?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Formaldehyde is a well-known toxic agent, therefore having a highly regulated status. Despite, some cosmetic firms recently introduced high and illegal concentrations of formaldehyde in hair treatments for increasing straightening and long-lasting performances. The objective of this study was to assess how and to which extent, these products may disperse formaldehyde in the environment of a hair salon, possibly exposing the consumer and the hair professional technician to hazardous airborne amounts of formaldehyde. A laboratory room was equipped with three air pumps located at three locations: close to the heads of a mannequin, nearby the hair technician and the whole volume of the room. Pumps were connected to cartridges apt at trapping airborne formaldehyde. The latter was further quantified through an HPLC procedure.
As compared to hair treatments free from formaldehyde that do not modify the airborne formaldehyde levels, products with a high concentration of formaldehyde (1.7% and 9.3%) disperse this compound in the environment to a high and hazardous concentration, detrimental to both consumer and hair professional. The brushing procedure on the hair led to a much higher dispersion of airborne formaldehyde than the hair straightening/ironing technique. To conclude, the use of hair treatments that contain high and illegal amounts of formaldehyde creates a hazardous environment to all people present within a hair salon.
Implications: This methodology adopted here might be used to evaluate during conception phase the level of Formaldehyde release and be used as part of safety data for the registration. However, the present work also strongly promotes the adoption of electronic detectors (commercially available) that continuously record the aerial concentration of Formaldehyde, when dealing with hair styling products that contain legal or illegal (> 0.2%w/w) content of Formaldehyde These detectors not only cover an adequate and realistic range of aerial Formaldehyde but can be used and transported at different locations of a given space. Such a cautious measure comes from common sense as it relates to the health status of consumers and operators present in a hair salon environment.
Introduction
Formaldehyde (HCHO), (CAS number 50–00-0, MW = 30) is one of the smallest volatile organic compounds (VOC’s). It is a highly reactive molecule that readily and partly transforms (up to 59%) into Methylene Glycol or Methanediol (CH2(OH)2), its hydrated form when dissolved in water. It is further oxidized to formic acid, the defense molecule of ants (in Latin “formica”). Of note, HCHO is a by-product of the normal human metabolism (degradation of amino acids) explaining its natural presence in blood serum at a 2.5–3 μg/mL concentration (Neuberger Citation1981). HCHO is widely used in various chemical synthetic products (polymers, paints, explosives etc). The aqueous solution is an effective disinfectant that acts as a bactericide, tuberculocide, fungicide, virucide and sporicide and remains a widely employed fixative that has been studied for decades.
Its toxicological profile, more related to inhalation than topical route, has been established by many official agencies (US EPA, ANSM France, Santé Canada], the exhaustive review of which has been performed by the World Health Organization (WHO), compiling 194 references (Kaden et al. Citation2010). With regard subjects prone to higher exposure conditions, through professional occupations, some official safety bureaus (NIOSH, National Institute for Occupational Safety and Health/US and AFSSET in France] recommended maximal limits of exposures to HCHO according to regular or short-term conditions as 1230 μg/m3 and 2460 μg/m3 (i.e. 1 and 2 ppm) respectively. The American Conference of Governmental Industrial Hygienists (ACGIH) in 2017 amended the HCHO exposure limits in workplaces as a result of the recent evolutions in the classification of this substance (https://catas.com/en-GB/news/acgih-sets-new-limits-for-exposure-to-formaldehyde-in-workplaces]. The Threshold Limit Values-TWA (time-weighted average) as per ACGIH is recommended at 0.1 ppm (corresponding to 123 μg/m3) which is the average concentration limit weighted throughout the working period (8 hours per day for 40 hours per week). The short-term exposure limit (STEL) which is the concentration limit for short exposures that shall be shorter than 15 min and only occasional is recommended at 0.3 ppm (corresponding to 370 μg/m3). A vast amount of toxicological data (based on animal models) converged to label HCHO as a dose-related potent toxic agent (acute toxicity, carcinogenicity, genotoxicity etc) that can seriously impact the human health at different levels (Bolt Citation1987; Bossetti et al. Citation2008; Harving et al. Citation1990; Krakowiak et al. Citation1998; Kriebel et al. Citation2001; Lang, Bruckner, and Triebig Citation2008; Paustenbach et al. Citation1997). These findings logically conveyed HCHO to being a highly regulated molecule in most countries. As an example, in EU the substance HCHO has a harmonized classification in Annex VI of Chemical Regulation (EC) No 1272/2008 (CLP Regulation) as Muta.2, H341/Carc.1B H350 and thus its use is banned in cosmetic products (article 15- EU Cosmetic Regulation N° 1223/2009/CE). In USA, HCHO is listed at the California’s Proposition 65 (Safe Drinking Water and Toxic Enf. Act, 1986) with a safe harbor level of 40 µg/day as carcinogenic.
As a gaseous compound, the airborne concentration of HCHO is often expressed in μg/m3 or mg/m3 rather than ppm with regard to the low density of air (≈ 1.3 g/L). At 20°C, 1 ppm of HCHO corresponds to 1246 μg/m3. The thresholds of its olfactory detection (an acrid odor) by non-anosmic humans (women and men, smokers or not) is in the range 60–200 μg/m3 i.e. 0.05– 0.16ppm (Kaden et al. Citation2010). As recorded in many countries, HCHO is constantly present in outdoor conditions at concentrations ranging from 1.5 −16.4 μg/m3, i.e. a variable background level (European commission, Citation2005; Kaden et al. Citation2010) as HCHO is decomposed into CO2 and CO by solar rays. In indoor conditions, HCHO logically concentrates arising from wooden furniture or paintings, glues, varnishes and smoking habit. Many studies carried out worldwide indicates that the background levels of HCHO ranges from 2 to 110 μg/m3 in indoor conditions (Dingle and Franklin Citation2009; Fromme, Heitmann, and Dietrich Citation2008; Jurvelin et al. Citation2001; Kaden et al. Citation2010; Marchand Citation2006; Osawa and Hayashi Citation2009; Salonen et al. Citation2009; Tang et al. Citation2009). These values are unsurprisingly of a rather large amplitude as the release of HCHO depends on various and variable factors such as ventilation, temperature, relative humidity, age of buildings etc. Of note, one smoked tobacco-based cigarette can disperse 1 to 2 mg of HCHO within the environment. E-cigarettes, that are now under much investigations, make no exception as their vapors were found containing 626 μg/m3of HCHO (Klager et al. Citation2017). All these safety considerations make HCHO a topic of constant survey in public environments (schools, kinder-gardens, offices etc).
Few years ago, some cosmetic firms introduced HCHO as an active ingredient at very high concentrations (1 to 12%, w/w) into products that aim at straightening hairs under hot conditions (iron plates) thereby fully breaching the regulation of the cosmetic industry (http://ansm.sante.fr/Dossiers/Securite-des-produits-cosmetiques/Risques-lies-a-l-utilisation-des-produits-de-lissage/(offset)/3]. Both the conditions of use (heat) and the high levels of HCHO present in the applied product raises a high probability of HCHO diffusing within the close aerial environment of the hair salon. In brief, the exposition of an aerial HCHO concerns equally the consumer under application, the hairdressers and possibly other persons present inside the salon.
Some sub-contracting laboratories use a different approach (unpublished) to detect the possible release of HCHO from a given product. The methodology consists in heating (170–180°C) the product for 30 min in a closed vial and trapping the traces of HCHO released in its head space. From our own experience, such a procedure is highly prone to give false-positive results as HCHO can be generated through the heat-induced transformation of the complex chemical matrix of a HCHO free cosmetic product. In addition, the concentration of HCHO obtained in a small closed vessel is difficult to extrapolate to a real hair salon considering the volume of the room, the diffusion of the compound in the salon etc.
For a better evaluation of HCHO, a methodology based on the chemical quantification of airborne HCHO was developed, derived from previously published technical approaches (Kennedy et al. Citation1985; Lipari and Swarin Citation1982; NIOSH Citation2016). The latter share in common the simulation of practical conditions that can be encountered in a private or public space such as a hair salon. In the present work, the quantification of aerial HCHO was simultaneously carried out at three different locations within a laboratory room that aims at modeling a hair salon. The results of this methodological approach, applied on few products are the objective of this study.
Experimental section
General design of the study
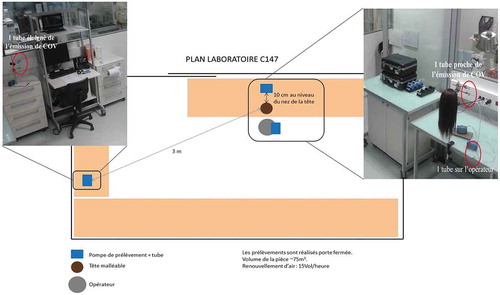
A laboratory room in our research facility (St Ouen, France) was specially dedicated for the study. This room presents a total volume of 72 m3 (L = 6 m, l = 4 m, h = 3 m), the ventilation of which is permanently provided (as other laboratory rooms) by our general services with a constant air flux of 1370 m3/h, i.e. ensuring an approximately 19 h−1 air renewal of this room every hour. Prior to each experiment, the T and RH of the room were regularly checked and were found reasonably stable, at an average 22 ±1°C, 58 ± 3% RH.
Three similar pumps (Gilair-5 Cleanair, Paris France) with a constant air flux of 0.7 L/min were used as air samplers during 20 minutes, providing an air flux regularly checked by an air flow meter (BIOS Defender 510, Brandt Instruments, Inc).
As previously mentioned, these were fixed at the three different locations of the room: i) close (≈ 10 cm) to the mannequin head simulating a consumer, ii) at a higher position (≈ 20 cm) from the head of the operator specialized in hair styling, and iii) at a high corner of the room for quantifying the content of HCHO in its whole volume, as illustrated in .
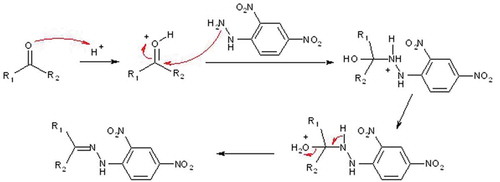
The propelled air by each pump was driven through firmly connected PTFE (Polytetrafluoroethylene) tubes that were fixed to two types of cartridges containing DNPH (2,4-dinitrophenylhydrazine) (NIOSH Citation2016)
These two types of cartridges were chosen for their adaptation to low or intense release (s) of HCHO (cartridges reference 505323 and reference 21014A from Supelco, France). Both were used for trapping and to further desorb ketones and aldehydes, HCHO included, in accordance to norm NFX43-264 and NIOSH.
The trapping of volatile aldehydes by DNPH results from the chemical reactions is depicted in .
Products applied
Four hair straightening products (referred in this study as 1, 2, 3, 4) were purchased from the market according to their respective full ingredient labeling. Products 3 & 4 contained HCHO as per their labeling and had a typical HCHO odor. Products 1 and 2 are HCHO free products from L’Oréal’s Portfolio while 3 and 4 are marketed samples. These 4 products were applied in real conditions according to their protocols using ceramic ironing plates (Babyliss pro Titanium ref BJADAJ0419) heated at 230°C pressed 1 to 5 times on hair for 20 minutes. An amount of 100 grams of Products 3 and 4 were additionally tested in a brushing procedure according to their claims.
Free HCHO determination in cosmetic products formulae
Free formaldehyde content in these products were analyzed by HPLC using post column derivatization with acetylacetone and detected at 420 nm according to European directive 90/207/CEE (4th April 1990) for cosmetic products analysis. Briefly, a XBridge shield RP18 (250 x 4.6 mm x5 µm) column was used with 1 mL/min eluent (Na2HPO4 at 6 mM pH 2.1). Post column heating was carried out at 100°C using a reactor and operated with a 0.5 mL/min flow of ammonium acetate/acetic acid using a second pump. Samples and standards were dissolved in ultrapure water and 10 µL of the sample was injected.
General protocol for air sampling
An operator specialized in hair styling was asked to brush or straighten the hairs of a mannequin elastomer head model where real human hairs are implanted/sawed at a 200/cm2 density (KAREN, Paris, France). Throughout these procedures, in order to guarantee absolute protection, the operator wore a respiratory panoramic mask that covered her whole face (Panoramic respiratory mask single cartridge DIN RD40, from SCOTT) equipped with filter AXB2P3 PRO2000 (SCOTT) for gas and vapors from organic compounds.
Sampling time was fixed at 20 minutes for these treatments (according to their respective procedures) at the end of which the three cartridges were removed from the pumps and further desorbed. For products involving a procedure without brushing, sampling was done during the use of flat iron. For products with a brushing procedure, sampling was done during brushing step and a second one during flat iron.
Prior to each experiment, to determine the background level (blank) of airborne HCHO, the three air sampling devices were run for the same time by replacing the product with water on mannequin head.
HPLC analysis for DNPH cartridges after trapping volatiles
The desorption of trapped HCHO-DNPH was carried out by eluting the cartridge with 10 mL of carbonyl-free Acetonitrile (gradient grade, ref 260290, VWR France). HCHO-DNPH was further chromatographed and quantified. Standard curves were established through a commercially available HCHO-DNPH standard (Carb Carbonyl DNPH Mix CRM 47649, Supelco, France) offering a linear response from 0.1 μg to 20 μg per desorbed cartridge. When necessary, all samples desorbed from the cartridges were diluted accordingly with pure acetonitrile to fit with the standard curve. In these conditions, cartridges extraction and HPLC determinations yielded an excellent Relative Standard Deviation (n = 4, <5% of the mean) with rather low Limits of Detection (LOD) and Quantification (LOQ) of HCHO, equal to 0.2 μg and 0.6 μg per trapping support, respectively.
The adopted analytical procedure follows the one recommended by the NIOSH or the French norm NFX43-264 published in 2011 (www.afnor.org), both being much comparable. All solvents were of analytical grade. A Waters™ HPLC equipment (Alliance 2695, St Quentin-en-Yvelines, France) equipped with a Diode Array Detector (DAD 2996, Waters, St Quentin-en-Yvelines, France) was used with a 4.6 × 250 mm stainless steel column packed with 5 µm particle size C18 (Waters XBridge C18, Ref 186003117, St Quentin-en-Yvelines, France). Mobile phase was 60% Acetonitrile/40%Water (v/v) with a flow rate of 1.2 mL/min and the injected volume was 60 μL. Column temperature was kept at 30°C and samples were stored at 6°C during analysis. HCHO-DNPH conjugate was quantified by UV absorption at 360 nm (based on Total Signal Acquired from 320 to 400 nm). The retention time of HCHO-DNPH was ~5 mins in our conditions. Data acquisition and treatment were carried out on software Empower (Waters, St Quentin-en-Yvelines, France).
Results
Products 1 and 2 (Formaldehyde free straighteners from L’Oréal’s Portfolio) as expected did not contain HCHO (below the LOD of 50 µg/g). Products 3 and 4 (Formaldehyde containing straighteners from the market) were found to contain 1.7% and 9.3% of HCHO (w/w), respectively according to this method.
illustrates the total amount of HCHO (in μg), present in each of the three cartridges used for the four products evaluated and those corresponding to the initial background level of aerial HCHO (blank values) before each procedure.
Table 1. Total amount of HCHO (in μg) present in the trapping cartridges during a 20 minute air collection according to procedures (B: Brushing, S = Straightening) at the three locations of the room (whole volume, close to mannequin head or close to operator). Blank values correspond to the background levels of HCHO. N.A: Not addressed
shows that the blank values oscillate between <0.1 and 0.15 μg. The raw values of trapped HCHO obtained for product 2 are almost twice that of the blank values, although remaining within a low noise/background level. As product 2 is a HCHO free product, it can be assumed with a fair degree of certainity that this very slight increase esults from an alteration of an ingredient contained in the formula of product 2 under the combined contact with the hair and the heated plate, thus generating traces of HCHO. For HCHO containing products 3 and 4, the amount of HCHO found during the applications appear rather linked to their respective content. Taking into account the times of application and the air volume collected by the three pumps in this 20 min duration, the values in allowed us to determine the airborne concentration of HCHO (in μg/m3) at the three locations of the room, i.e. their respective levels of exposure to HCHO.
Equation used for the determination is the following:
These concentrations are shown in .
Table 2. Aerial concentrations of F (in μg/m3) at the three different locations of the room according to applied products and procedures. In bold, values that largely exceed the 1,236 μg/m3 French limit value of professional exposure to F. B: brushing procedure, S: straightening procedure. N.A: Not-Addressed
Of note, the airborne HCHO values with products 3 and 4 generated during the brushing procedure increased by 7 to 11 fold compared the straightening procedure. The blank values, in all studied cases, represent low background levels (ranging 3–11 μg/m3) of aerial HCHO. Overall, the results obtained with products 3 and 4 is in accordance with their respective HCHO content in the formula. Although statistically irrelevant with regard such a low dataset, the 6 respective values (product 3 vs. 4) appear primarily highly correlated (r = 0.97). However, the brushing procedure is alarmingly more prone at releasing HCHO in the environment than the straightening routine.
Discussion
The methodology used to detect the aerial dispersion of HCHO in a close but well-ventilated room offers many positive facets. It allows to quantify the true airborne presence of HCHO at three distinct locations, i.e. to better assess the exposure to HCHO of the consumer, the hair technician and in the entire room. The laboratory room used here, as a mimic of a hair salon, was well ventilated as its aerial volume was renewed 19 times every hour. This aspect makes very likely that the data generated here, with regard HCHO containing products, underestimates the true values of airborne HCHO that can be determined in a location (hair salon) where ventilation is low or insufficient. Firstly the blank values of HCHO, naturally present within the room (3 to 11 μg/m3) is in accordance with the concentration-range of HCHO reported in outdoor conditions (1.5–16 μg/m3) (European commission, Citation2005; Kaden et al. Citation2010) reiterating the fact that a nil concentration of airborne HCHO can never be found in indoor or outdoor conditions. Logically, hair styling products 1 and 2, that do not contain HCHO do not lead to any changes in the blank values. Hair styling products 3 and 4 were voluntarily chosen as these indeed contain a high (and illegal, EU regulatory-wise) proportion of HCHO, used as an “active” ingredient for reinforcing the alteration of the hair shape induced by either a brushing or a straightening procedure. As expected, the two products, during these processes, disperse HCHO within the whole volume of the room. The latter dispersion, that appears globally proportional to their respective contents in HCHO, i.e. 1.7% and 9.3%, highly increased by 4 to 234 fold the blank values. As an example, product 4 used during the brushing procedure leads to disperse HCHO in the whole room at 2349 μg/m3 or 1.9 ppm. These values exceed the recommended maximal limits of exposures to HCHO as per NIOSH, USA and AFSSET, France. The latter figure (2349 μg/m3 or 1.9 ppm) is hazardous as apart from ocular irritation that occur at about 0.5 ppm, the 1–5 ppm range of HCHO is prone to seriously irritate and damage the respiratory tract (Kaden et al. Citation2010).
Of note, the airborne HCHO values with product 4 at all three locations of the room generated during the brushing procedure exceeds the short term exposure limit (STEL) recommended at 0.3 ppm (corresponding to 370 μg/m3) by ACGIH. The aerial environment of the whole room is indeed an important factor since that concerns all people susceptible to be affected on inhalation.
The airborne presence of HCHO clearly worsens with regard to the close environments of both the operator and the mannequin head that mimics a consumer. The uses of products 3 and 4 during the brushing procedure led to the creation of an HCHO rich environment which was 410 to 440 times those of the blank values, i.e. a local concentration of 3.3 and 3.5 ppm, respectively. These values are surprisingly much lower with the hair straightening process at a concentration of 42 to 527 µg/m3 (ie 0.04 to 0.35 ppm) in the whole room or the locations close to the operator and the “consumer”. These values are however not negligible in terms of hazardous exposure to HCHO and at par with the concentrations of HCHO that are normally smelt/detected by non-anosmic people. For the straightening process, it is possible that the strong heat (at least 100°C) delivered by the hair-drier favors a large evaporation of HCHO within the environment thereby decreasing the amount of HCHO on head before the use of heated plates. Hair-styling products that may contain the legal concentration of 0.2% (w/w), as preservative were commercially unavailable for us to analyze. It is however doubtful, taking into account the dose relationship observed here with products 3 and 4, that such low concentration would significantly increase the background level of aerial HCHO with both procedures.
Importantly, it is worth noting that the use of products 3 and 4 does not only concern airborne HCHO conditions. As fluids, under inappropriate gestures or application methods, formulae may well come into contact with skin or the eye cornea, thereby prone at inducing serious ocular damages.
The methodology adopted here was deliberately elaborated and conducted under laboratory conditions. It is however evident that these cannot be applicable in routine and transferable to professional hair salons. This methodology might be used to evaluate during conception phase the level of HCHO release and be used as part of safety data for the registration. However, the present work also strongly promotes the adoption of electronic detectors (commercially available) that can continuously record the aerial concentration of HCHO, when dealing with hair styling products that contain legal or illegal (> 0.2%w/w) content of HCHO. These detectors not only cover an adequate and realistic range of aerial HCHO but can be used and transported at different locations of a given space. Such a cautious measure comes from common sense as it relates to the health status of consumers and operators present in a hair salon environment.
Acknowledgments
The authors thank Jérôme Vial and especially Dr. Didier Thiebaut (UMR Chimie Biologie Innovation 8231, Laboratoire Sciences Analytiques, Bioanalytiques et Miniaturisation, ESPCI, Paris) for critical reading. They are also grateful to Irina Dumitrescu, Dr. Ratnadeep Paul Choudhury and Didier St leger for provided their expertise and assisting the research.
Disclosure statement
The authors, employees of L’Oréal research & Innovation group, state no conflict of interest.
Additional information
Funding
Notes on contributors
Patrick Henault
Patrick Henault works as a L’Oreal Expert in the Hair and Perfumes Development International Direction at L’Oreal R&I based in Saint Ouen, France. His field of activities focus on the development of new methodologies for analyzing and characterizing organic volatiles traces and aerosols.
Rémi Lemaire
Rémi Lemaire received the Ph.D. in Chemistry from Paris Sorbonne University in 2006. He heads the analytical chemistry teams in charge of the Cosmetics Development International Direction at L'Oréal R&I based in Chevilly-La-Rue, France. His field of activities focus on analytical chemistry on new products from conception phase to registration and post marketing support.
Aurélie Salzedo
Aurélie Salzedo works as a Senior Chemist in the Hair and Perfumes Development International Direction at L’Oreal R&I based in Saint Ouen, France. Her field of activities focus on Mass Spectrometry and High Resolution Mass Spectrometry (LC and GC) analyses to qualify and quantify organic volatiles traces.
Joel Bover
Joel Bover works as a Senior Chemist in the Hair and Perfumes Development International Direction at L’Oreal R&I based in Saint Ouen, France. His field of activities focus on Mass Spectrometry analyses to quantify organic volatiles traces in raw materials, finished products and air.
Gérard Provot
Gérard Provot received the Ph.D. in Chemistry from Paris Sorbonne University in 1993. After having held various positions in pharmaceutical development of Big Pharma for 15 years, since 2006 he joined the Hair and Perfumes Development International Direction at L'Oréal R&I based in Saint Ouen, France. He is Director of the Scientific and Technical Department in charge of analytical chemistry and quality support to Hair and Perfumes products development from innovation phase to market place.
References
- Bolt, H. M. 1987. Experimental toxicology of formaldehyde. J. Cancer Res. Clinical Oncol. 113:305–09. doi:10.1007/BF00397713.
- Bossetti, C., J. K. Mclaughlin, R. Tarone, E. Pira, and C. La Vecchia. 2008. Formaldehyde and cancer risk: A quantitative review of cohort studies. Ann. Oncol. 19:29–43. doi:10.1093/annonc/mdm202.
- Dingle, P., and P. Franklin. 2009. Formaldehyde levels and the factors affecting these levels in homes of Perth, Western Australia. Indoor Built Environ. 11:111–16. doi:10.1177/1420326X0201100206.
- European commission: Human exposure characterization of chemical substances. 2005. Quantification of exposure routes. Ispra: Physical and Chemical Unit., Joint research Center.
- Fromme, H., D. Heitmann, and S. Dietrich. 2008. Air quality in schools-classroom levels of carbon dioxide (CO2), volatile organic compounds (VOC), aldehydes, endotoxins and allergens. Gesundheitswesen 70:88–97. doi:10.1055/s-2008-1046775.
- Harving, H., J. Kongsgaard, O. F. Perdersen, et al. 1990. Pulmonary function and bronchial reactivity asthmatics during low level formaldehyde exposure. Lung 168:15–21. doi:10.1007/BF02719669.
- Jurvelin, J., M. Vartainen, M. Janlinen, and P. Pasanen. 2001. Personal exposure levels and microenviroenmental concentrations of formaldehyde and acetaldehyde in Helsinki metropolitan area, Finland. J. Air Waste Man. Assoc. 51:17–24. doi:10.1080/10473289.2001.10464251.
- Kaden, D. A., C. Mandin, G. D. Nielsen, and P. E. Wolkoff. 2010. WHO guidelines for indoor air quality: Selected pollutants. Geneva: WHO. ISBN-13:.
- Kennedy, E. R., T. J. Fischbach, R. Song, et al. 1985. Guidelines for air sampling and analytical method development and evaluation. Cincinnati, OH: National Institute for Occupational Safety and Health (NIOSH). Publication n° 95-117.
- Klager, S., J. Vallarino, P. MacNaughton, D. C. Christiani, Q. Lu, and J. G. Allen. 2017. Flavoring chemicals and aldehydes in E-cigarette emissions. Environ. Sci. Technol. 51 (18):10806–13. doi:10.1021/acs.est.7b02205.
- Krakowiak, A., P. Gorski, K. Pazdrak, and U. Ruta. 1998. Airway response to formaldehyde inhalation in asthmatic subjects with suspected respiratory formaldehyde sensitization. Am. J. Indust. Med. 33:274–81. doi:10.1002/(SICI)1097-0274(199803)33:3<274::AID-AJIM9>3.0.CO;2-W.
- Kriebel, D., D. Myers, M. Cheng, S. Woskie, and B. Cocanour. 2001. Short-term effects of formaldehyde on peak expiratory flow and irritant symptoms. Arch. Enviro.n Health 56:11–18. doi:10.1080/00039890109604049.
- Lang, I., T. Bruckner, and G. Triebig. 2008. Formaldehyde and chemosensory irritation in humans: A controlled human exposure study. Regul. Toxicol. Pharm. 50:23–36. doi:10.1016/j.yrtph.2007.08.012.
- Lipari, F., and S. Swarin. 1982. Determination of formaldehyde and other aldehydes in automobile exhaust with an improved 2,4 Dinitrophenylhydrazine method. J. Chromatogr. 247:297–306. doi:10.1016/S0021-9673(00)85953-1.
- Marchand, C. 2006. Aldehyde measurements in indoor environment in Strasbourg (France). Atm. Environ. 40:1336–45. doi:10.1016/j.atmosenv.2005.10.027.
- Neuberger, A. 1981. The metabolism of glycine and serine. In Amino acid metabolism and sulphur metabolism, ed. A. Neuberger and L. L. M. Van Deenen, Vol. 19A, 257–303.
- NIOSH. 2016. Manual of Analytical Methods (NMAM). 4th ed. Formaldehyde method, issue 2, 1–7.
- Osawa, H., and M. Hayashi. 2009. Status of the indoor air chemical pollution in Japanese houses based on the nationwide field survey from 2000 to 2005. Build. Environ. 44:1330–36. doi:10.1016/j.buildenv.2008.06.022.
- Paustenbach, D. J., A. Y. Kulle, N. Schachter, et al. 1997. A recommended occupational exposure limit for formaldehyde based on irritation. J. Tox Environ. Health 50:217–63. doi:10.1080/009841097160465.
- Salonen, H., A. L. Pasanen, S. Lappalainen, H. Riuttala, T. Tuomi, P. Pasanen, and K. Reijula. 2009. Volatile organic compounds and formaldehyde as explaining factors for sensory irritation in office environments. Hygiene 6:239–47.
- Tang, X., Y. Bai, A. Duong, M. T. Smith, L. Li, and L. Zhang. 2009. Formaldehyde in China, production, consumption, exposure levels and health effects. Environ. Int. 35:1210–24. doi:10.1016/j.envint.2009.06.002.