ABSTRACT
The study focused on exposure assessment to bacterial aerosols and organic dust in waste sorting plant. Samples were collected at different workplaces of waste sorting cycle i.e.: waste press, reloading area, loading of conveyor belt, sorting cabin, sorting hall, and control room. A quantitative analysis of aerobic and anaerobic bacteria was supplemented by qualitative analysis of anaerobic biota with the use of culture-based methods and biochemical tests. In addition, inhalable dust concentrations were also evaluated. To confirm the presence of Clostridium genus, the PCR reaction with specific primers (Chis150f and ClostIr) was performed. The average concentration of total bacteria in waste sorting plant was 4347 CFU m−3 (SD = 2439), of which 66% were anaerobic strains (2852 CFU m−3; SD = 2127). It was found that about 24% of anaerobic bacteria belonged to Clostridium genus (682 CFU m−3; SD = 633). The highest contamination with anaerobic bacteria was observed near the waste reloading plant (3740 CFU m−3), and the lowest in the control room (850 CFU m−3). The average concentration of inhalable dust in the waste sorting plant was 0.81 mg m−3 (SD = 0.59). The correlation analysis showed that the presence of anaerobic bacteria, including clostridia was significantly determined by the microclimate parameters. Qualitative analysis showed the presence of 16 anaerobic species belonging to 9 genera, of which Actinomyces, Clostridium, and Gemella were present at all workplaces. The molecular analysis confirmed the presence of Clostridium genus in both bioaerosol and settled dust samples.
Implications: The study showed that anaerobic bacteria should be taken into account as an important component of this microbiota when assessing the exposure of waste sorting workers to biological agents. However, future studies should investigate more precisely how the composition of sorted waste as well as the season can affect the diversity of anaerobic bacteria in this working environment. More attention should be paid to regular cleaning of equipment surfaces in the plant, as deposited organic dust is an important reservoir of anaerobic bacteria, including those of a potentially pathogenic nature.
Introduction
Municipal waste sorting plants are an important link in the waste management system, as they contribute to the recovery of many raw materials that are usually directly landfilled for a long time. The waste that reaches the sorting plant is in majority of cases a mixture of plastics, glass, and paper, which are usually moist and contaminated with food residues. During numerous activities in the sorting plant, such as unloading from cars, transport by conveyor belts or manual segregation, organic dust particles are released into the air forming bioaerosol. Several studies demonstrated that, the concentrations of airborne mesophilic bacteria in this work environment are highly variable (Poulsen et al. Citation1995; Searl Citation2008). Such exposure may have a relatively low level of 102 CFU m−3 (Lehtinen et al. Citation2013), but may also reach high concentrations of 105 CFU m−3 (Park et al. Citation2011). It has been also shown that among the identified bacteria there may be species that may pose a threat to human health (e.g., Alcaligenes faecalis, Enterococcus faecalis, or Staphylococcus aureus), including those resistant to many antibiotics (Brągoszewska, Biedroń, and Hryb Citation2020; Krajewski et al. Citation2001). A common element of these studies was the identification of microorganisms in aerobic conditions. Whereas, the study of Cyprowski et al. (Citation2016) revealed that, more than 80% of the bacterial aerosol emitted in the waste sorting plant was formed by anaerobic bacteria, which may indicate a large underestimation of exposure to biological agents in this occupational environment.
The knowledge about anaerobic bacteria that have temporarily or permanently restricted access to oxygen in the atmosphere is still scarse. In municipal environment, their presence is associated with the decay of waste, usually accompanied by biogas production (Liaquat et al. Citation2017). This is usually evident during composting processes, when, depending on the prevailing temperature and humidity, specific communities of microorganisms are formed. They are capable decomposing large molecular organic compounds (primarily cellulose and pectins), to produce hydrogen and methane. The most often mentioned in this context are anaerobic bacteria of Thermoanaerobacteriaceae and Clostridiaceae families (Nissilä et al. Citation2011), as well as of Methanosarcina, Methanosaeta, Methanobrevibacter, and Methanobacterium genera (Ike et al. Citation2010). Among these groups a special position has Clostridium genus, of which about 35 species can cause clinically important adverse effects, mainly due to the release of their toxins (Samul et al. Citation2013). In waste samples, both the common environmental strains (e.g., Clostridium butyricum) as well as species pathogenic for humans (such as Clostridium perfringens) can be found (Neuhaus, Shehata, and Krüger Citation2015). In total of 203 waste samples these researchers identified 11 species of the Clostridium genus, with C. perfringens being the most frequently isolated (nearly 60% of samples). Due to the production of endospores, which gives the ability to maintain its long-term viability, this species, along with Enterococcus genus and intestinal coliform rods, is considered as an indicator of fecal pollution of the environment (Lisle et al. Citation2004; Pillai et al. Citation1996; Unc, Niemi, and Goss Citation2015).
An important element of a proper exposure assessment is the use of appropriate analytical methods enabling to cover groups that have not been so far studied. For a long time, the combination of culture-based methods and biochemical tests, due to their acceptable cost and ease of use, has been the primary way of identifying environmental bacteria (Zaleski et al. Citation2005). Such approach enables identification of viable infectious strains responsible for numerous adverse outcomes and, due to that, was widely used in the studies of various working and living environments (Buczyńska, Cyprowski, and Szadkowska-Stańczyk Citation2011; Gołofit-Szymczak and Górny Citation2018; Góra et al. Citation2009; Naddafi et al. Citation2019; Reinthaler et al. Citation1997; Ugolini and Sander Citation2019). Today, molecular methods are faster, more sensitive and specific compared to culture-based techniques. The accuracy of the results obtained using biochemical tests is estimated to be 70–85% compared to molecular techniques (Ko et al. Citation2007). Considering the specificity and short time of analysis, polymerase chain reaction (PCR), including 16S rRNA analysis, seems to be a good way for identification of bacterial strains, including those with limited growth capacity (Mackay Citation2004). However, PCR analysis does not provide information on whether a particular microorganism is viable or not. In turn other molecular, methods such as Next Generation Sequencing (NGS) are successfully used in profiling of environmental bacterial communities (Eisen and Levin Citation2007), but short sequencing readings (usually around 300–500 base pairs) make difficult to recognize all species of specific bacterial genera (Rizal et al. Citation2020). Despite this drawback, the PCR method has significantly improved the precision of microbial identification in different occupational settings, including: wastewater treatment plants (Orsini et al. Citation2002), tanneries (Skóra et al. Citation2014), waste composting plants (Anedda et al. Citation2019), metalworking (Gilbert, Veillette, and Duchaine Citation2010) or animal production facilities (Dungan and Leytem Citation2009). In case of municipal waste sorting plants, molecular identification techniques have been so far used to diagnose Q fever cases caused among workers by Coxiella burnetii (Alonso et al. Citation2015); however, regarding anaerobic bacteria such analyzes have not been frequently performed (Degois et al. Citation2021). Detailed analyzes of anaerobic bacteria using the NGS as well as also culture-based methods in combination with the MALDI-TOF MS (Matrix-Assisted Laser Desorption/Ionization – Time of Flight Mass Spectrometry) technique were so far carried out on pig farms (White, Nielsen, and Madsen Citation2019).
Taking the above into account, the aim of this study was to assess the exposure to bacteria in municipal waste sorting plant. The particular focus was given on detailed identification of airborne anaerobic bacteria with the use of biochemical tests. In addition, to confirm the presence of anaerobic strains including those of Clostridium genus in bioaerosol, inhalable and settled dust samples were qualitatively assessed using molecular methods based on the analysis of 16S rRNA.
Material and methods
Sampling sites
The measurements were carried out during spring time (April 2020) in a municipal waste sorting plant with a capacity of about 170 tons of waste per day. Due to the SARS-CoV-2 virus pandemic, it was not possible to perform measurements in other seasons of the year, as the access to the plant was strictly limited. On-site sampling points were determined taking into account the major dust generated activities at workplaces i.e.: waste press, reloading area, loading of conveyor belt, sorting cabin, sorting hall, and control room. In addition, to relativize the concentrations of microorganisms measured at workplaces the external background air samples were also collected in the point located approx. 50 m from the border of the plant. In total, 23 sampling points were selected, to collect bioaerosols as well as inhalable and settled dust samples. Their detailed description is given in .
Table 1. Description of sampling points and characteristics of samples collected at the waste sorting plant
Bioaerosol sampling
Bioaerosol samples (BAS) were stationary taken in duplicates using a one-stage MAS impactor (100NT, Merck KGaA, Germany) placed about 1.5 m above the ground. At each studied point, the bioaerosol was sampled for 30 seconds. Impactor was operated at a flow rate of 100 l min−1. For the measurement of anaerobic bacteria, impactor was loaded with Petri plates containing Schaedler agar (SCS) with 5% additive of sheep blood (bioMérieux, France). For quantitative determination of aerobic bacteria Tryptic Soy Agar (TSA) with 5% additive of sheep blood was used (bioMérieux). In total 40 samples were taken for the above mentioned purposes.
Simultaneously with bioaerosol measurements, at each sampling point, the temperature and relative humidity were measured in duplicates with the use of portable thermo-hygrometer (model TFA 30.5024, Conrad Electronic GmbH, Germany). In total 20 measurements of each microclimate parameter were performed.
Dust sampling
Inhalable and settled dust samples were collected and directly used for the isolation of bacterial DNA to confirm the presence of Clostridium genus strains. Inhalable dust samples (IDS) were taken using CIS Sampler (Conical Inhalable Sampler, Casella Measurements Inc., UK) loaded with PTFE membrane filter (SKC Inc., USA) with a pore size of 2 µm and diameter of 37 mm. Each sampler was connected with APEX (Casella Measurements Inc.) and placed also about 1.5 m above the ground. The flow rate of the air was set at 4 l min−1, and the sampling was performed for 18 hours. The use of extended measurement time was aimed to cover the morning and afternoon work shift, and thus to collect more dust for further analysis (Blackley et al. Citation2019). In total, eight inhalable dust samples were collected using this method. For determination of organic dust concentration a gravimetric method was used. In the laboratory, the filters before and after the measurement were conditioned for 24 hours in a controlled microclimate room (mean temperature = 23.5°C; standard deviation, SD = 0.21; relative humidity = 27.5%; SD = 9.19) and weighed using microbalance XS-105DU (Mettler-Toledo GmbH, Switzerland), with accuracy of 0.01 mg.
Settled dust (SDS) samples were collected from the surfaces of 25–100 cm2, using eSwab ™ sterile swabs (Copan Diagnostics Inc., USA), having the tip of the nylon fibers and liquid Amies transport medium (1 ml volumes) dedicated to the collection of anaerobic bacteria. In total, five settled dust samples were collected using this method. After the measurement, the material was directly delivered to the laboratory for further analysis.
Laboratory analysis of bioaerosol samples
Petri plates with TSA medium were incubated under the following conditions: 1 day (37°C) + 3 days (22°C) + 3 days (4°C). Prolonged incubation of the samples was intended to allow the development of the whole spectrum of bacterial strains including psychrophilic ones. In turn, SCS plates were incubated in an anaerobic atmosphere with the use of AnaeroGen™ system (Oxoid Ltd., UK) under the following conditions: 2 days (37°C) + 2 days (30°C). After incubation, all growing colonies were counted and the bacterial concentrations were expressed as colony forming units per 1 cubic meter of the air (CFU m−3). Finally, isolated colonies of anaerobic bacteria from bioaerosol samples were identified to the genus and species level using the biochemical API 20A test (bioMérieux). SCS medium plates were also used to isolate bacterial DNA to confirm presence of Clostridium strains.
Isolation of DNA from samples
Genomic DNA from IDS and SDS samples was extracted using Syngen Soil DNA Mini Kit (Syngen Biotech Ltd., Poland). After weighing, each of PTFE filters was cut into small pieces. In the same time, all swabs immersed in Amies medium were shaken vigorously for 10 minutes. The cut filters as well as 50 µl aliquots of the samples obtained after shaking the swabs were transferred to the previously prepared tubes containing 200 mg of glass beads. All samples prepared in this way were flooded with 600 µl of DLS1 buffer and vortexed for 5 minutes. Further DNA isolation steps were carried out according to protocol recommended by the manufacturer.
For isolation of DNA from MAS samples, A&A Genomic Mini Kit (A&A Biotechnology, Poland) was used. After enumeration, each plate was flooded with 2 ml PBS and the colonies were transferred into suspension by scraping. The resulting suspension was transferred into a sterile 2 ml tube. Then 200 μl of the suspended material was transferred to a new tube and centrifuged for 3 minutes at 7000 rpm. In the next step, the supernatant was removed and the remaining pellet was suspended in 145 μl digestive tap (to which 25 μl of lysozyme (10 mg ml−1) and 10 μl of lysostaphin (1 mg ml−1) were added) and subsequently incubated for 40 min at 37°C. Further DNA isolation steps were performed according to the manufacturer’s protocol.
The concentration of the isolated DNA (ng ml−1) as well as its purity were checked with spectrophotometer DS-11 (DeNovix Inc., USA) at wave lengths of 260 nm and 280 nm.
Molecular confirmation of Clostridium isolates
Isolated DNA was used as a template for PCR reaction with primer pair specific to Clostridium genus: Chis150f (5ʹ-AAAGGRAGATTAATACCGCATAA-3ʹ) and ClostIr (5ʹ-TTCTTCCTAATCTCTACGCA-3ʹ) (Hung et al. Citation2008), which allow amplification of the gene fragment encoding 16S rRNA. The reaction mixture (20 μl) contained 2 μl of 10× reaction buffer with MgCl2, 0.5 U of RUN-HS Taq Polymerase (A&A Biotechnology), 250 µM of each deoxynucleotide (dNTP), 50 pmol of each primer and 0.5 μl of template DNA. Amplification included 35 cycles preceded by initial denaturation (95°C, 5 min). Each cycle included denaturation (95°C, 15 sec), annealing (58°C, 60 sec) and elongation (72°C, 60 sec) steps. The reaction ended with a final elongation (72°C, 5 min). The size of PCR product and the specificity of the primers were checked using electrophoretic analysis in 1.5% agarose gel (Certified™ Molecular Biology Agarose, BioRad, USA) and comparing the product size to DNA fragment marker (GeneRuler 1kb DNA Ladder, Thermo Scientific, USA).
Statistical analysis
The obtained results were presented as means with standard deviations as well as medians together with the concentration ranges. As all the independent variables were not normally distributed (based on Shapiro–Wilk test), the nonparametric Mann–Whitney (M-W) and Kruskal–Wallis (K-W) tests as well as Spearman’s rank correlation coefficient were used to confirm statistical importance of the observed relationships. The Chi-square test was also used to assess differences in microbial diversity. All calculations were performed using Statistica data analysis software system, version 10 (StatSoft, Inc., USA), assuming a value of p < .05 as statistically significant.
Results and discussion
The average concentration of total bacteria in the waste sorting plant was 4347 CFU m−3 (SD = 2439), of which 66% were anaerobic (2852 CFU m−3; SD = 2127) and 34% aerobic bacteria (1494 CFU m−3; SD = 630). In addition, it was found that about 24% of anaerobic bacteria belonged to Clostridium genus (682 CFU m−3; SD = 633) (). Comparative analysis showed statistically significant differences between workplace and background bacterial aerosol concentrations in all groups of microorganisms (p < .05).
Table 2. Bacterial aerosol and inhalable dust concentrations in a waste sorting plant
The bacterial concentrations found in the waste sorting plant were at a comparable level to results previously demonstrated by other authors (Brągoszewska Citation2019; Cyprowski et al. Citation2016; Krajewski et al. Citation2002). As previous work by Cyprowski et al. (Citation2016), this study also proved that anaerobic bacteria predominated in the air at workplaces in waste sorting facilities; however, their contribution in the total pool of bacteria was 20% lower than that in previous research. The current results have again confirmed the phenomenon of the abundant presence of this bacterial group, which so far has been rarely taken into account in exposure assessment to biological agents in this kind of occupational environment.
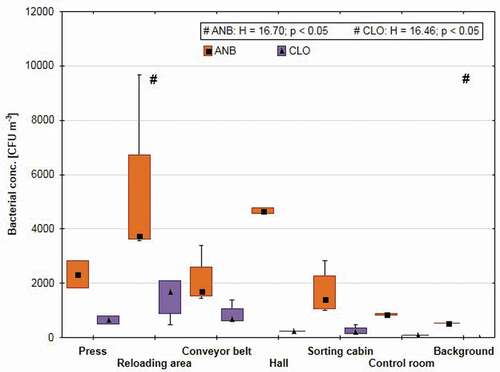
The degree of contamination with bacterial aerosols was variable depending on the measurement site. Among different areas in the sorting plant, the highest contamination with anaerobic bacteria was observed near the waste reloading plant (3740 CFU m−3) and the lowest in the control room (850 CFU m−3). Anaerobic bacteria (540 CFU m−3) were also present in the background, but strains of Clostridium genus were not identified in those samples (). The correlation analysis showed that the presence of anaerobic bacteria, including those of the Clostridium genus, was significantly determined by the microclimate parameters (). During bioaerosol measurements, the average temperature was 13.2°C (SD = 3.3), of which the lowest readings were found next to the press for segregated waste (10.1°C; SD = 0.2), and the highest in the control room (20.4°C; SD = 0.9). In the case of relative air humidity, its mean value during bioaerosol measurements was 46.3% (SD = 5.8) with the lowest readings found in background point (35.0%; SD = 2.8) and with the highest at the press (52.0%; SD = 2.8).
Table 3. Spearman’s rank correlation coefficient analysis for the microclimate parameters and bioaerosol concentrations measured in the waste sorting plant
Figure 1. Concentrations of airborne anaerobic bacteria and genus Clostridium (medians) in the waste sorting plant, by workplace. ANB – anaerobic bacteria, CLO – Clostridium genus, boxes – 25 and 75 percentile, whiskers – range of values, top rectangle – values of Kruskal–Wallis H statistics, # – sampling points with significance difference
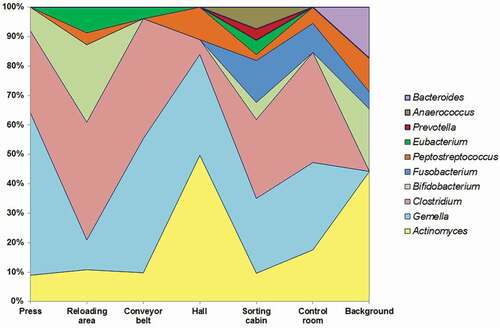
The qualitative analysis with the use of biochemical tests allowed to identify in this plant a total of 16 bacterial species, belonging to 9 genera, including: Actinomyces, Anaerococcus, Bifidobacterium, Clostridium, Eubacterium, Fusobacterium, Gemella, Peptostreptococcus, and Prevotella (). The microbial diversity of background samples was much smaller (seven species from five genera) and this difference was statistically significant (Chi-square = 7.71, p < .01; 95% CI: 23.2–76.8).
Table 4. Qualitative characteristics (based on biochemical tests) of anaerobic bacteria present in the waste sorting plant samples collected with MAS impactor
The spatial analysis also showed that occurrence of anaerobic bacteria strictly depended on the workplaces in the sorting plant. Three of the above-mentioned genera (Actinomyces, Clostridium, and Gemella) were present in various proportions at all sampling points; however, only in the sorting cabin the entire spectrum of the identified microorganisms was observed (). The presence of Bacteroides, Bifidobacterium, Fusobacterium, and Peptostreptococcus genera in background samples may indicate waste transported with garbage trucks and/or emitted from the waste sorting plant as their source; however, this would require further verification, as in the current study the wind direction was not controlled. It cannot be ruled out that the other sources of these bacteria may be birds, insects (Ventura et al. Citation2014) and rodents (Carlstedt-Duke et al. Citation1986).
Strains of anaerobic bacteria identified in the studied plant, including those from Actinomyces, Bifidobacterium, Clostridium, Eubacterium, and Gemella genera, were similar to those found in another waste management plant described by Cyprowski et al. (Citation2016). It may suggest that the structure of delivered municipal waste was comparable. However, due to the limited scope of this study, it is difficult to say whether this would be also found in other plants of this type. The new knowledge on this subject, however, is provided by one-year-follow-up study conducted at waste sorting plant in France (Degois et al. Citation2021). A detailed qualitative analysis of microorganisms showed that in the unloading area where waste is delivered as well as in the first sorting cabin, the predominant bacteria also included anaerobic strains belonging to Prevotella, Lactobacillus, and Lactococcus genera, with a small share of members of Clostridium genus. The demonstrated differences in the bacterial diversity may result from various morphological composition of the waste. The provided description showed that in France, the sorting plant received pre-sorted waste, including cardboard, different types of plastic packing, cartons and metals. In Poland, the waste continue to have a mixed character and thus it is significantly contaminated with organic fraction, including the food remnants. This necessitates the pre-separation of the organic fraction with the use of special drum screens in order to be able to conduct further manual segregation of glass, paper, plastics, or problematic waste (e.g., batteries) in the sorting cabins. Such characteristics of the waste usually forces construction of additional composting installation in sorting plants (Cyprowski et al. Citation2016). As no such installation has been present in the currently studied plant, the separated organic parts, and waste that cannot be reused were sent to a nearby ballast dump.
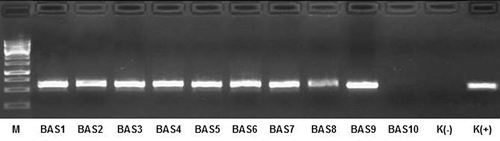
Due to the preliminary nature of this study, the analyses of collected samples based on the bacterial DNA were narrowed to confirm the presence of Clostridium genus, as a representative of bacteria with pathogenic properties. The obtained PCR reaction products () confirmed the presence of Chis150f and ClostIr genes in all bioaerosol samples collected at workplaces (BAS1-10), which was fully consistent with the results of biochemical API tests. In addition, Clostridium strains were detected in some of the settled dust samples (SDS1, SDS4, and SDS5), but not in any of the inhalable dust samples taken with CIS samplers. These results showed that some dust samples gave the opportunity to identify bacteria without the need for microbial cultivation. As it was shown by Degois et al. (Citation2021), the diagnostics based on molecular techniques allows the identification of a very wide spectrum of microorganisms. However, this approach, in contrast to classical analytical methods, does not provide information on whether the identified species are viable, and thus whether they can actively infect (especially when they are inhaled) exposed workers.
Figure 3. Electrophoretic analysis of PCR product using primers specific to the genus Clostridium (Chis150f/ClostIr). Description: M – DNA marker; BAS1-9 – isolates of bioaerosols from workplaces in waste sorting plant; BAS10 – isolate from outdoor background; K(-) – negative control; K(+) – positive control
Our study showed that some of isolated airborne bacteria belonged to risk group 2, according to the Commission Directive (UE) 2019/1833, and as such they pose a health threat to people exposed to them. Among others, bacteria of Actinomyces genus exhibit strong allergenic properties, and the species A. israelii is considered to be an etiological factor of actinomycosis (Valour et al. Citation2014). In turn, strains of Prevotella genus may be responsible for hematogenous osteoarthritis as well as wound and genital tract infections (Kierzkowska et al. Citation2016). It is extremely important considering the fact that women were part of the employees in the sorting cabins. Among the identified bacteria there were also those responsible for opportunistic infections of soft tissues, especially in the oral cavity, as well as sinusitis and pneumonia (Fusobacterium and Peptostreptococcus) (Nagy, Boyanova, and Justesen Citation2018). It is also important that some of the identified strains (Fusobacterium and Prevotella) are Gram-negative bacteria, which are a source of endotoxin. Endotoxin has strong pro-inflammatory properties and may adversely affect efficiency of the respiratory system (manifested by a reduction in spirometric parameters, mainly forced expiratory volume in one second – FEV1).
Considering the potential health hazards, it is worrying that all workplaces were rich in clostridia, of which only C. beijerinckii was identified by biochemical methods. Usually, this species in low concentrations is not harmful to workers and its existence in the environment of waste sorting plants confirms that organic matter of plant origin is present in the waste (Sanguanchaipaiwonga and Leksawasdi Citation2018). However, among the identified Clostridium representatives, about 10% were strains, which species affiliation could not be determined by biochemical tests. Thus, it cannot be ruled out that bacteria with pathogenic properties, e.g. C. perfringens, were present in bioaerosol and dust samples. This species may be responsible for food poisoning, inflammation of the small intestine, and diarrhea following antibiotic treatment (Nagy, Boyanova, and Justesen Citation2018). Hence, the analytical method applied for its identification should enable to confirm its environmental presence. A good solution in this regard may be e.g. the quantitative polymerase chain reaction (qPCR) technique, which appears as a method of choice for the quantification of target organisms (Karpowicz et al. Citation2010), or multiplex PCR (Neuhaus, Shehata, and Krüger Citation2015). The latter may be characterized by even two times higher sensitivity in detecting Clostridium strains than cultural methods, but the high efficiency of this method depends on the proper design of primers for such reaction.
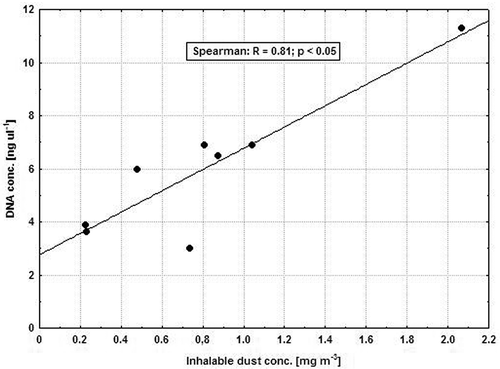
Special processing of dust samples without cultivation was aimed at searching for genes characteristic for the entire Clostridium genus. Such analytical elaboration was successful for some of the settled dust samples only. The lack of appropriate PCR products in inhalable dust samples could be limited by obtaining genetic material from environmental samples. It must be always ensured that the amount of isolated DNA is sufficient. In the present study, with a relatively small number of samples, a significant correlation (R = 0.81; p < .05) was shown between the concentrations of inhalable dust and bacterial DNA (). Perhaps, in order to increase the amount of biological material, it is necessary to use highly efficient dust sampling techniques, extending simultaneously sampling flow rate from traditional 4 l min−1 to about 10 l min−1 (Degois et al. Citation2021) and in some environmental situations even up to 300 l min−1 (Lecours et al. Citation2012; Rinsoz et al. Citation2008). However, simple increase of sample volume does not solve all the problems related to qualitative identification of microbiota. For example, despite the collection of approximately 4,000 liters of air during sampling of inhalable dust, the genetic material isolated in the present study did not allow for the obtaining of PCR products when searching for genes of Clostridium bacteria. In turn, in the work of Degois et al. (Citation2021), the amount of aspirated air was about 3,000 liters, and the isolated DNA enabled the use of the sequencing technique. Moreover, the process of DNA extraction itself, during which it is necessary to neutralize various inhibitors that hinder the release of DNA from bacterial cells seems to be of great importance as well. This could be influenced by, for example, the use of a detergent in the elution of filters, which helps to dissolve denatured DNA (Vingataramin and Frost Citation2015). In addition, the set of reagents used in the analysis should remove all unnecessary inhibitors of the reaction, which should improve the purity of the isolated genetic material. The most important inhibitors are usually humic acids, various types of proteins and heavy metals (Felczykowska et al. Citation2015). Researchers also indicate that some microorganisms, e.g. anaerobic bacteria of Porphyromonas gingivalis species, are able to produce nucleases that degrade purified DNA (Nadkarni et al. Citation2009). Their removal from environmental samples increases the chances that the subsequent application of molecular identification techniques will be highly effective.
Conclusion
The study showed that anaerobic bacteria should be taken into account as an important component of airborne microbiota when assessing the exposure of waste sorting workers to biological agents. However, future studies should investigate more precisely how the composition of sorted waste as well as the seasonal variation of microclimate parameters can affect the diversity of anaerobic bacteria in this working environment. More attention should be paid to regular cleaning of equipment surfaces in the plant, as deposited organic dust is an important reservoir of anaerobic bacteria, including those of a potentially pathogenic nature, and the use of molecular techniques based on the analysis of bacterial DNA may be helpful in this regard.
Acknowledgment
The authors wish to give special thanks to their colleague, Aleksandra Bakal-Kijek, MSc, who worked in the Central Institute for Labour Protection – National Research Institute when the project was performed, for participation and assistance in carrying out laboratory analyzes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Marcin Cyprowski
Marcin Cyprowski, Ph.D., is a research assistant in the Laboratory of Biohazards at the Central Institute for Labour Protection–National Research Institute (CIOP-PIB), Warsaw, Poland. He deals with the problems of biological hazards in the working and living environments. His professional interest is assessment of toxic effects of bioaerosols on workers’ health.
Anna Ławniczek-Wałczyk
Anna Ławniczek-Wałczyk, Ph.D., is a research assistant in the Laboratory of Biohazards at the CIOP-PIB, Warsaw, Poland. Her research activities focus on occupational exposure to biological hazards including the methods of their quantification, monitoring of pathogens transmission using genotypic methods and antimicrobial susceptibility testing.
Agata Stobnicka-Kupiec
Agata Stobnicka-Kupiec, Ph.D., is a research assistant in the Laboratory of Biohazards at the CIOP-PIB, Warsaw, Poland. She has been working in the field of the qualitative and quantitative assessment of microbial contamination of indoor spaces. Her professional interest is application of molecular methods in the detection of viruses in bioaerosols and on fomites.
Rafał L. Górny
Rafał L. Górny is a full professor of medical sciences, is currently head of the Laboratory of Biohazards at the CIOP-PIB, Warsaw, Poland. He has been engaged in numerous studies on the health-related aspects of exposure to particulate, including biological, and fibrous aerosols in occupational and non-occupational environments. Since 2002, he has been working as a World Health Organization and European Commission adviser within the field of biological agents.
References
- Alonso, E., I. Lopez-Etxaniz, A. Hurtado, P. Liendo, F. Urbaneja, I. Aspiritxaga, J. I. Olaizola, A. Piñero, I. Arrazola, J. F. Barandika, et al. 2015. Q fever outbreak among workers at a waste-sorting plant. PLoS One 10 (9):e0138817. doi:https://doi.org/10.1371/journal.pone.0138817.
- Anedda, E., G. Carletto, G. Gilli, and D. Traversi. 2019. Monitoring of air microbial contaminations in different bioenergy facilities using cultural and biomolecular methods. Int. J. Environ. Res. Public Health. 16 (14):2546. doi:https://doi.org/10.3390/ijerph16142546.
- Blackley, B. H., J. L. Gibbs, K. J. Cummings, A. B. Stefaniak, J. Y. Park, M. Stanton, and M. A. Virji. 2019. A field evaluation of a single sampler for respirable and inhalable indium and dust measurements at an indium-tin oxide manufacturing facility. J. Occup. Environ. Hyg. 16 (1):66–77. doi:https://doi.org/10.1080/15459624.2018.1536826.
- Brągoszewska, E. 2019. Exposure to bacterial and fungal aerosols: Microorganisms indices in a waste-sorting plant in Poland. Int. J. Environ. Res. Public Health. 16:3308. doi:https://doi.org/10.3390/ijerph16183308.
- Brągoszewska, E., I. Biedroń, and W. Hryb. 2020. Microbiological air quality and drug resistance in airborne bacteria isolated from a waste sorting plant located in Poland – A case study. Microorganisms 8 (2):202. doi:https://doi.org/10.3390/microorganisms8020202.
- Buczyńska, A., M. Cyprowski, and I. Szadkowska-Stańczyk. 2011. Indoor disposal of household waste as a source of environmental biohazard exposure. Polish J. Environ. Stud. 20 (4):851–56.
- Carlstedt-Duke, B., T. Midtvedt, C. E. Nord, and B. E. Gustafsson. 1986. Isolation and characterization of a mucin-degrading strain of Peptostreptococcus from rat intestinal tract. Acta Pathol. Microbiol. Immunol. Scand. B. 94 (5):293–300. doi:https://doi.org/10.1111/j.1699-0463.1986.tb03056.x.
- Cyprowski, M., A. Stobnicka, R. L. Górny, M. Gołofit-Szymczak, and A. Ławniczek-Wałczyk. 2016. Aerosols of bacterial origin in working rooms of waste management facility. Rocz. Ochr. Srodowiska. 18:294–308. (in Polish).
- Degois, J., X. Simon, F. Clerc, C. Bontemps, P. Leblond, and P. Duquenne. 2021. One-year follow-up of microbial diversity in bioaerosols emitted in a waste sorting plant in France. Waste Manag. 120:257–68. doi:https://doi.org/10.1016/j.wasman.2020.11.036.
- Dungan, R. S., and A. B. Leytem. 2009. Qualitative and quantitative methodologies for determination of airborne microorganisms at concentrated animal-feeding operations. World J. Microbiol. Biotechnol. 25:1505–18. doi:https://doi.org/10.1007/S11274-009-0043-1.
- Eisen, J. A., and S. Levin. 2007. Environmental shotgun sequencing: Its potential and challenges for studying the hidden world of microbes. PLoS Biol. 5 (3):e82. doi:https://doi.org/10.1371/journal.pbio.0050082.
- European Commission. 2019. Commission Directive (EU) 2019/1833 of 24 October 2019 amending Annexes I, III, V and VI to Directive 2000/54/EC of the European Parliament and of the Council as regards purely technical adjustments. OJ L 279:54–79.
- Felczykowska, A., A. Krajewska, S. Zielińska, and J. M. Łoś. 2015. Sampling, metadata and DNA extraction – Important steps in metagenomic studies. Acta Biochim. Pol. 62 (1):151–60. doi:https://doi.org/10.18388/abp.2014_916.
- Gilbert, Y., M. Veillette, and C. Duchaine. 2010. Metalworking fluids biodiversity characterization. J. Appl. Microbiol. 108 (2):437–49. doi:https://doi.org/10.1111/j.1365-2672.2009.04433.x.
- Gołofit-Szymczak, M., and R. L. Górny. 2018. Microbiological air quality in office buildings equipped with different ventilation systems. Indoor Air 28:792–805. doi:https://doi.org/10.1111/ina.12495.
- Góra, A., B. Mackiewicz, P. Krawczyk, M. Golec, C. Skórska, J. Sitkowska, G. Cholewa, L. Larsson, M. Jarosz, A. Wójcik-Fatla, et al. 2009. Occupational exposure to organic dust, microorganisms, endotoxin and peptidoglycan among plants processing workers in Poland. Ann. Agric. Environ. Med. 16:143–50.
- Hung, C. H., C. H. Cheng,C. H L. H. Cheng, C. M. Liang, and C. Y. Lin. 2008. Application of Clostridium-specific PCR primers on the analysis of dark fermentation hydrogen-producing bacterial community. Int. J. Hydrog. Energy 33:1586–92.
- Ike, M., D. Inoue, T. Miyano, T. T. Liu, K. Sei, S. Soda, and S. Kadoshin. 2010. Microbial population dynamics during startup of a full-scale anaerobic digester treating industrial food waste in Kyoto eco-energy project. Bioresour. Technol. 101 (11):3952–57. doi:https://doi.org/10.1016/j.biortech.2010.01.028.
- Karpowicz, E., A. Novinscak, F. Bärlocher, and M. Filion. 2010. qPCR quantification and genetic characterization of Clostridium perfringens populations in biosolids composted for 2 years. J. Appl. Microbiol. 108 (2):571–81. doi:https://doi.org/10.1111/j.1365-2672.2009.04441.x.
- Kierzkowska, M., A. Majewska, A. Sawicka-Grzelak, and G. Młynarczyk. 2016. Gram-negative anaerobic rods – Diagnostics and clinical significance. Post. Microbiol. 55 (1):91–98. (in Polish).
- Ko, K. S., T. Kuwahara, L. Haehwa, Y. J. Yoon, B. J. Kim, K. H. Lee, Y. Ohnishi, and Y. H. Kook. 2007. RNA polymerase beta-subunit gene (rpoB) sequence analysis for the identification of Bacteroides spp. Clin. Microbiol. Infect. 13 (1):48–54. doi:https://doi.org/10.1111/j.1469-0691.2006.01553.x.
- Krajewski, J. A., J. Szarapińska-Kwaszewska, B. Dudkiewicz, and M. Cyprowski. 2001. Alive microorganisms in the workplace ambient air in plants disposing communal waste. Med. Pr. 52 (5):343–49. (in Polish).
- Krajewski, J. A., S. Tarkowski, M. Cyprowski, J. Szarapińska-Kwaszewska, and B. Dudkiewicz. 2002. Occupational exposure to organic dust associated with municipal waste collection and management. Int. J. Occup. Med. Environ. Health. 15 (3):289–301.
- Lecours, P. B., M. Veillette, D. Marsolais, and C. Duchaine. 2012. Characterization of bioaerosols from dairy barns: Reconstructing the puzzle of occupational respiratory diseases by using molecular approaches. Appl. Environ. Microbiol. 78 (9):3242–48. doi:https://doi.org/10.1128/AEM.07661-11.
- Lehtinen, J., O. Tolvanen, U. Nivukoski, A. Veijanen, and K. Hänninen. 2013. Occupational hygiene in terms of volatile organic compounds (VOCs) and bioaerosols at two solid waste management plants in Finland. Waste Manag. 33 (4):964–73. doi:https://doi.org/10.1016/j.wasman.2012.11.010.
- Liaquat, R., A. Jamal, I. Tauseef, Z. Qureshi, U. Farooq, M. Imran, and M. I. Ali. 2017. Characterizing bacterial consortia from an anaerobic digester treating organic waste for biogas production. Pol. J. Environ. Stud. 26 (2):709–16. doi:https://doi.org/10.15244/pjoes/59332.
- Lisle, J. T., J. J. Smith, D. D. Edwards, and G. A. McFeters. 2004. Occurrence of microbial indicators and Clostridium perfringens in wastewater, water column samples, sediments, drinking water, and weddell seal feces collected at McMurdo Station, Antarctica. Appl. Environ. Microbiol. 70 (12):7269–76. doi:https://doi.org/10.1128/AEM.70.12.7269-7276.2004.
- Mackay, I. M. 2004. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 10 (3):190–212. doi:https://doi.org/10.1111/j.1198-743x.2004.00722.x.
- Naddafi, K., R. Nabizadeh, A. N. Baghani, and M. Fazlzadeh. 2019. Bioaerosols in the waterpipe cafés: Genera, levels, and factors influencing their concentrations. Environ. Sci. Pollut. Res. Int. 26 (20):20297–307. doi:https://doi.org/10.1007/s11356-019-05413-6.
- Nadkarni, M. A., F. E. Martin, N. Hunter, and N. A. Jacques. 2009. Methods for optimizing DNA extraction before quantifying oral bacterial numbers by real-time PCR. FEMS Microbiol. Lett. 296 (1):45–51. doi:https://doi.org/10.1111/j.1574-6968.2009.01629.x.
- Nagy, E., L. Boyanova, and U. S. Justesen; ESCMID Study Group of Anaerobic Infections. 2018. How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories. Clin. Microbiol. Infect. 11:1139–48. doi:https://doi.org/10.1016/j.cmi.2018.02.008.
- Neuhaus, J., A. A. Shehata, and M. Krüger. 2015. Detection of pathogenic clostridia in biogas plant wastes. Folia Microbiol. 60 (1):15–19. doi:https://doi.org/10.1007/s12223-014-0334-2.
- Nissilä, M. E., H. P. Tähti, J. A. Rintala, and J. A. Puhakka. 2011. Effects of heat treatment on hydrogen production potential and microbial community of thermophilic compost enrichment cultures. Bioresour. Technol. 102:4501–06. doi:https://doi.org/10.1016/j.biortech.2010.12.072.
- Orsini, M., P. Laurenti, F. Boninti, D. Arzani, A. Lanni, and V. Romano-Spica. 2002. A molecular typing approach for evaluating bioaerosol exposure in wastewater treatment plant workers. Water Res. 36 (5):1375–78. doi:https://doi.org/10.1016/s0043-1354(01)00336-0.
- Park, D.-U., S.-H. Ryu, S.-B. Kim, and C.-S. Yoon. 2011. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. J. Air Waste Manag. Assoc. 61 (4):461–68. doi:https://doi.org/10.3155/1047-3289.61.4.461.
- Pillai, S. D., K. W. Widmer, S. E. Dowd, and S. C. Ricke. 1996. Occurrence of airborne bacteria and pathogen indicators during land application of sewage sludge. Appl. Environ. Microbiol. 62 (1):296–99. doi:https://doi.org/10.1128/AEM.62.1.296-299.1996.
- Poulsen, O. M., N. O. Breum, N. Ebbehøj, A. M. Hansen, U. I. Ivens, D. Van Lelieveld, P. Malmros, L. Matthiasen, B. H. Nielsen, E. M. Nielsen, et al. 1995. Sorting and recycling of domestic waste. Review of occupational health problems and their possible causes. Sci. Total Environ. 168 (1):33–56. doi:https://doi.org/10.1016/0048-9697(95)04521-2.
- Reinthaler, F. F., E. Marth, U. Eibel, U. Enayat, O. Feenstra, H. Friedl, M. Köck, F. P. Pichler-Semmelrock, G. Prodnig, and R. Schlacher. 1997. The assessment of airborne microorganisms in large-scale composting facilities and their immediate surroundings. Aerobiologia 13:167–75. doi:https://doi.org/10.1007/BF02694504.
- Rinsoz, T., P. Duquenne, G. Greff-Mirguet, and A. Oppliger. 2008. Application of real-time PCR for total airborne bacterial assessment: Comparison with epifluorescence microscopy and culture-dependent methods. Atmos. Environ. 42 (28):6767–74. doi:https://doi.org/10.1016/j.atmosenv.2008.05.018.
- Rizal, N. S. M., H.-M. Neoh, R. Ramli, P. R. A. L. K. Periyasamy, A. Hanafiah, M. N. A. Samat, T. L. Tan, K. K. Wong, S. Nathan, S. Chieng, et al. 2020. Advantages and limitations of 16S rRNA next-generation sequencing for pathogen identification in the diagnostic microbiology laboratory: Perspectives from a middle-oncome country. Diagnostics 10 (10):816. doi:https://doi.org/10.3390/diagnostics10100816.
- Samul, D., P. Worsztynowicz, K. Leja, and W. Grajek. 2013. Beneficial and harmful roles of bacteria from the Clostridium genus. Acta Biochim. Pol. 60 (4):515–21.
- Sanguanchaipaiwonga, V., and N. Leksawasdi. 2018. Butanol production by Clostridium beijerinckii from pineapple waste juice. Energy Procedia 153:231–36. doi:https://doi.org/10.1016/j.egypro.2018.10.006.
- Searl, A. 2008. Exposure response relationships for bioaerosols emissions from waste treatment processes. Defra project WR0606, Institute of Occupational Medicine, Edinburgh, UK. Accessed February 22, 2021. http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&Completed=0&ProjectID=15140.
- Skóra, J., B. Gutarowska, Ł. Stepień, A. Otlewska, and K. Pielech-Przybylska. 2014. The evaluation of microbial contamination in the working environment of tanneries. Med. Pr. 65 (1):15–32. doi:https://doi.org/10.13075/mp.5893.2014.005.
- Ugolini, M., and L. E. Sander. 2019. Dead or alive: How the immune system detects microbial viability. Curr. Opin. Immunol. 56:60–66. doi:https://doi.org/10.1016/j.coi.2018.09.018.
- Unc, A., J. Niemi, and M. J. Goss. 2015. Soil and waste matrix affects spatial heterogeneity of bacteria filtration during unsaturated flow. Water 7 (12):836–54. doi:https://doi.org/10.3390/w7030836.
- Valour, F., A. Sénéchal, C. Dupieux, J. Karsenty, S. Lustig, P. Breton, A. Gleizal, L. Boussel, F. Laurent, E. Braun, et al. 2014. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 7:183–97. doi:https://doi.org/10.2147/IDR.S39601.
- Ventura, M., F. Turroni, G. A. Lugli, and D. Van Sinderen. 2014. Bifidobacteria and humans: Our special friends, from ecological to genomics perspectives. J. Sci. Food Agric. 94:163–68. doi:https://doi.org/10.1002/jsfa.6356.
- Vingataramin, L., and E. H. Frost. 2015. A single protocol for extraction of gDNA from bacteria and yeast. Biotechniques 58:120–25. doi:https://doi.org/10.2144/000114263.
- White, J. K., J. L. Nielsen, and A. M. Madsen. 2019. Microbial species and biodiversity in settling dust within and between pig farms. Environ. Res. 171:558–67. doi:https://doi.org/10.1016/j.envres.2019.01.008.
- Zaleski, K. J., K. L. Josephson, C. P. Gerba, and I. L. Pepper. 2005. Survival, growth, and regrowth of enteric indicator and pathogenic bacteria in biosolids, compost, soil, and land applied biosolids. J. Residuals Sci. Technol. 2 (1):49–63.