?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Recycling of valuable metals from spent catalysts in a green way is gaining extensive interest for economic and environment reasons. In this study, we developed novel hydrophobic deep eutectic solvents to extract Mo from spent catalysts. The hydrophobic DESs have been designed and synthesized by mixing one molar of the quaternary ammonium salt and two molars of various saturated fatty acids with different carbon chain lengths. The extraction ability and extraction mechanism of these DESs were studied, some factors influencing the extraction efficiency, including the structure of hydrogen bond acceptors and hydrogen bond donors, initial aqueous pH, reaction time and temperature, phase ratios were investigated. It is found that the synthesized hydrophobic DESs exhibit excellent extraction performance toward Mo, where the Mo distribution ratio is more than 2200 in the presence of other metals, corresponding to an extraction efficiency of 99% at optimal reaction conditions. This work reveals a distinct class of materials, guiding an effective and green way for spent catalyst treatment.
Implications: Novel hydrophobic deep eutectic solvents have been developed to extract Mo from spent catalysts, the synthesized hydrophobic DESs possess several advantages, such as green, low price, low toxicity, and biodegradability. It exhibits excellent extraction performance under an optimized extraction condition. This work reveals a distinct class of materials, guiding a promising way for green and economical utilization of spent catalysts.
Introduction
Spent catalysts have already been classified as hazardous wastes and are subjected to strict waste disposal regulations, since potential environmental problems may be triggered by improper treatment of these spent catalysts. While a large amount of valuable metals like Mo and Co are contained in spent catalysts, these metals are highly in demand in industrial production (Akcil et al. (Citation2015), Banda et al. (Citation2013), and Yang et al. (Citation2019)). Thus, re-utilization and recycling of Mo are attractive for economic benefits and environment protection (Li et al. (Citation2018), Zhang et al. (Citation2020)). To date, several strategies have been proposed to recover Mo from spent catalysts, including precipitation (Zeng and Cheng (Citation2009), Sahu, Agrawal, and Mishra (Citation2013), Valverde Júnior, Paulino, and Afonso (Citation2008)), ion exchange (Nguyen and Lee, Citation2015), as well as solvent extraction (Wang et al. (Citation2014), Lasheen et al. (Citation2014), Zeng and Cheng (Citation2010), Shah, Phadke, and Kocher (Citation1995)). Among these methods, solvent extraction is widely used because of its good separation capability and simple operation. Researchers have investigated the extraction of Mo with various of extractants, including amine extractants (Parhi et al. (Citation2011), Ning, Cao, and Zhang (Citation2010)) which are widely used and exhibit a good extraction performance, phosphorus extractants (Cheema et al. (Citation2018), Batchu et al. (Citation2017), Xia et al. (Citation2015), Padhan and Sarangi (Citation2014)), chelate extractants (Ning et al. (Citation2014), Park, Kim, and Parhi (Citation2010)), as well as ionic liquids (Onghena et al. (Citation2017), Wejman-Gibas et al. (Citation2016), Quijada-Maldonado, Torres, and Romero (Citation2016)).
However, previous extractants used for solvent extraction exhibit inherent disadvantages, such as flammability, high toxicity, non-biodegradability, and high cost. Therefore, green extractant with outstanding performance has become of great importance. At the beginning of this century, deep eutectic solvents (DESs) are known as a new generation of solvent that has emerged. They can be easily obtained by mixing together two or three components in combination through hydrogen-bonding interactions (Smith, Abbott, and Ryder, Citation2014). DESs are a kind of eutectic mixtures consisting of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), they have a lower melting point than the individual component (Abbott et al., Citation2004). Quaternary ammonium salts are one of the most widespread components used as HBAs, organic acids, alcohols and urea are often used as HBDs (Basaiahgari, Panda, and Gardas (Citation2018), Basaiahgari, Panda, and Gardas (Citation2017)). Generally, DESs possess the advantages of ionic liquids, and they also offer additional benefits such as green, low price, biocompatible, and simple synthetic process, since they do not require purification and waste disposal. Moreover, they pose several advantages like low toxicity, diversity, and biodegradable (Jiang et al., Citation2019). Because of these outstanding merits, DESs have already been applied to many applications, such as separation process, catalysis, organic synthesis, electrochemistry, and preparation of materials (Zhang et al., Citation2012).
In this regard, DESs stand for an appealing, potentially sustainable, and unexplored opportunity Phelps et al. (Citation2018). However, most of the DESs reported today are hydrophilic, limiting their application to separation processes. In recent years, hydrophobic DESs emerged and immediately attracted great attention (Zhang et al. (Citation2019a, Citation2019b)). Kroon et al. (Osch et al., Citation2015) presented a series of hydrophobic DESs for the first time and used to recover volatile fatty acids. They also investigated the effect on two-phase system; Cao, Wang, and Ez (Citation2017) studied bioactive compounds extraction by hydrophobic DESs; Tereshatov et al. (Boltoeva, Folden, and Tereshatov (Citation2016)) demonstrated quaternary ammonium and menthol-based DES extracted indium from aqueous solution; Schaeffer et al. (Nicolas et al., Citation2018) use terpene-based DESs to extract and separate copper and transition metals; trace pertechnetate extraction by hydrophobic DESs has been studied by Phelps et al. (Citation2018), quantitative (>99%) removal of tracer levels of 99mTcO4− from an aqueous phase can be realized. Shi et al. (Citation2020) studied Cr extraction with hydrophobic DESs, the extraction capacity can be reached as high as 66.7 mg/g, and the extraction efficiency can be greater than 90%.
Nevertheless, there are few studies that focus on the extraction of metals with hydrophobic DESs. In this present work, we study the extraction of Mo from a spent catalyst leaching solution using hydrophobic DESs for the first time. The spent catalyst leaching solution with multiple components was obtained from former research (Zhang et al., Citation2020). We use quaternary ammonium salt as HBAs due to its excellent metal extraction ability and good hydrophobic performance, while fatty acids are used as HBDs due to its high hydrophobic behavior in combination with its moderate ability to undergo hydrogen bonding interactions (Osch et al., Citation2015), where Mo extraction performance to a large extent depends on alkyl chain lengths. The effects of operation conditions, including initial aqueous pH, reaction time and temperature, phase ratios of hydrophobic DES to aqueous phase, were investigated, aiming to reach optimal ones.
Experimental section
Material
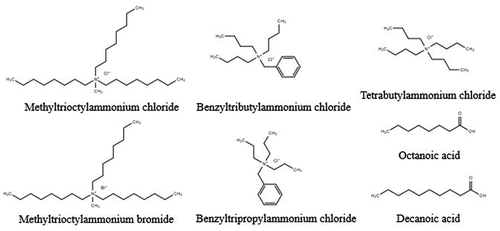
Quaternary ammonium salts were chosen as HBA and a series of fatty acids were chosen as HBD in this work. The structures of HBAs and HBDs are shown in . Tetrabutylammonium chloride, methyltrioctylammonium chloride, methyltrioctylammonium bromide, benzyltripropylammonium chloride, benzyltributylammonium chloride, heptanoic acid, octanoic acid, n-nonanoic acid, decanoic acid, undecanoic acid, and dodecanoic acid were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. The samples were used as received without further purification.
Experimental procedure
The synthesis of DESs was carried out in a round-bottomed flask with temperature control and magnetic stirrer. HBAs and HBDs were mixed at a certain molar ratio under the atmospheric pressure. The synthetic reaction was processed at a certain temperature, ranging from 70°C to 90°C about 2–3 hours, the combinations of HBA and HBD formed a clear liquid after cooling to room temperature. All DESs were kept under vacuum for another 24 hours to reduce the water content. Synthesized DESs were stored well, in order to avoid contact with air, moisture, and contaminants. Bruker Vertex 70 FTIR spectrometer was used for infrared spectra measurement. Thermo iCAP6300 was used for ICP-OES measurements to analyze the metal ions content.
The spent catalyst solution obtained from previous research shows that the main components of aqueous phase are Al, Mo, and Co with a weight fraction of 8.48‰, 5.65‰, 1.47‰, respectively. DES phase and aqueous phases with different volume ratio were mixed well in a round-bottomed flask under certain temperature and time. When equilibrium was reached, the mixture was then separated by a separating funnel. The content of metal ions was analyzed by ICP-OES. The metal content in the loaded organic phase was estimated by mass balance, and the distribution ratio (D) was calculated as follows:
The extraction efficiency (E%) was calculated as follows:
Results and discussion
The extraction and related properties of DES are closely determined by HBA and HBD compositions Basaiahgari, Panda, and Gardas (Citation2017), Jiang et al. (Citation2019), Zhang et al. (Citation2012). In order to study the influence of HBAs on Mo selective extraction performance, several DESs consisting of the same HBD and five different HBAs were synthesized. Specifically, DES A consists of a 1:2 molar ratio of tetrabutylammonium chloride with decanoic acid; DES B consists of a 1:2 molar ratio of methyltrioctylammonium chloride with decanoic acid; DES C consists of a 1:2 molar ratio of methyltrioctylammonium bromide with decanoic acid; DES D consists of a 1:2 molar ratio of benzyltripropylammonium chloride ([BTPA]Cl) with decanoic acid; DES E consists of a 1:2 molar ratio of benzyltributylammonium chloride ([BTBA]Cl) with decanoic acid. The Mo extraction process was carried out at 50°C and mixed into two phases well for a specified period of 10 minutes using a 1:1 volume ratio of hydrophobic DESs to aqueous phase; an aqueous phase contains three different metals of Al, Mo, and Co; and pH value was adjusted to 2. The distribution ratios of three metals were used to evaluate the selective extractive performance of different DESs. As shown in , DES D and DES E show excellent selective extraction ability of Mo, and Mo distribution ratios reach 2141 and 2253, respectively, almost all the Mo was extracted, while Al and Co distribution ratios remain at a very low level. Because Mo polynuclear anionic species possess a strong binding ability with quaternary ammonium salts, the Mo polynuclear anionic species are predominant in the pH range between 2 and 3; under these conditions, Co and Al exist in cationic form. Only Mo anionic species in the aqueous phase can be combined with DES to form immiscible complexes, and the complex can be easily dissolved in the organic phase. The Mo distribution ratios of the remaining DESs are in the order of DES B> DES C> DES A, and Al and Co distribution ratios of these three DESs are all higher than those of DES D and DES E.
Figure 2. Distribution ratios of Mo extracted by five hydrophobic DESs (DES A, DES B, DES C, DES D, DES E) after 10 min extraction at 50 °C using a 1:1 volume ratio of DES phase to aqueous phase at pH of 2
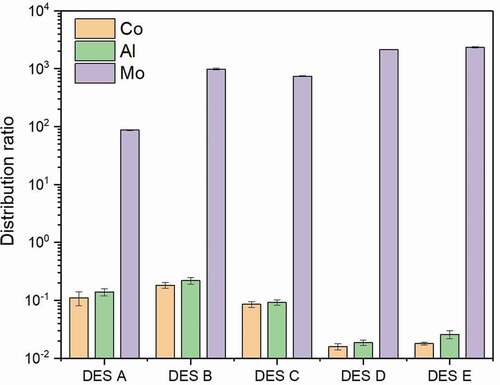
The results indicate that the Mo selective extraction ability is obviously affected by the contents of different HBAs. DES A shows a weak Mo extraction ability due to the solubility of tetrabutylammonium chloride in water, while DES B and DES C show an improved distribution ratio due to the unique methyltrioctylammonium structure, since the longer carbon chains of methyltrioctylammonium chloride and bromide endow higher hydrophobicity. Moreover, the methyl group in the quaternary ammonium salt also induces more steric hindrance for the water to contact with charged groups (Osch et al., Citation2015). On the other hand, benzyl groups are believed to enhance the selective ability, demonstrated by superior performance related to DES D and DES E, which are formed by quaternary ammonium salts with benzyl groups. It demonstrates excellent performance for the extraction of Mo. The operation conditions also have a substantial influence on Mo extraction. For further research, [BTPA]Cl and [BTBA]Cl are chosen to be HBA.
The alkyl chain length of the HBD component also appears to play a role in metal extraction ability Phelps et al. (Citation2018); thus, hydrophobic DESs consist of [BTPA]Cl or [BTBA]Cl as HBAs and a series of fatty acids with different lengths of carbon chain as HBDs were synthesized. In order to observe a clear variation of Mo extraction efficiency, we use a lower volume ratio of hydrophobic DESs to aqueous phase and extraction experiments were tested at 50°C for 10 min with a 1:20 volume ratio of DES phase to aqueous phase at pH of 2. In , it shows that increasing HBD alkyl chain length enhances Mo extraction ability of DES; however, a steady decrease in the Mo extraction efficiency is observed, starting from the 8-carbon and 10-carbon fatty acids for [BTBA]Cl and [BTPA]Cl, respectively. This is due to the decreasing stability and increasing viscosity of DES with increasing alkyl chain. Generally, DESs exhibit relatively high viscosity, which can be attributed to the extensive hydrogen bonding network inside them, causing lower mobility for the free species Taysun, Sert, and Atalay (Citation2017). The shorter chained fatty acids as HBD show higher water solubility, resulting in poor Mo extraction. In this case, we choose DES D consisting of [BTPA]Cl and decanoic acid, DES F consisting of [BTBA]Cl and octanoic acid for further research.
Figure 3. Extraction efficiency of Mo extracted by hydrophobic DESs consist of HBA([BTPA]Cl and [BTBA]Cl) and a series of fatty acids with different alkyl chain length after a 10 min extraction at 50°C using a 1:20 volume ratio of DES phase to aqueous phase at pH of 2
![Figure 3. Extraction efficiency of Mo extracted by hydrophobic DESs consist of HBA([BTPA]Cl and [BTBA]Cl) and a series of fatty acids with different alkyl chain length after a 10 min extraction at 50°C using a 1:20 volume ratio of DES phase to aqueous phase at pH of 2](/cms/asset/f5a18404-8287-420e-8775-954b5bc7453a/uawm_a_1937379_f0003_oc.jpg)
FT-IR is an effective way for analyzing the structural information and determining the functional groups present in synthesized DESs. As shown in , the spectra of DES D and DES F are familiar, they both show characteristic absorptions at 3471 and 2897 cm−1, which is attributed to OH stretching vibrations and aliphatic stretching vibrations, as is clear evidence of hydrogen bonding present in synthesized DESs Basaiahgari, Panda, and Gardas (Citation2018). There are several significant vibrations like C = C and C-C ring stretching, C-OH bending, C-O and C-O-C stretching Aromatic CH wagging are observed and presented in . The results of FT-IR spectra demonstrate that the functional groups of HBA and HBD are maintained after the synthesis process, and the structure of DESs is stabilized.
Table 1. IR spectra regions of synthesized DES
Figure 4. FT-IR spectra for the synthesized DESs (a)DES D consisting of [BTPA]Cl and decanoic acid; (b) DES F consisting of [BTBA]Cl and octanoic acid
![Figure 4. FT-IR spectra for the synthesized DESs (a)DES D consisting of [BTPA]Cl and decanoic acid; (b) DES F consisting of [BTBA]Cl and octanoic acid](/cms/asset/b2af43bb-a35e-4a84-9d3e-cba7ae4b7d1d/uawm_a_1937379_f0004_oc.jpg)
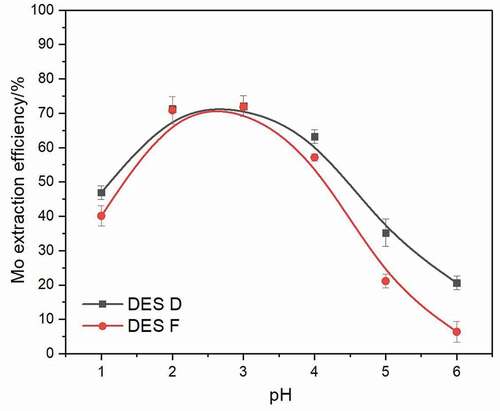
The pH of the aqueous phase plays a crucial role in the Mo extraction process, and it is essential to find out the influence of pH on the aqueous phase. Therefore, the Mo extraction experiments were carried out under aqueous phase pH ranging from 1 to 6, DES D and DES F were used as extractants, and the extraction reaction was performed at 50°C for 10 min with a 1:50 volume ratio of DES phase to aqueous phase. The variation of Mo extraction ability is shown in . It is obvious that the Mo extraction efficiency with DES D and DES F increases to 72.2% and 71.4%, respectively, when the aqueous phase pH raises from 1 to 3. As the aqueous phase pH increases to 6, Mo extraction efficiency decreases to 20.1% and 4.8%, respectively, this phenomenon can be demonstrated by the change of Mo species. Generally, Mo polynuclear anionic species possess a strong binding ability with quaternary ammonium salts, according to the distribution of Mo, the Mo polynuclear anionic species are predominant in the pH range between 2 and 3, while mononuclear anionic species are predominant when aqueous pH > 4, and mononuclear cationic species are predominant when aqueous pH < 19. The latter two have inferior binding trend with DESs, resulting in a declined extraction efficiency.
Figure 5. Extraction efficiency of Mo extracted by hydrophobic (DES D, DES F) after a 10 min extraction at 50°C using a 1:50 volume ratio of DES phase to aqueous phase under the pH range from 1 to 6
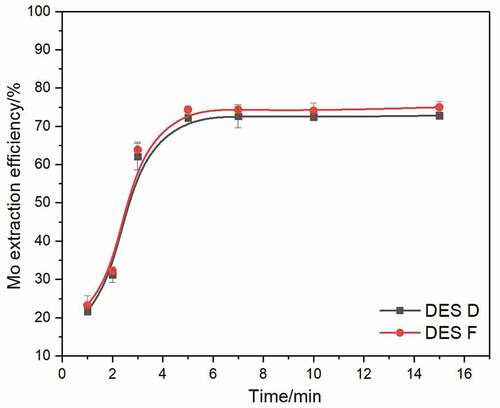
All the above experiments were conducted for a fixed time of 10 min to reach the equilibrium. However, for practical applications, fast extract reaction is required; hence, experiments were carried out with DES D and DES F to determine the equilibrium time needed. These two DESs were studied at 50°C using a 1:50 volume ratio of DES phase to the aqueous phase at a pH of 2. As shown in , DES D and DES F display a similar Mo extraction kinetics, and the extraction process of Mo with these two DESs is speedy, the reaction reaches the equilibrium within 6 minutes. Further increasing reaction time results in unchangeable efficiency. For extraction efficiency and economic benefit reasons, all further experiments were carried out within 6 min.
Figure 6. Extraction efficiency of Mo extracted by DES D and DES F after a various extraction time at 50°C using a 1:50 volume ratio of DES phase to aqueous phase at pH of 2
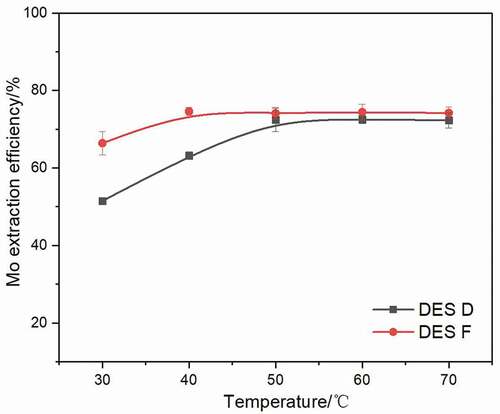
In order to determine the effect of temperature on Mo extraction, the extraction experiments were performed at various temperatures using a 1:50 volume ratio of DES phase to aqueous phase at pH of 2. As presented in , Mo extraction efficiencies of both DES D and DES F increase with the rising temperature. The Mo efficiency of DES D increases from 51.1% to 70.3% with temperature increases from 30°C to 50°C, while the efficiency of DES F reaches 71.5% when the temperature is 40°C. The Mo extraction efficiencies of DES D and DES F both stay stable above 50°C. However, DES F shows a better extraction ability when the temperature below 50°C. This is because the intermolecular interaction among DES is sensitive to temperature; with the temperature rising, the kinetic energy of molecules also increases, which is a benefit for metal extraction due to the faster ions movements Basaiahgari, Panda, and Gardas (Citation2018). Moreover, the viscosity and surface tension of DES will also reduce as the temperature rises, and it is known that low viscosity accelerates the mass transport process, promotes Mo transfer into DES phase, while low surface tension allows the growing interfacial area of DES, facilitating the jumping of metal component into the DES phase Yi et al. (Citation2019).
Figure 7. Extraction efficiency of Mo extracted by DES D and DES F after a 6 min extraction at various temperature using a 1:50 volume ratio of DES phase to aqueous phase at pH of 2
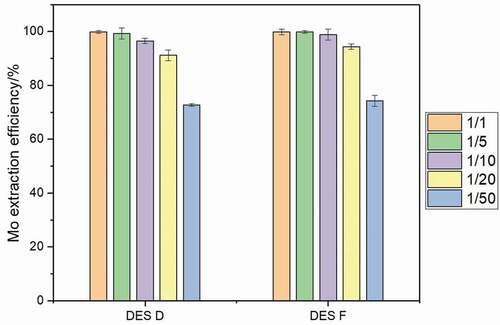
The influence of the phase ratio on the extraction performance was investigated after a 5 min extraction at 50°C at pH of 2. As can be seen in , two DES show similar tendency, and Mo extraction efficiency decreases with reduction in the volume ratio of DES phase to aqueous phase. Mo can be almost completely extracted by two DES with a volume ratio of 1:1 and 1:5; when the volume ratio is below 1:10, the amount of DES is not enough to extract all the Mo, the extraction efficiency decreases rapidly with reduction in volume ratio, and the influence of phase ratio on extraction efficiency is dependent on the extraction capacity of DES. According to the extraction results, the synthesized DESs show good Mo extraction performance with a wide range of volume ratio from 1:1 to 1:10, these DESs possess an excellent Mo extraction ability. Considering extraction efficiency and economic benefits, the 1:10 volume ratio is chosen as a suitable ratio.
Conclusion
We present a method to extract transition metal Mo from spent catalyst leaching solution with hydrophobic DES. This Mo extraction method has several attractive characteristics, including high extraction efficiency, excellent Mo selectivity, low temperature, and short reaction time. The interaction between Mo and hydrophobic DES is stronger than other metals, promoting the selective extraction process. The consistency of DES has an evident influence on Mo extraction. Among the synthesized DESs, a 1:2 molar ratio of [BTBA]Cl and octanoic acid demonstrates excellent performance for the extraction and separation of Mo. The operation conditions also have a substantial influence on Mo extraction, and the optimal result was obtained under 6 min extraction at 50°C with a 1:10 volume ratio of DES phase to aqueous phase at pH of 2. These conditions result in a Mo extraction efficiency of 98.8%, where other metals are barely extracted in the meantime. The synthesized DES in this work can realize efficient Mo extraction from spent catalyst leaching solution, it provides a new way for green and economical utilization of spent catalysts.
Additional information
Funding
Notes on contributors
Menglei Zhang
Menglei Zhang is a Ph.D. candidate in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Hao Song
Hao Song is a postdoctoral fellow in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Chenghang Zheng
Chenghang Zheng is a professor in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Shaojun Liu
Shaojun Liu is an associate professor in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Zhenglong Lin
Zhenglong Lin is a Master degree candidate in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Yi Liu
Yi Liu is a Master degree candidate in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Weihong Wu
Weihong Wu is an associate professor in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
Xiang Gao
Xiang Gao is a professor in State Key Laboratory of Clean Energy Utilization, State Environmental Protection Center for Coal-Fired Air Pollution Control, Zhejiang University.
References
- Akcil, A., F. Vegliò, F. Ferella, M. D. Okudan, and A. Tuncuk. 2015. A review of metal recovery from spent petroleum catalysts and ash. Waste Manage. 45 (Urban Mining):420–33. doi:https://doi.org/10.1016/j.wasman.2015.07.007.
- Abbott, A. P. D. Boothby, G. Capper, D. L Davies. 2004. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 126 (29):9142–47. doi:https://doi.org/10.1021/ja048266j.
- Banda, R., T. H. Nguyen, S. H. Sohn, and S. L. Man. 2013. Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction. Hydrometallurgy 133 (2):161–67. doi:https://doi.org/10.1016/j.hydromet.2013.01.006.
- Basaiahgari, A., S. Panda, and R. L. Gardas. 2017. Acoustic, volumetric, transport, optical and rheological properties of Benzyltripropylammonium based Deep Eutectic Solvents. Fluid Phase Equilib. 448:41–49. doi:https://doi.org/10.1016/j.fluid.2017.03.011.
- Basaiahgari, A., S. Panda, and R. L. Gardas. 2018. Effect of ethylene, diethylene, and triethylene glycols and glycerol on the physicochemical properties and phase behavior of benzyltrimethyl and benzyltributylammonium chloride based deep eutectic solvents at 283.15–343.15 K. J. Chem. Eng. Data 63 (7):2613–27. doi:https://doi.org/10.1021/acs.jced.8b00213.
- Batchu, N. K., T. V. Hoogerstraete, D. Banerjee, and K. Binnemans. 2017. Separation of rare-earth ions from ethylene glycol (+LiCl) solutions by non-aqueous solvent extraction with Cyanex 923. RSC Adv. 7 (72):45351–62. doi:https://doi.org/10.1039/C7RA09144C.
- Boltoeva, M. Y., C. M. Folden III, and E. E. Tereshatov. 2016. First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: Indium extraction from hydrochloric and oxalic acids. Green Chem. 18 (17):4616–22. doi:https://doi.org/10.1039/C5GC03080C.
- Cao, Y., F. Wang, and J. Ez. 2017. Well-designed hydrophobic deep eutectic solvents as green and efficient media for the extraction of artemisinin from Artemisia annua leaves. ACS Sustain. Chem. Eng. 5 (4):3270–78. doi:https://doi.org/10.1021/acssuschemeng.6b03092.
- Cheema, H. A., S. Ilyas, S. Masud, M. A. Muhsan, I. Mahmood, and J.-C. Lee. 2018. Selective recovery of rhenium from molybdenite flue-dust leach liquor using solvent extraction with TBP. Sep. Purif. Technol. 191:116–21. doi:https://doi.org/10.1016/j.seppur.2017.09.021.
- Jiang, W., H. Jia, H. Li, L. Zhu, R. Tao, W. Zhu, H. Li, and S. Dai. 2019. Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel. Green Chem. 21 (11):3074–80. doi:https://doi.org/10.1039/C9GC01004A.
- Lasheen, T. A., M. E. Ibrahim, H. B. Hassib, and A. S. Helal. 2014. Recovery of molybdenum from uranium bearing solution by solvent extraction with 5-Nonylsalicylaldoxime. Hydrometallurgy 146 (3):175–82. doi:https://doi.org/10.1016/j.hydromet.2014.03.011.
- Li, Z., G. Zhang, W. Guan, L. Zeng, L. Xiao, Q. Li, Z. Cao, and X. Lu. 2018. Separation of tungsten from molybdate using solvent extraction with primary amine N1923. Hydrometallurgy 175:203–07. doi:https://doi.org/10.1016/j.hydromet.2017.10.018.
- Nguyen, T. H., and M. S. Lee. 2015. Separation of molybdenum(VI) and tungsten(VI) from sulfuric acid solution by ion exchange with TEVA resin. Sep. Sci. Technol. 50 (13):2060–65.
- Nicolas, S., M. Martins, S. A. N. Catarina, S. O. P. Pinho, and J. A. P. Coutinho. 2018. Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem. Commun. 54 (58):8104–07. doi:https://doi.org/10.1039/C8CC04152K.
- Ning, P., H. Cao, and Y. Zhang. 2010. Selective extraction and deep removal of tungsten from sodium molybdate solution by primary amine N1923. Sep. Purif. Technol. 70 (1):27–33. doi:https://doi.org/10.1016/j.seppur.2009.08.006.
- Ning, P., X. Lin, H. Cao, and Y. Zhang. 2014. Selective extraction and deep separation of V(V) and Cr(VI) in the leaching solution of chromium-bearing vanadium slag with primary amine LK-N21. Sep. Purif. Technol. 137 (nov):109–15. doi:https://doi.org/10.1016/j.seppur.2014.08.033.
- Onghena, B., S. Valgaeren, T. Vander Hoogerstraete, and K. Binnemans. 2017. Cobalt (II)/nickel (II) separation from sulfate media by solvent extraction with an undiluted quaternary phosphonium ionic liquid. RSC Adv. 7 (57):35992–99. doi:https://doi.org/10.1039/C7RA04753C.
- Osch, D. J. G. P. V., L. F. Zubeir, A. V. D. Bruinhorst, M. A. A. Rocha, and M. C. Kroon. 2015. Hydrophobic deep eutectic solvents: Water-immiscible extractants. Green Chem. 17 (9):4518–21. doi:https://doi.org/10.1039/C5GC01451D.
- Padhan, E., and K. Sarangi. 2014. Separation of molybdenum and cobalt from spent catalyst using Cyanex 272 and Cyanex 301. Int. J. Min. Proc. 127 (2):52–61. doi:https://doi.org/10.1016/j.minpro.2014.01.003.
- Parhi, P. K., K. H. Park, H. I. Kim, and J. T. Park. 2011. Recovery of molybdenum from the sea nodule leach liquor by solvent extraction using Alamine 304-I. Hydrometallurgy 105 (3):195–200. doi:https://doi.org/10.1016/j.hydromet.2010.09.004.
- Park, K.-H., H.-I. Kim, and P. K. Parhi. 2010. Recovery of molybdenum from spent catalyst leach solutions by solvent extraction with LIX 84-I. Sep. Purif. Technol. 74 (3):294–99. doi:https://doi.org/10.1016/j.seppur.2010.06.018.
- Phelps, T. E., N. Bhawawet, S. S. Jurisson, and G. A. Baker. 2018. Efficient and selective extraction of 99mTcO4- from aqueous media using hydrophobic deep eutectic solvents. ACS Sustain. Chem. Eng. 6 (11):13656–61. doi:https://doi.org/10.1021/acssuschemeng.8b03950.
- Quijada-Maldonado, E., M. J. Torres, and J. Romero. 2016. Solvent extraction of Molybdenum (VI) from aqueous solution using ionic liquids as diluents. Sep. Purif. Technol. 200:200–06.
- Sahu, K. K., A. Agrawal, and D. Mishra. 2013. Hazardous waste to materials: Recovery of molybdenum and vanadium from acidic leach liquor of spent hydroprocessing catalyst using alamine 308. J. Environ. Manage. 125:68–73. doi:https://doi.org/10.1016/j.jenvman.2013.03.032.
- Shah, D. B., A. V. Phadke, and W. M. Kocher. 1995. Lead removal from foundry waste by solvent extraction. J. Air Waste Manage. Assoc. 45 (3):150–55. doi:https://doi.org/10.1080/10473289.1995.10467354.
- Shi, Y., D. Xiong, Y. Zhao, T. Li, K. Zhang, and J. Fan. 2020. Highly efficient extraction/separation of Cr (VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 241:125082. doi:https://doi.org/10.1016/j.chemosphere.2019.125082.
- Smith, E. L., A. P. Abbott, and K. S. Ryder. 2014. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 114 (21):11060–82. doi:https://doi.org/10.1021/cr300162p.
- Taysun, M. B., E. Sert, and F. S. Atalay. 2017. Effect of hydrogen bond donor on the physical properties of benzyltriethylammonium chloride based deep eutectic solvents and their usage in 2-ethyl-hexyl acetate synthesis as a catalyst. J. Chem. Eng. Data 62 (4):1173–81. doi:https://doi.org/10.1021/acs.jced.6b00486.
- Valverde Júnior, I. M., J. F. Paulino, and J. C. Afonso. 2008. Hydrometallurgical route to recover molybdenum, nickel, cobalt and aluminum from spent hydrotreating catalysts in sulphuric acid medium. J. Hazard. Mater. 160 (2):310–17. doi:https://doi.org/10.1016/j.jhazmat.2008.03.003.
- Wang, M. Y., X. W. Wang, C. J. Jiang, and F. Chao. 2014. Solvent extraction of molybdenum from acidic leach solution of Ni-Mo ore. Rare Metals 33 (1):107–10. doi:https://doi.org/10.1007/s12598-013-0061-x.
- Wejman-Gibas, K., K. Wieszczycka, A. Wojciechowska, K. Ochromowicz, and P. Pohl. 2016. Extraction of molybdenum(VI) from sulfate media by 3-pyridineketoxime and its quaternary salts. Sep. Purif. Technol. 158:71–79. doi:https://doi.org/10.1016/j.seppur.2015.11.037.
- Xia, Y., L. Xiao, C. Xiao, and L. Zeng. 2015. Direct solvent extraction of molybdenum(VI) from sulfuric acid leach solutions using PC-88A. Hydrometallurgy 158:114–18. doi:https://doi.org/10.1016/j.hydromet.2015.10.016.
- Yang, Z., P. Ji, Q. Li, Y. Jiang, C. Zheng, Y. Wang, X. Gao, and R. Lin. 2019. Comprehensive understanding of SO3 effects on synergies among air pollution control devices in ultra-low emission power plants burning high-sulfur coal. J. Clean. Prod. 239:118096. doi:https://doi.org/10.1016/j.jclepro.2019.118096.
- Yi, L., J. Feng, W. Li, and Z. Luo. 2019. High-Performance Separation of Phenolic Compounds from Coal-Based Liquid Oil by Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 7 (8):7777–83. doi:https://doi.org/10.1021/acssuschemeng.8b06734.
- Zeng, L., and C. Y. Cheng. 2009. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part II: Separation and purification. Hydrometallurgy 98 (1):10–20. doi:https://doi.org/10.1016/j.hydromet.2009.03.012.
- Zeng, L., and C. Y. Cheng. 2010. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction. Hydrometallurgy 101 (3):141–47. doi:https://doi.org/10.1016/j.hydromet.2009.12.008.
- Zhang, K., S. Li, C. Liu, Q. Wang, Y. Wang, and J. Fan. 2019a. A hydrophobic deep eutectic solvent‐based vortex‐assisted dispersive liquid–liquid microextraction combined with HPLC for the determination of nitrite in water and biological samples. J. Sep. Science. 42 (2):574–81. doi:https://doi.org/10.1002/jssc.201800921.
- Zhang, K., C. Liu, S. Li, and J. Fan. 2019b. A hydrophobic deep eutectic solvent based vortex-assisted liquid-liquid microextraction for the determination of formaldehyde from biological and indoor air samples by high performance liquid chromatography. J. Chromatogr. A 1589:39–46. doi:https://doi.org/10.1016/j.chroma.2018.12.063.
- Zhang, M., H. Song, C. Zheng, Z. Lin, Y. Liu, W. Wu, and X. Gao. 2020. Highly efficient recovery of molybdenum from spent catalyst by an optimized process. J. Air Waste Manage. Assoc. 70 (10):971–79. doi:https://doi.org/10.1080/10962247.2020.1792377.
- Zhang, Q., D. O. V. Karine, S. Royer, and F. O. Jérome. 2012. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 41 (21):7108–46. doi:https://doi.org/10.1039/c2cs35178a.