption capability reduction for COD and ammoniacal nitrogen were a better indication of GM:ZE combination mixture potentially used in its adsorption process. The equilibrium conditions were well-fitted with the Langmuir--Freundlich models. The Langmuir data is better fitted over Freundlich. Therefore, it is proposed that kinetic adsorption study be taken into consideration for further research to examine adsorption processes of COD and ammoniacal nitrogen onto GM:ZE.
Acknowledgment
The authors would like to express thanks to the Ministry of Higher Education, Universiti Tun Hussein Onn Malaysia under Research Fund E15501, Research Management Centre (RMC) UTHM and Quaid-e-Awam University of Engineering, Science & Technology (QUEST) Nawabshah, Sindh, Pakistan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Zawawi Daud
Zawawi Daud works as an Associate Professor at Faculty of Civil Engineering and Built Environmental, Universiti Tun Hussein Onn Malaysia. Dr. Zawawi received his Ph.D. in Civil Engineering (Environment) from Universiti Sains Malaysia in 2009. Dr. Zawawi’s research focuses on alleviating problems associated with water pollution issues from industrial wastewater and landfill leachate. His latest interest is on natural adsorbent material in water and wastewater treatments.
Amir Detho
Amir Detho works as a Lab Engineer at Energy & Environment Engineering Department, Quaid-e-Awam University of Engineering, Science & Technology, Nawabshah, Pakistan. Engr. Detho received his Bachelor of Engineering (Mechanical Engineering) in the year 2010 and Master of Engineering (Energy & Environment Engineering) in the year 2018 from Quaid-e-Awam University of Engineering, Science & Technology Nawabshah, Sindh, Pakistan. He is currently pursuing Ph.D in Faculty of Civil Engineering and Built Environment, Universiti Tun Hussein Onn Malaysia. His research areas include solid waste management and treatment. Engr. Detho does research in Environmental Engineering, Transportation Engineering, and Mechanical Engineering.
Mohd Arif Rosli
Mohd Arif Rosli is currently a lecturer in the Faculty of Engineering Technology, Universiti Tun Hussein Onn Malaysia. His current research interest includes landfill leachate/water and wastewater treatment technology, building services and performance; water, air, noise and indoor environmental quality. Mohd Arif holds a Ph.D degree in Civil Engineering from Universiti Tun Hussein Onn Malaysia. He is a member of The Clean Air Forum Society of Malaysia (MyCAS).
Halizah Awang
Halizah Awang received the Ph.D. in Educational Curriculum from Universiti Sains Malaysia in 2010. Since January 2011, she has been with Faculty of Technical and Vocational Education, Universiti Tun Hussein Onn Malaysia as an Associate Professor. Her research focuses on Curriculum and Instruction in Technical and Vocational Education and Training (TVET), Job and career development in TVET and teaching and learning in TVET.
Mohd Baharudin Bin Ridzuan
Mohd Baharudin Bin Ridzuan works as a senior lecturer at Faculty of Civil Engineering and Built Environment, Universiti Tun Hussien Onn Malaysia. Mohd Baharudin is currently doing Ph.D in Environmental Engineering field at Universiti Tun Hussein Onn Malaysia. His research focuses on environmental issues including water pollution, wastewater treatment, erosion and sedimentation control and rainwater harvesting.
Husnul Azan Tajarudin
Husnul Azan Tajarudin is Associate Professor in Division of Bioprocess, School of Industrial Technology, Universiti Sains Malaysia. He obtained his Ph.D (Chemical and Biological Engineering) from Swansea University, United Kingdom, Master of Environmental Engineering from Universiti Sains Malaysia and Bachelor of Civil Engineering from Universiti Tun Hussein Onn. He also a registered environmental engineer under Board Engineer Malaysia, Member of Society Biological Engineering, United Kingdom and Member of Asia-Pacific Chemical, Biological & Environmental Engineering Society (APCBEES). He has published more than 100 publications such as articles and paper reviews, chapters in book, books and technical reports. His research interests are biological treatment and production of materials from waste by biological process.
References
- Adeleke, A. R. O., A. A. Abdul Latiff, Z. Daud, B. Ridzuan, and N. F. Mat Daud. 2016. Remediation of raw wastewater of palm oil mill using activated cow bone powder through batch adsorption. Key Eng. Mater. 705:380–84. doi:https://doi.org/10.4028/.scientific.net/KEM.705.380.
- Agenson, K. O., and T. Urase. 2007. Change in membrane performance due to organic fouling in nanofiltration (NF)/reverse osmosis (RO) applications. Sep. Purif. Technol. 55 (2):147–56. doi:https://doi.org/10.1016/j.seppur.2006.11.010.
- Aziz, H. A., M. N. Adlan, M. S. M. Zahari, and S. Alias. 2004. Removal of ammoniacal nitrogen (N-NH3) from municipal solid waste leachate by using activated carbon and limestone. Waste Manage. Res. 22 (5):371–75. doi:https://doi.org/10.1177/0734242X04047661.
- Aziz, H. A., A. A. Foul, M. H. Isa, and Y. T. Hung. 2010. Physico-chemical treatment of anaerobic landfill leachate using activated carbon and zeolite: Batch and column studies. Int. J. Environ. Waste Manag. 5 (3–4):269–85. doi:https://doi.org/10.1504/IJEWM.2010.032008.
- Azmi, N. B., M. J. Bashir, S. Sethupathi, and C. A. Ng. 2016. Anaerobic stabilized landfill leachate treatment using chemically activated sugarcane bagasse activated carbon: Kinetic and equilibrium study. Desalination Water Treat. 57 (9):3916–27. doi:https://doi.org/10.1080/19443994.2014.988660.
- Barakat, M. 2011. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4 (4):361–77. doi:https://doi.org/10.1016/j.arabjc.2010.07.019.
- Bohdziewicz, J., E. Neczaj, and A. Kwarciak. 2008. Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 221 (1–3):559–65. doi:https://doi.org/10.1016/j.desal.2007.01.117.
- Budhiarta, I., C. Siwar, and H. Basri. 2012. Current status of municipal solid waste generation in Malaysia. International Journal on Advanced Science, Engineering and Information Technology 2 (2):16–21. doi:https://doi.org/10.18517/ijaseit.2.2.169.
- Daud, Z., M. H. Abubakar, A. A. Kadir, A. A. Latiff, H. Awang, A. A. Halim, and A. Marto. 2016. Optimization of leachate treatment with granular biomedia: Feldspar and zeolite. Indian J. Sci. Technol. 9 (37). doi:https://doi.org/10.17485/ijst/2016/v9i37/91845.
- Daud, Z., M. H. Abubakar, A. A. Kadir, A. A. A. Latiff, H. Awang, A. A. Halim, and A. Marto. 2017. Batch study on COD and ammonia nitrogen reduction using granular activated carbon and cockle shells. Int. J. Eng. 30 (7):937–44.
- Daud, Z., A. Detho, M. A. Rosli, M. H. Abubakar, K. A. Samo, N. F. M. Rais, A. A. Halim, and H. A. Tajarudin. 2020a. Ammoniacal nitrogen and COD removal from stabilized landfill leachate using granular activated carbon and green mussel (Perna viridis) shell powder as a composite adsorbent. Desalination Water Treat. 192:111–17. doi:https://doi.org/10.5004/dwt.2020.25470.
- Daud, Z., A. Detho, M. A. Rosli, M. B. Ridzuan, H. Awang, M. A. Kamaruddin, H. A. Tajarudin, and A. A. Halim. 2020b. COD and ammoniacal nitrogen reduction from stabilized landfill leachate using carbon mineral composite adsorbent. Desalination Water Treat. 205:916–25.
- Demirbas, A. 2008. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 157 (2–3):220–29. doi:https://doi.org/10.1016/j.jhazmat.2008.01.024.
- Federation WE. 2012. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association (APHA).
- Foo, K. Y., and B. H. Hameed. 2010. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156 (1):2–10. doi:https://doi.org/10.1016/j.cej.2009.09.013.
- Halim, A. A., N. N. Z. Abidin, N. Awang, A. Ithnin, M. S. Othman, and M. I. Wahab. 2011. Ammonia and COD reduction from synthetic leachate using rice husk composite absorbent. J. Urban Environ. Eng. 5 (1):24–31. doi:https://doi.org/10.4090/juee.2011.v5n1.024031.
- Ibrahim, F. N. D., Z. Daud, M. B. Ridzuan, Z. Ahmad, H. Awang, and A. Marto. 2016. Ammoniacal nitrogen and COD reduction using zeolite-feldspar mineral composite adsorbent. Int. J. Integr. Eng. 8 (3):9–12.
- Johari, A., H. Alkali, H. Hashim, S. I. Ahmed, and R. Mat. 2014. Municipal solid waste management and potential revenue from recycling in Malaysia. Mod. Appl. Sci. 8 (4):37–49. doi:https://doi.org/10.5539/mas.v8n4p37.
- Kamaruddin, M. A., M. S. Yusoff, L. M. Rui, A. M. Isa, M. H. Zawawi, and R. Alrozi. 2017. An overview of municipal solid waste management and landfill leachate treatment: Malaysia and Asian perspectives. Environ. Sci. Pollut. Res. 24 (35):26988–7020. doi:https://doi.org/10.1007/s11356-017-0303-9.
- Kurniawan, T. A., W. Lo, G. Chan, and M. E. Sillanpää. 2010. Biological processes for treatment of landfill leachate. J. Environ. Monit. 12 (11):2032–47. doi:https://doi.org/10.1039/c0em00076k.
- Latiff, A. A. B. A., A. T. Bin Abdul Karim, M. B. B. Ridzuan, D. E. C. Yeoh, and Y. T. Hung. 2010. Heavy metal removal by crops from land application of sludge. In Environmental bioengineering, edited by L. K. Wang, J.-H. Tay, S. T. Lee Tay, Y.-T. Hung, 211–32. Totowa, NJ: Humana Press.
- Liu, M., Q. Yang, Y. Peng, T. Liu, H. Xiao, and S. Wang. 2015. Treatment performance and N2O emission in the UASB-A/O shortcut biological nitrogen removal system for landfill leachate at different salinity. J. Ind. Eng. Chem. 32:63–71. doi:https://doi.org/10.1016/j.jiec.2015.07.017.
- Qi, Y., A. F. Hoadley, A. L. Chaffee, and G. Garnier. 2011. Characterization of lignite as an industrial adsorbent. Fuel 90 (4):1567–74. doi:https://doi.org/10.1016/j.fuel.2011.01.015.
- Renou, S., S. Poulain, J. G. Givaudan, and P. Moulin. 2008. Treatment process adapted to stabilized leachates: Lime precipitation–prefiltration–reverse osmosis. J. Memb. Sci. 313 (1–2):9–22. doi:https://doi.org/10.1016/j.memsci.2007.11.023.
- Sreenivasan, J., M. Govindan, M. Chinnasami, and I. Kadiresu. 2012. Solid waste management in Malaysia–A move towards sustainability. Waste Manage. Integr. Vision 2005:55–70.
- Yu, D., J. Yang, X. Fang, and H. Ren. 2015. Simultaneous efficient removal of high‐strength ammonia nitrogen and chemical oxygen demand from landfill leachate by using an extremely high ammonia nitrogen–resistant strain. Biotechnol. Appl. Biochem. 62 (3):357–68. doi:https://doi.org/10.1002/bab.1284.
- Zukri, N. I., M. H. Khamidun, M. S. Sapiren, S. Abdullah, and M. A. A. Rahman. 2018. Lake water quality improvement by using waste Mussel shell powder as an adsorbent. IOP Conf. Ser.: Earth Environ. Sci. 140 (1):1–6.
- Zulfikar, M. A., E. Novita, R. Hertadi, and S. D. Djajanti. 2013. Removal of humic acid from peat water using untreated powdered eggshell as a low cost adsorbent. Int. J. Environ. Sci.Technol. 10 (6):1357–66. doi:https://doi.org/10.1007/s13762-013-0204-5.