?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Landfill leachate is a liquid generated due to rainwater percolation through the waste in a landfill or dumping site that may contain high levels of organic matter, both biodegradable and non-biodegradable, which are the major sources of water pollution. Chemical oxygen demand (COD) and Ammoniacal Nitrogen (NH3-N) contents have been relevant indicators of severity and pollution potential of landfill leachate. The reductions of COD and NH3-N were investigated in this study using different combinations of media ratios of green mussel (GM) and zeolite (ZEO). Generally, ZEO is considered as a renowned adsorbent but with a relatively high in cost. In Malaysia, mussel shell is abundantly available as a by-product from the seafood industry, is regarded as waste, and is mostly left at the dumpsite to naturally deteriorate. Its quality and availability make GMs a cost-effective material. In this research study, leachate samples were characterized and found to contain high concentrations of COD and NH3-N. The adsorption process was conducted to find out the best combination media ratio between GM and ZEO. The removing efficiency was determined at different amounts of composite media ratios. The optimal adsorbent mixture ratios between (GM: ZEO) of 1.0:3.0 and 1.5:2.5 were considered as a more efficient technique in removing COD and NH3-N compared to exploiting these adsorbents individually. The optimal extenuation removal reduction was found at an approximately 65% of COD and 78% of NH3-N. The adsorption Isotherm Langmuir model exhibited a better fit with high regression coefficient for COD (R2 = 0.9998) and NH3-N (R2 = 0.9875), respectively. This means that the combination of GM: ZEO adsorption of landfill leachate in this analysis is homogeneous with the monolayer. The mixture of GMs and ZEO was observed to provide an alternative medium for the reduction of COD and NH3-N with comparatively lower cost.
Implications: The concentration of organic constituents (COD) and ammoniacal nitrogen in stabilized landfill leachate have significantly strong influences of human health and the environment. The combination of mixing media green mussel and zeolite adsorbent enhancing organic constituents (COD) and ammoniacal nitrogen reduction efficiency from leachate. This would be greatly applicable in future research as well as conventionally minimizing high cost materials like zeolite, thereby lowering the operating cost of leachate treatment.
Introduction
Landfilling is the most efficient method and a widely used technique for organizing waste disposal in most countries. It is the most economical and environmentally acceptable technique for eliminating and disposing of municipal and industrial solid wastes (Bashir et al. Citation2012; Daud et al. Citation2016; Naveen and Malik Citation2019; Sharma and Katoch Citation2019). The leachate is generally characterized by an elevated concentration of dissolved organic matters (chemical oxygen demand, COD), inorganic macro constituents, halogenated hydrocarbons, ammonia, xenobiotic organic substances, suspended solids, and a substantial concentration of heavy metals in addition to inorganic salts (Bashir et al. Citation2012). Leachate is discharged directly into the atmosphere, becaming percolate into soil and subsoil, posing serious threats to humans and the ecosystem. If inadequately managed, the landfill leachate may become the source of hydro-geological pollution. Therefore, over the recent decade, the reduction of pollutant has become the most important concern in the treatment of leachate (Naveen and Malik Citation2019). There are several factors that influence the characterization of leachate from landfills, i.e., landfill age, hydro-geological site, climate and season, quantity and quality of waste, rainwater precipitation and percolation, landfill conditions, landfill’s morphology, biology and chemical processes occurring at landfill site, depth of waste, and operation facilities (Sharma and Katoch Citation2019). Stabilized or old landfill leachate characterization has a (less biodegradability) ratio that is difficult to proceed biologically. Variations of biodegradable ratio (BOD5/COD) in landfill leachate were categorized to different groups of landfill leachate according to landfill age and decomposition of leachate. In general, the biodegradability ratio of young leachate (< 1 years), intermediate leachate (1–5 years) and stabilized leachate (> 5 years) as reported are 0.5–1.0, 0.1–0.55, and <0.1, respectively (Alvarez‐Vazquez, Jefferson, and Judd Citation2004). shows characteristics of leachate versus the age of landfill (Li, Zhou, and Hua Citation2010).
Table 1. Characterization of leachate at different age of landfills (Li, Zhou, and Hua Citation2010)
Adsorption is relatively a physiochemical technique widely used for stabilized landfill leachate treatment. Adsorption is basically a process of mass transferring substance from liquid to solid surface and to become bounded by physical-chemical interactions. Nowadays, focus have been increasing since the usage of low cost material (e.g., natural polymer or agricultural waste and industrial process by product) to achieve adequate leachate treatment as an alternative approaches to the conventional adsorbent for the treatment of water because of its availability locally and eco-friendly material. Application of zeolite (ZEO)-based adsorbents has certain advantages over conventional method used for treating the water. ZEO typically hydrated mineral aluminosilicate that belong to tectosilicates minerals and porous material (Latiff and Rahman Citation2016). ZEO has an advantage of natural – VE charge which allows it to adsorb cation. ZEO have potential as an efficient adsorbent in various treatment process of drinking water (Lakdawala and Patel Citation2015), reduction of ammoniacal nitrogen, color, dissolved organic matter, heavy metal from landfill leachate (Aziz et al. Citation2010; Rosli et al. Citation2017), and many others.
It is noted that the mixture of green mussel (GM) and ZEO has been used effectively for the reduction of COD and ammoniacal nitrogen from leachate (Aziz et al. Citation2010; Rosli et al. Citation2017). The utilization of GM and ZEO mixture in the application of leachate treatment is not well known. Author (Rosli et al. Citation2017) reported that the composite materials have been produced to many applications, like improvising the adsorption property or producing cost effective adsorbent. In accordance to the above studies, the combination of two minerals improves the adsorption property and reduce treatment costs by partial replacement of ZEO with GM. This study explores the impact of GM and ZEO mixing ratio against the reduction of COD and ammoniacal nitrogen. This was obtained by identifying the optimum composition of the adsorbate medium using batch technique. In addition, adsorption capacity was analyzed using Langmuir and Freundlich isotherm models. Therefore, the finding results will be used in evaluating the pattern of reduction of each parameter in leachate.
The aim of this present research study, leachate was characterized and checked its concentration of COD and ammoniacal nitrogen. Adsorption was performed to determine the combination of different media ratios between GM and ZEO. The removing efficiency was determined at different amounts of composite media ratios for removing concentration of COD and ammoniacal nitrogen.
Materials and method
Site location
The research study was carried out on leachate samples that were collected from the Simpang Renggam Landfill Site (SRLS) found at lat. 10 53ʹ 41.64” N and lon. 1030 22ʹ 34.68” E in Kluang district, Johore, Malaysia. A total land area of SRLS is distributed approximately 6-hectares, and the aerated logoon technique method is used for the treatment of leachate. The SRLS is operated over 12 years and about 250 tonnes of waste is collected from three designated regions namely Simpang Renggam district, Kluang district, and Batu Pahat district, respectively (Bashir et al. Citation2012; Le and Le Citation2019).
Landfill Leachate Sampling
The leachate sample has been taken manually into 30-L high density polyethylene plastic container (HDPE) from (SRLS) according to the procedure guidelines described in standard Water and Wastewater Examination Method (Standard Methods for the Examination of Water and Wastewater Citation2012). The collected samples of leachate were immediately transported to the laboratory and preserved for experimental purposes in a cold storage room at room temperature, preventing deterioration or minimizing further characterization changes. In this study all chemical analyses for leachate characterization were of analytical grade. The leachate characterization are presented in .
Table 2. Characterization of leachate obtained from SRLS
Preparation of adsorbents
Green mussel and zeolite adsorbent is used in this study. Green mussel were collected from Ceria Maju Restaurant located in Parit Raja, Johor and zeolite were purchase from PT. Anugerah Alam Sdn. Bhd at a rate of RM4000 and RM400 per ton, respectively” (Aziz et al. Citation2010; Rosli et al. Citation2017; Zukri et al. Citation2018). The preparation was carried out in accordance with the procedure mentioned in (Aziz et al. Citation2010; Rosli et al. Citation2017). The adsorbent density media has been calculated conventionally (i.e., mass by media volume). Both GM and ZEO has been ground to achieve particle size <150 µm using ceramic ball mill.
Optimum ratio
In this analysis of research study, the optimum mixing ratio of GM: ZEO was determined by varying media ratio (by weight) based on the previous research studies. The total weight of mixing media used was 4.0 g for each volumetric glass. For the experimental purpose, the mixing ratios of GM: ZEO used were 0.0:0.4, 0.5:3.5, 1.0:3.0, 1.5:2.5, 2.0:2.0, 2.5:1.5, 3.0:1.0, 3.5:0.5, and 4.0:0.0. The static batch experiment was conducted at the combination of a fixed media ratio (as mentioned above) with 100 mL sample of leachate in a 250 mL volumetric glass shaken for 120 minutes with 200 rpm shaking speed at pH7 (Daud et al. Citation2020a, Citation2020b; Detho et al. Citation2021). The optimum mixing ratio of GM: ZEO was evaluated, which revealed optimal reduction of COD and NH3-N parameters. For the experimental run, three replicates were performed for each sample, and the average result was utilized. The reduction percentages of parameters were determined using the following EquationEquation (1)(1)
(1) :
where is the initial concentration and
is the final equilibrium concentration and leachate in mg/L, respectively.
Adsorption equilibrium
The isotherm model experiment was conducted by contacting 4.0 g of the GM: ZEO adsorbent in 250 mL Erlenmeyer flask with 100 mL of varying leachate concentrations (100 to 10 degrees dilution) allowing adequate time, 2 hours adsorption equilibrium. The rates of adsorbed GM: ZEO were determined by mass balance EquationEquation (2)(2)
(2) based on the following formula:
whereas qe denotes adsorption equilibrium sorption capacity (mg/g), Co denotes initial concentration, Cf denotes final equilibrium concentration of COD and NH3-N in (mg/L), m denotes mass of adsorbent GM: ZEO in (g), and V denotes leachate volume (Lit). In order to define the relation between COD and NH3-N amounts, Langmuir isotherm and Freundlich isotherm models were applied.
Adsorption isotherm models
Equilibrium data from adsorption experiment for COD and NH3-N results were investigated in relations with Langmuir isotherm and Freundlich isotherm adsorption models (Halim and Mohd Citation2013).
The conventional parameters of the Langmuir isotherm equation was found suitable for the experiment results to the linear isotherm equation derived from EquationEquation (3)(3)
(3) :
The parameters of qm and KL have been derived from a linear plot of 1/qe versus 1/Ce, which gives slope as 1/qe and intercept as 1/Ce, respectively.
The parameters of the Freundlich model equation was found suitable for the experiment results to the linear isotherm equation derived from EquationEquation (4)(4)
(4) :
The parameters of and
were derived from a linear plot of lnqe versus
, which gives slope as and intercept as
, respectively.
According to the prediction of adsorption capacity and the adsorption favorability process, the equilibrium dimensionless parameter was identified as separation factor (). The
was calculated using EquationEquation (5)
(5)
(5) .
For separation factor (), four numbers of probabilities values indicate the nature of adsorption: (
=0) as irreversible; (
=1) as linear; (0 <
<1) as favorable; and (
>1) as un-favorable (Halim and Mohd Citation2013).
Leachate analysis
COD analysis was conducted by using Model HACH DR6000 spectrophotometer according to the procedure described in the standard method (Standard Methods for the Examination of Water and Wastewater Citation2012). Meanwhile, NH3-N analysis was conducted by using Nessler’s method, using the Model HACH DR6000 spectrophotometer. All experiments were conducted in triplicates to obtain consistent results at room temperature 25 ± 2°C.
Results and discussion
Leachate characteristics
Many previous research studies described variations of leachate qualities from different landfills (Aopreeya et al. Citation2013). Leachate characteristics utilized in this study were shown in . The leachate has a high volume of NH3-N and COD, as seen in the . The average value of COD and BOD5 are (1829 mg/L) and (163 mg/L), respectively. The raw leachate has a biodegradability ratio (BOD5/COD) that ranges from 0.07 to 0.08 with an average of 0.07, as shown in . The results show that the value of COD and BOD5 demonstrate that the leachate was clearly stabilized. The physicochemical properties of the composite adsorbents (GM and ZEO) have been presented in .
Table 3. Physicochemical characteristics of composite adsorption media
Stabilized leachate has high concentration of COD (<3,000 mg/L) and NH3-N (>400 mg/L) but low concentration of BOD5/COD (Aziz and Mojiri Citation2014). The pH concentrations of leachate range from 7.65 to 8.27 with an average of 7.96, and it was increasing usually with time (Aeslina et al. Citation2014). A previously published study described that the concentration of pH of a stabilized leachate was higher than 7.5 (Foo and Hameed Citation2009; Moideen et al. Citation2020).
Optimum ratio
Effect of different ratio toward COD
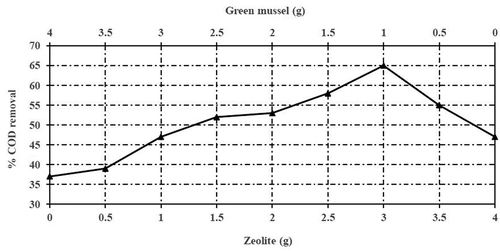
shows COD reduction efficiency against varying ratio of GM and ZEO. The use of ZEO as a single media resulted in the reduction of COD at 39.48% with an intensity value of 1140 mg/L from stabilized leachate. The use of GM as a single media with a mixing ratio of (4.0:0.0 g) was producing very low results of 30% COD reduction as compared to the ZEO mixing ratio (0.0:4.0 g) as a single adsorbent. Both adsorbents have various mixing ratios. The lowest ratio of ZEO which achieved the greatest reduction of COD was known as optimal mixture. The obtained result indicated that the ideal optimum mixing ratio of GM: ZEO was 1.0:3.0 g. It consisted of 25% GM and 75% ZEO, whereas the reduction of COD remain unchanged (although ZEO percentage increases) with the reduction of COD at 65%. In another words, 25% of ZEO was replaced by GM at the maximum reduction of COD.
Effect of different ratio toward NH3-N
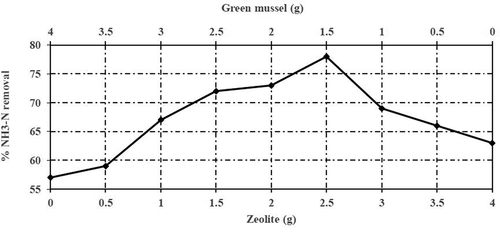
According to , the lowest ratio of ZEO that achieved the highest reduction of NH3-N was considered as the optimal mixture. A mixture of GM: ZEO adsorbent with a media ratio of 1.5:2.5 g resulted in a reduction of NH3-N at 78% with an intensity value of 4,748 mg/L. This optimal mixture consisted of 37.5% of GM and 62.5% of ZEO. The reduction percentage of NH3-N remained unchanged although ZEO percentage increased. In other words, approximately 37.5% of ZEO was replaced by GM at the maximum reduction of NH3-N, while the use of GM as a single media with a mixing ratio of (4.0:0.0 g) produced a very low result with 48% of COD reduction.
Adsorption isotherm
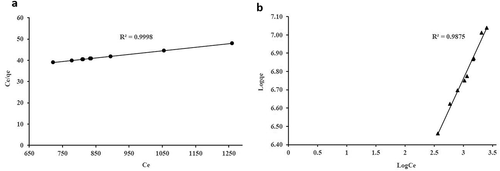
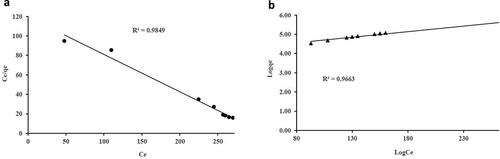
As presented in , Langmuir adsorption isotherm data better fits the model as compared to Freundlich adsorption. The regression coefficient (R2) value in terms of Langmuir adsorption for COD (0.9998) and NH3-N (0.9875) shows higher results as compared to Freundlich adsorption for COD (0.9849) and for NH3-N (0.9663). This indicates that the adsorption of both parameters onto GM: ZEO experienced adsorption of monolayer to a homogeneous surface in adsorption equilibrium (affinity). The maximum uptake capacities () of COD and NH3-N are shown in .
Table 4. Langmuir and Freundlich adsorption isotherm parameters
In the present study, the value of was higher than the unity that indicates the process of adsorption was highly favorable under study condition (Aziz et al. Citation2010; Aopreeya et al. Citation2013).
Conclusion
In this present research study, the experimental results show that the combination of GM and ZEO possessed an ability for the reduction of COD and NH3-N from leachate. It was found that the optimum mixing ratio of the adsorbent of GM: ZEO was at 1.0:3.0, which shows the maximum reduction of COD at 65%, while the optimum mixture ratio of 1.5:2.5 shows the maximum reduction of NH3-N of 78%. It was observed from the equilibrium data that the Langmuir--Freundlich isotherm models was better fitted to the results. The adsorption capacity obtained for COD and NH3-N indicates that the potential use of GM: ZEO for the adsorption process. For further research, therefore, it is proposed that kinetic adsorption is taken into consideration to examine the adsorption process of COD and NH3-N on GM: ZEO.
Acknowledgment
The authors would like to express their gratitude to Ministry of Higher Education Malaysia, Universiti Tun Hussein Onn Malaysia under Research Fund E15501, Research Management Centre (RMC) UTHM, and Quaid-e-Awam University of Engineering, Science & Technology (QUEST) Nawabshah, Sindh, Pakistan.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Amir Detho
Amir Detho works as a Lab Engineer at Energy & Environment Engineering Department, Quaid-e-Awam University of Engineering, Science & Technology, Nawabshah, Pakistan. He received his Bachelor of Engineering (Mechanical Engineering) in the year 2010 and Master of Engineering (Energy & Environment Engineering) in the year 2018 from Quaid-e-Awam University of Engineering, Science & Technology Nawabshah, Sindh, Pakistan. He is currently pursuing Ph.D. in Faculty of Civil Engineering and Built Environment, Universiti Tun Hussein Onn Malaysia. His current research interest includes landfill leachate/water and wastewater treatment technology. He is a registered member of the Pakistan Engineering Council (PEC).
Zawawi Daud
Zawawi Daud works as an Associate Professor at Faculty of Civil Engineering and Built Environment, Universiti Tun Hussein Onn Malaysia. Dr. Zawawi received his Ph.D. in Civil Engineering (Environment) from Universiti Sains Malaysia in 2009. Dr. Zawawi’s research focuses on alleviating problems associated with water pollution issues from industrial wastewater and landfill leachate. His latest interest is on natural adsorbent material in water and wastewater treatments.
Mohd Arif Rosli
Mohd Arif Rosli is currently a lecturer in the Faculty of Engineering Technology, Universiti Tun Hussein Onn Malaysia. His current research interest includes landfill leachate/water and wastewater treatment technology, building services and performance; water, air, noise and indoor environmental quality. Mohd Arif holds a Ph.D. degree in Civil Engineering from Universiti Tun Hussein Onn Malaysia. He is a member of The Clean Air Forum Society of Malaysia (MyCAS).
Halizah Awang
Halizah Awang received the Ph.D. in Educational Curriculum from Universiti Sains Malaysia in 2010. Since January 2011, she has been with Faculty of Technical and Vocational Education, Universiti Tun Hussein Onn Malaysia as an Associate Professor. Her research focuses on Curriculum and Instruction in Technical and Vocational Education and Training (TVET), Job and career development in TVET and teaching and learning in TVET.
References
- Aeslina, A. K., A. B. A. Mohd Mustafa, A. V. Sandu, M. N. Norazian, A. L. Alia Lisanah, and H. Kamarudin. 2014. Usage of palm shell activated carbon to treat landfill leachate. Int. J. Conserv. Sci. 5 (1):117–26.
- Alvarez‐Vazquez, H., B. Jefferson, and S. J. Judd. 2004. Membrane bioreactors vs conventional biological treatment of landfill leachate: A brief review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 79 (10):1043–49. doi:https://doi.org/10.1002/jctb.1072.
- Aopreeya, M., C. Arunlertaree, C. Yuwaree, R. Boonprasert, and R. Hutacharoen. 2013. Blood cockle shell: An agro-waste for N and P removal of shrimp farm effluent. Environ. Nat. Res. J. 11 (1):58–69.
- Aziz, H. A., A. A. Foul, M. H. Isa, and Y. T. Hung. 2010. Physico-chemical treatment of anaerobic landfill leachate using activated carbon and zeolite: Batch and column studies. Int J Environ Waste Manag 5 (3–4):269–85. doi:https://doi.org/10.1504/IJEWM.2010.032008.
- Aziz, H. A., and A. Mojiri, Eds. 2014. Wastewater engineering: Advanced wastewater treatment systems. Penang, Malaysia: IJSR Publications.
- Bashir, M. J., H. A. Aziz, M. S. Yusoff, and S. Q. Aziz. 2012. Color and chemical oxygen demand removal from mature semi-aerobic landfill leachate using anion-exchange resin: An equilibrium and kinetic study. Environ. Eng. Sci. 29 (5):297–305. doi:https://doi.org/10.1089/ees.2010.0187.
- Daud, Z., A. Detho, M. A. Rosli, M. H. Abubakar, K. A. Samo, N. F. M. Rais, and H. A. Tajarudin. 2020a. Ammoniacal nitrogen and COD removal from stabilized landfill leachate using granular activated carbon and green mussel (Perna viridis) shell powder as a composite adsorbent. Desalin. Water Treat. 192:111–17. doi:https://doi.org/10.5004/dwt.2020.25470.
- Daud, Z., A. Detho, M. A. Rosli, H. Awang, M. B. B. Ridzuan, and H. A. Tajarudin. 2020b. Optimization of mixing ratio, shaking speed, contact time, and pH on reduction of COD and ammoniacal nitrogen in leachate wastewater treatment. J. Air Waste Manage. Assoc. 195.
- Daud, Z., N. Nasir, A. Aziz Abdul Latiff, M. B. Ridzuan, and H. Awang. 2016. Treatment of biodiesel wastewater by coagulation-flocculation process using polyaluminium chloride (PAC) and polyelectrolyte anionic. ARPN J. Eng. Appl. Sci. 11 (20):11855–59.
- Detho, A., Z. Daud, M. A. Rosli, M. B. Ridzuan, H. Awang, M. A. Kamaruddin, H. A. Tajarudin, and A. A. Halim. 2021. COD and ammoniacal nitrogen reduction from stabilized landfill leachate using carbon mineral composite adsorbent. Desalin. Water Treat. 210:143–51. doi:https://doi.org/10.5004/dwt.2021.26500.
- Environmental Quality (Control of Pollution from Solid Waste Transfer Station and Landfill) Regulations 2009; Under the Laws of Malaysia-Malaysia Environmental Quality Act 1974. 2010. Kuala Lumpur, Malaysia: Department of Environment.
- Foo, K. Y., and B. H. Hameed. 2009. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 171 (1–3):54–60. doi:https://doi.org/10.1016/j.jhazmat.2009.06.038.
- Halim, A. A., and F. A. Mohd. 2013. Isotherm and kinetic adsorption of boron onto limestone as a low-cost adsorbent. Sains Malaysiana 42 (12):1689–96.
- Lakdawala, M. M., and Y. S. Patel. 2015. Studies on adsorption capacity of zeolite for removal of chemical and bio-chemical oxygen demands. Chem. J. 1 (4):139–43.
- Latiff, A. A. A., and A. A. Rahman. 2016. O., Daud, Z., Ridzuan, MB, & Mat Daud, NF, Batch adsorption of manganese from palm oil mill effluent onto activated cow bone powder. ARPN J. Eng. Appl. Sci. 11 (4):2627–31.
- Le, S. T., and K. C. Le. 2019. Reduction of cod in nam son landfill leachate by electro-fenton as secondary treatment after electrocoagulation pretreatment. Vietnam J. Sci. Technol. 57 (6):724–33. doi:https://doi.org/10.15625/2525-2518/57/6/13883.
- Li, W., Q. Zhou, and T. Hua. 2010. Removal of organic matter from landfill leachate by advanced oxidation processes: A review. Int. J. Chem. Eng. 2010:10 pp. doi:https://doi.org/10.1155/2010/270532.
- Moideen, S. N. F., M. F. M. Din, S. Rezania, M. Ponraj, A. Abd Rahman, L. W. Pei, and D. Komori. 2020. Dual phase role of composite adsorbents made from cockleshell and natural zeolite in treating river water. J. King Saud Univ. Sci. 32 (1):1–6. doi:https://doi.org/10.1016/j.jksus.2017.06.001.
- Naveen, B. P., and R. K. Malik. 2019. Assessment of contamination potential of leachate from municipal solid waste landfill sites for metropolitan cities in India. Pollution 5 (2):313–22.
- Rosli, M. A., Z. Daud, H. Awang, A. Zainorabidin, and A. A. Halim. 2017. The effectiveness of peat-AC composite adsorbent in removing SS, colour and fe from landfill leachate. Int. J. Integr. Eng. 9 (3):35–38.
- Sharma, A., and S. S. Katoch. 2019. Study of ammoniacal nitrogen removal from leachate of sanitary landfills in hilly terrain. Int. Res. J. Eng. Technol. 6 (9):299–303.
- Standard Methods for the Examination of Water and Wastewater. 2012. Washington, DC: American Public Health Association (APHA).
- Zukri, N. I., M. H. Khamidun, M. S. Sapiren, S. Abdullah, and M. A. A. Rahman. 2018. Lake water quality improvement by using waste mussel shell powder as an adsorbent. Earth Environ. Sci. 140 (1):012057.