ABSTRACT
Both natural and anthropogenic fugitive dust can cause serious hazards to the environment and human health. In this study, the development of biodegradable dust suppressants and their environmental impacts were evaluated. Biodegradable dust suppressants were prepared using various biomass-based polymeric materials such as crude glycerol (a by-product of biodiesel manufacturing), biodiesel, palm oil, cooking oil, seaweed mixtures, wakame (Undaria pinnatifida), and red algae. The results of wind-tunnel tests with Korean standard sand demonstrated that spraying diluted mixtures of crude glycerol and biomass materials can significantly reduce the generation of dust. The optimal molar mixing ratio of crude glycerol and the biomass materials was 1:1, and the optimal dilution concentration was determined to be 100 times for the mixture of crude glycerol and biodiesel, palm oil, and cooking oil and 50 times for the mixture of crude glycerol with a seaweed mixture, wakame, and red algae. The suppression ability was 83.4%, 60.4%, 99.5%, and 98.1% for the mixtures of glycerol with soybean oil, palm oil, wakame, and red algae, respectively. The mixtures of glycerol plus wakame or red algae were the most efficient suppressants; they also have substantial biodegradability. Our results suggest that the mixture of crude glycerol with the various oils or the seaweeds may be a promising option to develop nontoxic biodegradable dust suppressants.
Implications: Since the early 2010s, anthropogenic fugitive dust from industrial activities has become a serious environmental issue due to its serious hazards to the environment and human health in South Korea. The origin and responsibility of the dusts is still disputable to prepare appropriate actions to take, which could be solved by scientific collaboration with surrounding countries. Regardless, domestic efforts to reduce the generation of fine dust from various sources should also be made. So far, several dust suppressants (mostly salts) were made and used for field application. However, due to their toxic effects, it is necessary to develop a new eco-friendly suppressant that can be biodegraded in the soil and that is not hazardous to human health or the environment. In this study, we try to develop an eco-friendly dust suppressant with low toxicity, to evaluate various potential dust suppressants, and to propose promising candidate products for commercialization and mass production. Ingredients and by-products of biodiesel production, marine biomass, and commercial vegetable oils were selected for the synthesis of suppressants. The optimal mixing ratio was determined, and the suppression ability was evaluated via wind tunnel tests. Considering biodegradability, the most effective suppressants were determined.
Introduction
Fugitive dust is a serious environmental issue that can arise from the mechanical disturbance of soils, which injects fine particles into the air. Atmospheric particulate matter can contain various metals, depending on natural or anthropogenic factors, which are harmful to the human body. These suspended particles have been shown to constitute a large fraction of PM10 (particles with aerodynamic diameters less than 10 micrometers) in many urban and non-urban areas (Schins et al. Citation2004; Schwarze et al. Citation2007). These fine dusts are generated from various sources, such as roads, construction sites, combustion processes, and yellow dust (incoming from China and Mongolia), and may cause harm to humans in the short or long term. In particular, the fugitive dust generated from construction sites near residential areas needs to be controlled immediately, because it has a significant impact on human health (Gillies et al. Citation2005). A 2008 study found that PM10 generated by the erosion of road pavement by studded tires provoked inflammatory responses in the cells of human bodies as potent as the response caused by diesel particles (Gustafsson et al. Citation2008). Severe health effects that have been linked to long-term exposure to moderate concentrations of PM10 include a significant reduction in the average life expectancy of a population by several months (Abbey et al. Citation1999).
Various methods have been employed to suppress the dust, including street washing with water, street sweeping, and spreading chemicals as suppressants. Street washing with water has been considered by several studies as a method of reducing the mobility of dust deposited on street surfaces (Amato et al. Citation2010). However, watering has a short restraint time due to drying and is limited in its suppression ability against mud-like sticky particles. It also has the disadvantage of the secondary pollution arising from the spent water. One study reviewed more than 60 commercial suppressants in the categories of salts, asphalt/petroleum emulsions, tree resin, and organic emulsions, lignin sulfonates, polymers, fibers, and mulches (Gillies et al. Citation1999). According to other previous studies (Chang, Hwang, and Chou Citation2003; Kirk Citation2018), compared with watering, applying a general dust reducer showed superior efficiency in dust suppression over a longer period. Salts and brines are the most common type of dust suppressants used. It has been reported that 75–80% of all dust suppressants used were chloride salts and salt brine products, 5–10% were lignin sulfonates, and 10–15% were petroleum-based products (Travnik Citation1991). Calcium chloride (CaCl2) and magnesium chloride (MgCl2) are commonly used as dust suppressants.
The known interactions of salt with the environment include its tendency to easily dissolve in water, and its rapid mobility through soils (Piechota et al. Citation2004). In the area near the application of salts, there have been negative impacts on the growth of fruit trees (Neill Citation2004), and alterations in the plant nutrition due to increases in the osmotic pressure of soils (Addo, Sanders, and Chenard Citation2004). Chloride concentrations as low as 40 mg/L are toxic to trout, and concentrations up to 10,000 mg/L are toxic to other fish species (Foley, Cropley, and Giummarra Citation1996; Golden Citation1991). High levels of lignin sulfonate in water have high coloring effects, increase biochemical oxygen demand, reduce biological activity, and retard fish growth (Addo, Sanders, and Chenard Citation2004; Golden Citation1991; Heffner Citation1997; Raabe Citation1968). It appears that the environmental impacts of dust suppressants are determined by their chemical composition, application rates, and interactions with other environmental components. Potential environmental impacts include the following: surface and groundwater quality deterioration, soil contamination, toxicity to soil and water biota, toxicity to humans during and after application, air pollution, accumulation in soils, changes in hydrologic characteristics of the soils, and impacts on native flora and fauna populations (Gillies et al. Citation1999). Therefore, it is imperative to develop a new dust suppressant that can be biodegraded in the soil and that is not hazardous to human health or the environment when blown in the wind or absorbed into a body of water.
Biomass-based materials, such as lignocellulose or organic ligands are important raw materials for making functional materials and applying them in many applications (Dutta et al. Citation2017; Liao et al. Citation2020; Liao, Matsagar, and Wu Citation2018). Among them, glycerol is an attractive material for dust suppressants due to its high viscosity. Glycerol is a principal by-product of biodiesel production. For each gallon of biodiesel produced, approximately 0.3 kg of crude glycerol is generated. Due to impurity, the produced crude glycerol has little value (Thompson and He Citation2006). Instead, an effort has been made to investigate the crude glycerol for a dust suppressant by developing suppressing materials including crude glycerol, natural and synthetic polymers, and their mixtures of surfactants and organic solvents (Grogan Citation2009; Kinast Citation2003; Ogzewalla Citation2009; Rath and Verrall Citation2008; Talamoni Citation2010). The mixture of crude glycerol, water, sodium chloride, and fatty acid methyl ester (FAME) showed the excellent performance of dust control in field application (Kinast Citation2003). By using borate and boric acid as cross-linked agents, the mixture of glycerol, surfactants, and starch also successfully prevented dust generation in treated roads (Rath and Verrall Citation2008). Grogan (Citation2009) revealed that the mixture of polyhydric alcohols (including glycerin) with acrylic compounds and polyhydric esters effectively reduced the generation of dust by binding particles to the stationary structure. Ogzewalla (Citation2009) suggested polymeric derivatives synthesized from glycerol and polybasic acid. Compared with other commercial dust suppressants, the derivatives showed the greatest performance in the reduction of dust.
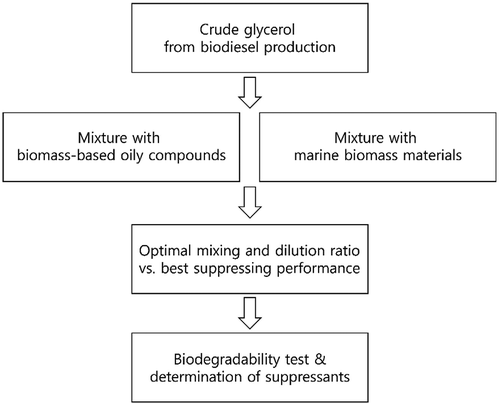
In this study, the application of crude glycerol from biodiesel production for dust suppressants was investigated using various types of biomass materials. Glycerol is reported to be readily degraded in the natural environment and easily stored over a long period (Fountoulakis and Manios Citation2009; Yan and Hoekman Citation2012), which may be advantageous for dust suppressants. It has been hypothesized that the combination of high viscous glycerol and readily biodegradable oily compounds may perform well as long-lasting, high-performance suppressants. Therefore, this study aims to develop a biodegradable dust suppressant with low toxicity, evaluate various potential dust suppressants, and propose promising candidate products for commercialization and mass production. By-products of biodiesel production (as well as biodiesel ingredients themselves), various types of marine biomasses, and commercial vegetable oils were selected for the preparation of suppressants. The optimal mixing ratio was determined, and the suppression ability was evaluated via wind tunnel tests. Considering biodegradability, the most effective suppressants were determined ().
Materials and methods
Materials
Potassium permanganate (KMnO4, 99%) was purchased from Daejung Chemical (Gyeonggi, South Korea). Sodium oxalate (Na2C2O4, 99%) and sulfuric acid (H+ SO4, 95%) were purchased from OCI (Seoul, South Korea). Silver sulfate (Ag2SO4, 99.5%) was provided by Kojima Chemicals (Aichi, Japan). PolySeed® BOD seed inoculum capsules, containing a minimum of 100 mg of specialized microbial cultures were purchased from Hach (Loveland, CO, USA). The raw materials crude glycerol, biodiesel, and palm oil were supplied through JC Chemical (Ulsan, South Korea), a company that develops and produces alternative fuels such as biodiesel to be added to diesel. The color of crude glycerol was dark yellow, and the pH was 10.0. Chemical compositions of crude glycerol were glycerol (82.5%), water (0.04%), ash (2.64%), matter organic none-glycerol (MONG, 14.52%), methanol (0.3%). Chemical compositions of biodiesel were FAME (98.4%), ash (0.001%), total glycerol (0.181%), monoglyceride (0.588%), diglyceride (0.063%), triglyceride (0.038%), and methanol (<0.01%). Palm oil is composed of fatty acid (99.8%), acid (0.18%), and water (0.02%). All compositions were provided by JC chemical. Crude biodiesel and palm oil were selected as possible oily compounds due to their low viscosity and considerable biodegradability. The kinematic viscosity values of crude glycerol and biodiesel were 42.4 and 3.9 mm2/s, respectively. Among various types of cooking oil, the most commonly used soybean oil was also obtained from a grocery store (Ottugi Co., Gyeonggi, South Korea). The molar mass of crude glycerol, palm oil, biodiesel, and soybean oil is 92, 256, 270, and 874 g/mol, respectively (Hong and Hong Citation2007). The seaweed mixture used is a washed mixture of various seaweeds obtained from the East Sea (Sea of Japan) shore. Wakame (Undaria pinnatifida) is a species of edible seaweed and a type of marine algae and is easily purchased from grocery stores in South Korea. Red algae powder is an ingredient in desserts throughout Asia and is a substitute for gelatin. It was also purchased from a grocery store.
Preparation of dust suppressant
Crude glycerol exists in a solid-state at room temperature; therefore, it was heated in an oven at 120°C for 30 min. To prepare 1 M solution, in a 1 L volumetric flask, approximately 200 ml of deionized (DI) water and 92 g of pre-heated crude glycerol were introduced and vigorously stirred. After 1 L of the solution was made by adding DI water to the volumetric line, the 1 M glycerol solution was stirred at 180 rpm for 1 h. The solution was mustard-yellowish in color and completely miscible. Pre-heating was not required for palm oil, biodiesel, and soybean oil, as they are liquid at room temperature. The 0.5, 1, 2, and 3 M solutions were prepared in the same way as the crude glycerol, according to their molar mass. It was observed that the prepared solutions were immiscible; the oil and water components were divided into layers. Therefore, 20 g of sodium dodecyl sulfate (SDS) surfactant was added and stirred at 180 rpm for 3 h to make the solutions completely miscible. To find the optimum mixing ratio of suppressants, crude glycerol and palm oil (or biodiesel) were mixed with ratios ranging from 1:0.5 to 1:3. The results indicated that the optimal molar ratio of glycerol-to-palm oil (or biodiesel) was 1:1, which was maintained between glycerol and biomass/oily materials throughout the experiments. All mixtures were shaken at 180 rpm for 3 h and stored in a bottle for wind tunnel tests.
To prepare the seaweed mixture and wakame solutions, the two types of seaweed were dried at room temperature for 24 h. Then, the dried seaweeds were ground and passed through a sieve (#4 mesh, <4.75 mm). Then, 50 g of the ground seaweeds were added to 1 L of DI water, and boiled for 4 to 5 h, with constant stirring at 180 rpm. The solutions became visibly slimy and clammy. After cooling at room temperature, the solutions were diluted to 10 g/L with DI water. Because the prepared solutions included suspended materials, they were filtered through a glass fiber filter (Type B) (Millipore, Burlington, MA, USA) and the filtrates were stored in a refrigerator. To prepare the red algae solution, 10 g of the powder was added to 1 L of DI water. The mixture was heated at 100°C with constant stirring (180 rpm) for 10 min and cooled to room temperature. After filtration with the glass fiber filter, the algae solution was stored for further experiments. The addition of linkers (oily compounds and marine biomass materials) did not change the stability of the dust suppressants at room temperature for at least one week. As long as stored in the refrigerator, it was stable for more than a month.
Wind tunnel tests
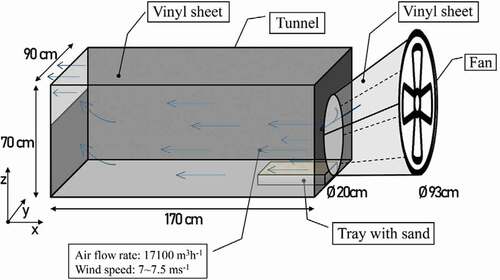
A wind tunnel test was designed to measure whether the dust suppressant candidate samples could sufficiently suppress scattering dust generation. A laboratory-scale tunnel test model was designed (). According to the Big Spring testing tunnel model suggested by (Fryreat Citation1971), a rectangular prism-tunnel was made, using a wooden table with a size of 1.7 m (length) × 0.7 m (height) × 0.9 m (width) as a structural frame, and its end side was covered with a vinyl sheet. A commercial drum-type fan (Hans Electronics, Busan, South Korea) was installed as the blower and placed at one end of the tunnel. The wind speed was maintained at 7.5 m/s by measuring with an anemometer (Testo 410i, Lenzkirch, Germany). As a scattering dust material, Jumunjin sand, (Korean standard sand) was chosen because preliminary tests with fine particles such as clay and silt did not show any significant difference between suppressants and water. Jumunjin sand is classified as poorly graded sand (SP) with a specific gravity of 2.65 and mean grain size (D50) of 0.52 mm, and with a uniformity coefficient (Cu) of 1.94 (Chang et al. Citation2015). The tunnel test procedures were as follows. First, an aluminum tray (area 0.16 m2) was filled up with the pre-weighed Korean standard sand. Then, the sand-filled tray was placed in the tunnel, in front of the fan, and experiments were started by turning on the fan to mimic wind blowing. After a pre-determined period (15, 30, 45, 60, and 120 min), the weight of the remaining sand was recorded, which was calculated by subtracting the weight of the sand remaining in the tray from the initial weight of sand. To determine the effect of the suppressant, 1 kg/m3 of the suppressant was spread evenly on the sand. Considering the total volume of sand (including pore volume), 1 kg of suppressant was applied to 1 m3 of sand. As a control experiment, identical experiments were conducted with and without water. Preliminary tests using DI water without suppressant showed no weight loss in the wind tunnel tests (without drying periods). When the suppressant solution-spread sands were dried at room temperature for 3 days, clear distinctions among the suppressants were observed in the wind tunnel tests. Since further drying time did not increase the effects, the 3-day drying step was inserted between suppressant adding and wind tunnel testing. All tests were conducted in duplicate.
Results and discussion
The mixture of glycerol and oily compounds
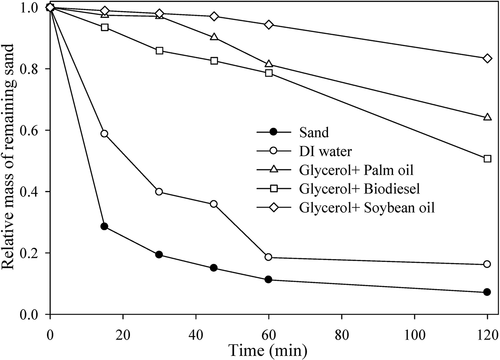
The control test results are shown in . For the sand control experiment, after 15 min, over 71% of the sand mass was lost, and after 2 h, more than 90% of the sand mass was lost. When DI water was sprayed, the suppression effect was evident at 1 h, with only 3.60% of sand mass loss. However, at 2 h, the water started to evaporate, and the loss of sand mass increased to 60.9%. For a 72 h drying time after DI water was applied, 41.3% and 83.8% of the sand mass was lost in 15 min and 2 h, respectively. It was confirmed that the loss of sand mass increased as the water dried. The wind tunnel tests were conducted using the mixtures of crude glycerol plus palm oil, biodiesel, or soybean oil, to evaluate whether they could act as dust suppressants. As a control test, a pre-prepared 1 M glycerol solution was diluted 10 and 100 times and sprayed onto sands. In 2 h, the sample sprayed with 0.1 M glycerol solution lost approximately 19.8% of its initial sand mass, and for the sample sprayed with 0.01 M glycerol solution, the mass loss was 45.4%, indicating a dust suppression ability more than two times higher than that of DI water alone (). Similarly, palm oil, biodiesel, and soybean oil were also diluted and applied to the sand, and their suppression results are shown in . All the 0.1 M diluted solutions had no loss of sand mass, and among the 0.01 M diluted solutions, soybean oil had the highest suppression ability (100% with 0.01 M soybean oil). All the solutions had a better suppression effect than DI water.
Figure 3. Relative remaining sand mass, with the Korean standard sand and deionized water, in the wind tunnel test
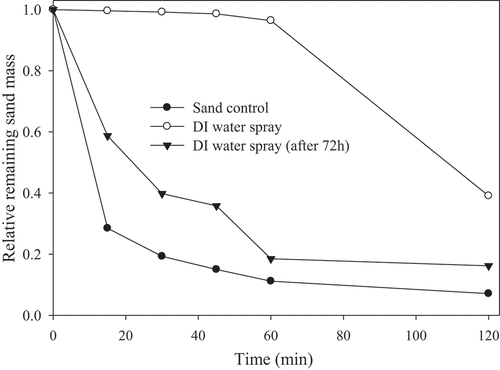
Figure 4. Relative remaining sand mass in the wind tunnel test, with: (a) crude glycerol control, and (b) biodiesel and palm oil sprayed without glycerol
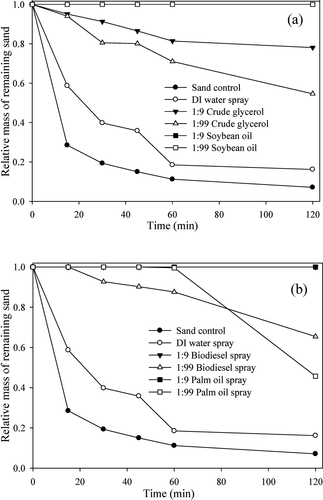
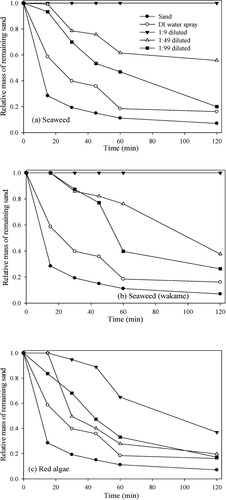
Two types of seaweed and red algae were used for the control experiment, to ensure each of the materials could act as dust suppressants. Pre-prepared 10 g/L solutions were diluted 10, 50, and 100 times and applied to the sand. The results of wind tunnel tests showed that all seaweed solutions had a suppression effect (). For the 10 times-diluted seaweed mixture and wakame samples, no loss of sand mass was detected. For the 50 times-diluted seaweed mixture, the loss of sand mass was 44.3%, (two times higher dust suppression ability than DI water). For the 50 and 100 times-diluted wakame samples, the loss of sand mass was 62.3% and 73.6%, respectively. The red algae showed lower suppression ability than 2 other seaweeds, but higher suppression than DI water (). All materials have shown better suppression ability than DI water. To obtain a stable suppressant with high suppression ability and biodegradability, the crude glycerol was mixed with other oily materials in a molar ratio of 1:1, and we then added 20 g/L surfactants as described in the experimental section. These mixtures were diluted by 10 (0.05 M) and 100 (0.005 M), and the dust suppression efficiency was evaluated with wind tunnel testing. The 0.05 M mixtures had no loss of sand mass. For the 0.005 M mixtures of glycerol plus palm oil, biodiesel, or soybean oil, the loss of sand mass at 2 h was 39.6%, 49.3%, and 16.6%, respectively (). The addition of palm oil and soybean oil to the glycerol enhanced the suppression ability of glycerol, but biodiesel did not have the same effect. Theoretically, viscosity is the main parameter to determine the ability to suppress dust. According to measured kinematic viscosity values of palm oil and soybean oil are 4.0 and 4.5 mm2/s (Kim et al. Citation2013), respectively. In the actual application for sands, suppressing performance with the mixture of glycerol and soybean oil was the better of the two. Although the mixture of glycerol and soybean oil was the most effective in suppression ability, due to the lowest biodegradability of the mixture of glycerol and palm oil (data will be shown and discussed later), the loss of sand mass by the 100 times-diluted mixture of glycerol and palm oil was used as a baseline for suppression ability.
The mixture of glycerol and seaweeds
The 10 g/L solutions of seaweed mixture, wakame, and red algae were prepared in advance, and each solution was mixed with a 1 M glycerol solution, in a ratio of 1:1, to create dust suppressant candidates. The mixed solutions were diluted 10, 50, and 100 times, and the wind tunnel tests were conducted. The 10 times-diluted mixtures had no loss of sand mass (data not shown). The loss of sand mass was 34.8% in 2 h for the 50 times-diluted samples of glycerol plus seaweed mixture, indicating higher suppression ability than the mixture of glycerol plus palm oil (). For the 50 times-diluted mixtures of glycerol plus wakame and glycerol plus red algae, the loss of sand mass was 0.51% and 1.90%, respectively (), and the suppression ability was 60 and 20 times higher, respectively than that of the mixture of glycerol plus palm oil. As explained above, the viscosity of seaweed is responsible for the enhancement of suppressing ability. According to a previous report (Bono et al. Citation2014), the kinematic viscosity of seaweed extract was more than 2000 mm2/s, much higher than those of palm oil (4.0 mm2/s). Thus, the increase in the stickiness of the mixture resulted in enhancing the suppression significantly. The addition of glycerol to the seaweed mixture, wakame, and red algae solutions resulted in a synergistic increase of suppression ability; the loss of sand mass decreased by 21.4%, 99.1%, and 97.6%, respectively, when the glycerol solution was included (). However, when the mixtures were diluted by 100 times, the suppression ability was relatively low. The loss of sand mass with the mixtures of glycerol plus seaweed mixture, wakame, and red algae was 65.9%, 73.6%, and 73.4%, respectively (). It is therefore suggested that the mixtures of glycerol plus wakame and glycerol plus red algae show the highest suppression ability and that the 50-times dilution may be optimal for the mixture of glycerol plus seaweeds. Finally, the suppressing performance by selected dust suppressants was compared with a commercial suppressant (MgCl2). Under identical conditions, 75.0 and 91.1% of the initial sand mass were lost with 1.0 and 0.1 M of MgCl2, clearly indicating that the mixture of glycerol and seaweeds showed excellent performance. It should be noted that in addition to viscosity, surface films, surface tension effects, and drying property may also be involved in the suppressing mechanisms. Additional studies may be needed to explore the relationship between the immobilization of dust and the properties of suppressants.
Drying time effect and biodegradability of the suppressants
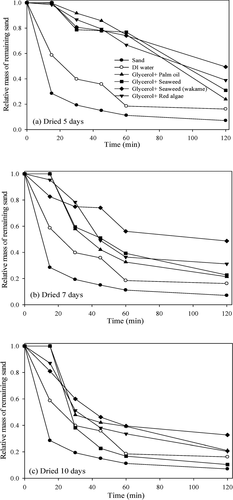
To determine the effect of drying time (between suppressor application and the wind tunnel test) on suppression ability, the drying time was increased from 3 days to 5, 7, and 10 days for the mixtures of glycerol plus palm oil, seaweed mixture, and wakame. As the drying time was extended, the ability to suppress the dust was reduced for all mixtures. After 7 days, the suppression ability of the mixture of glycerol plus palm oil was significantly reduced so it is near that of water (). After 10 days, its suppression effect was lower than that of DI water. The suppression ability of the other biomass-based candidates also decreased steadily. However, their suppression ability did not become less than that of water. To determine the environmental effects of the prepared dust suppressant samples, their biodegradability was evaluated via 5-day biochemical oxygen demand (BOD5) and chemical oxygen demand (COD) tests (). The BOD5 values of surfactant and glycerol were 2.00 ± 0.10 and 6.80 ± 0.50 mg/L, respectively, much lower than expected. The BOD5values for the mixtures of glycerol plus palm oil, biodiesel, and soybean oil were 17.0 ± 2.10, 21.1 ± 0.90, and 0.60 ± 0.30 mg/L, respectively. The mixtures of glycerol plus seaweed mixture, glycerol plus wakame, and glycerol plus red algae had BOD5 values of 32.4 ± 0.10, 35.6 ± 0.60, and 25.2 ± 0.30 mg/L respectively. As expected, marine biomass-derived materials showed higher BOD5 values than those of biodiesel and oily products. Considering BOD5 and COD values, the palm oil, seaweed mixture, and wakame had high biodegradability, with 0.51, 0.57, and 0.78 BOD5-to-COD ratios, respectively. In contrast, soybean oil’s BOD5 to COD ratio was 0.13, as low as that of glycerol (0.14). Due to its low BOD5 value, the addition of glycerol resulted in lower BOD5 values. However, the extremely low BOD5 value of the mixture of glycerol plus soybean oil could make it unfavorable as a suppressant. In contrast, the BOD5 to-COD ratio indicated that the mixture of glycerol and either wakame or red algae had good suppression efficiency and biodegradability ().
Table 1. Biodegradability of suppressants used in this study
Figure 8. Relative remaining sand mass in the wind tunnel test, with the mixtures of glycerol plus palm oil, glycerol plus seaweed mixture, glycerol plus wakame, and glycerol plus red algae, after drying for (a) 5 days, (b) 7 days, and (c) 10 days
It should be noted that the application of glycerol may have a negative effect on natural environments. Because the glycerol-based dust suppressants have high BOD values, the presence of glycerol in water can significantly deplete the oxygen content of water, resulting in suffocation of fish and other organisms (Yan and Hoekman Citation2012). Similar oxygen depletion problems can occur in wet soil (U.S. Epa Citation2008). Therefore, it is strongly necessary to monitor the concentrations of glycerol and dissolved oxygen in natural water in the vicinity of the field application area to assure the least effect of glycerol and other readily biodegradable ingredients in dust suppressants. The control of those compounds to minimize the oxygen depletion in water and soil remains to be examined.
Conclusion
This study was conducted to investigate the following biomass-based polymeric materials as dust suppressants: crude glycerol, biodiesel, palm oil, soybean oil, a seaweed mixture, wakame, and red algae. Using crude glycerol as a base material, potential biodegradable suppressants were prepared and tested. In accordance with the hypothesis, all of the polymeric materials had better suppression ability than DI water. Under the given conditions, the 100 times-diluted mixture of glycerol plus palm oil showed a loss of sand mass of 36.0% in 2 h. The 50 times-diluted mixture of glycerol plus the seaweed mixture showed a loss of sand mass of 34.8% in 2 h. The 50 times-diluted mixtures of glycerol plus wakame and glycerol plus red algae showed the highest suppression ability, with 0.51% and 1.90% loss, respectively. The suppressing ability of additives was greatly related to their viscosity values. According to the BOD5 values, the biodegradability of the suppressants using materials derived from marine biomass was higher than that of the suppressants prepared with oil materials or byproducts from biodiesel production processes. Both the glycerol plus wakame and the glycerol plus red algae suppressants showed good suppression ability and biodegradability. Our results suggest that the mixtures of glycerol plus either wakame or red algae may be good biodegradable suppressants for fugitive dust in urbanized and industrial areas.
Supplemental Material
Download MS Word (34.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Baigali Tsogt
Baigali Tsogt is a Ph.D. Student at the University of Ulsan, B.S. from National University of Mongolia, M.S. from University of Ulsan.
Seok-Young Oh
Seok-Young Oh is Professor at the University of Ulsan (2007-present), B.S. and M.S. from Seoul National University. Ph.D. from the University of Delaware.
References
- Abbey, D. E., N. Nishino, W. F. McDonnell, R. J. Burchette, S. F. Knutsen, L. W. Beeson, and J. X. Yang. 1999. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am. J. Respir. Crit. Care. Med. 159:373–82. doi:https://doi.org/10.1164/ajrccm.159.2.9806020.
- Addo, J. Q., T. G. Sanders, and M. Chenard. 2004. Road dust suppression: Effect on maintenance stability, safety and the environment, Phases 1-3. No. MPC Report No. 04-156, Colorado State University.
- Amato, F., X. Querol, C. Johansson, C. Nagl, and A. Alastuey. 2010. A review on the effectiveness of street sweeping, washing and dust suppressants as urban PM control methods. Sci. Total Environ. 408:3070–84. doi:https://doi.org/10.1016/j.scitotenv.2010.04.025.
- Bono, A., S. M. Anisuzzama, and O. W. Ding. 2014. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Eng. Sci. 26:3–9. https://doi.org/10.1016/j.jksues.2012.06.001
- Chang, I., A. K. Prasidhi, J. Im, and G. C. Cho. 2015. Soil strengthening using thermo-gelation biopolymers. Constr. Build. Mater. 77:430–38. doi:https://doi.org/10.1016/j.conbuildmat.2014.12.116.
- Chang, Y. M., J. S. Hwang, and C. M. Chou. 2003. Study on short-term prevention efficiencies of chemical binders to reduce fugitive dust. Environ. Eng. Sci. 20:265–80. doi:https://doi.org/10.1089/109287503322148546.
- Dutta, S., J. Kim, Y. Ide, J. H. Kim Md, S. A. Hossain, Y. Bando, Y. Yamuchi, and K. C. W. Wu. 2017. #d network of cellulose-based energy storage devices and related emerging applications. Mat. Horiz. 4:522–45. doi:https://doi.org/10.1039/C6MH00500D.
- Foley, G., S. Cropley, and G. Giummarra. 1996. Road dust control techniques - Evaluation of chemical dust suppressants’ performance. Vermont South, VIC: Australian Road Research Board.
- Fountoulakis, M. S., and T. Manios. 2009. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresources Technol. 100:3043–47. doi:https://doi.org/10.1016/j.biortech.2009.01.016.
- Fryreat, D. W. 1971. Survival and growth of cotton plants damaged by windblown sand. Agron. J. 63:638–42. doi:https://doi.org/10.2134/agronj1971.00021962006300040038x.
- Gillies, J. A., J. G. Watson, C. F. Rogers, D. DuBois, J. C. Chow, R. Langston, and J. Sweet. 1999. Long-term efficiencies of dust suppressants to reduce PM10 emissions from unpaved roads. J. Air Waste Manage. 49:3–16. doi:https://doi.org/10.1080/10473289.1999.10463779.
- Gillies, J. A., V. Etyemezian, H. Kuhns, D. Nikolic, and D. A. Gillette. 2005. Effect of vehicle characteristics on unpaved road dust emissions. Atmos. Environ. 39:2341–47. doi:https://doi.org/10.1016/j.atmosenv.2004.05.064.
- Golden, B. J. 1991. Impact of magnesium chloride dust control product on the environment. In Proceedings of the 1991 Annual Conference of the Transportation Association of Canada, Winnipeg, Manitoba, September 15-19, 1991, Vol. 1.
- Grogan, R. 2009. Mixtures, compositions and methods of suing and preparing same. U.S. Patent Application No. 0269499 A1.
- Gustafsson, M., G. Blomqvist, A. Gudmundsson, A. Dahl, E. Swietlicki, M. Bohgard, J. Lindbom, and A. Ljungman. 2008. Properties and toxicological effects of particles from the interaction between tyres, road pavement and winter traction material. Sci. Total Environ. 393:226–40. doi:https://doi.org/10.1016/j.scitotenv.2007.12.030.
- Heffner, K. 1997. Water quality effects of three dust-abatement compounds. Eng. Field Notes 29:35–43.
- Hong, Y. K., and W. H. Hong. 2007. Biodiesel production technology and its fuel properties. Korean Chem. Eng. Res. 45:424–32.
- Kim, J. K., J. Y. Park, C. H. Jeon, K. I. Min, E. S. Yim, C. S. Jung, and J. H. Lee. 2013. Fuel properties of various biodiesels derived vegetable oil. J. Korean Oil Chem. Soc. 30:35–48. doi:https://doi.org/10.12925/jkocs.2013.30.1.035.
- Kinast, J. A. 2003. Production of biodiesels from multiple feedstocks and properties of biodiesels and biodiesel/ diesel blends. NREL/SR-510-31460, National Renewable Energy Laboratory.
- Kirk, T. K. 2018. Lignin biodegradation: Microbiology, chemistry, and potential applications. Boca Raton, FL: CRC Press.
- Liao, Y. T., B. M. Matsagar, and K. C. W. Wu. 2018. Metal-organic framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustainable Chem. Eng. 6:13628–43. doi:https://doi.org/10.1021/acssuschemeng.8b03683.
- Liao, Y. T., N. V. Chi, N. Ishiguro, A. P. Young, C. K. Tsung, and K. C. W. Wu. 2020. Engineering a homogeneous alloy-oxide interface derived from metal-organic frameworks for selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Appl. Catal. B: Environ. 270:118805. doi:https://doi.org/10.1016/j.apcatb.2020.118805.
- Neill, C. R. 2004. Guide to bridge hydraulics. 2nd ed. London, UK: Thomas Telford Publishing.
- Ogzewalla, M. 2009. Dust control of solid granular materials. U.S. Patent No. 0178452 A1.
- Piechota, T., J. Van Ea, J. Batista, K. Stave, and D. James. 2004. United States Environmental Protection Agency. EPA 600/R-04/031. Potential environmental impacts of dust suppressants: Avoiding another timesbeach. An Expert Panel Summary. Las Vegas, NV. May 30-31, 2002.
- Raabe, E. 1968. Biochemical oxygen demand and degradation of lignin in natural waters. J. Water Pollut. Control. Fed. 40:145–50.
- Rath, C., and A. P. Verrall. 2008. Method of dust abatement. U.S. Patent Application, No. 055290A1.
- Schins, R. P., J. H. Lightbody, P. J. Borm, T. Shi, K. Donaldson, and V. Stone. 2004. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharmacol. 195:1–11. doi:https://doi.org/10.1016/j.taap.2003.10.002.
- Schwarze, P. E., J. Øvrevik, R. B. Hetland, R. Becher, F. R. Cassee, M. Låg, M. Løvik, E. Dybing, and M. Refsnes. 2007. Importance of size and composition of particles for effects on cells in vitro. Inhalation Toxicol. 19:17–22. doi:https://doi.org/10.1080/08958370701490445.
- Talamoni, J. R. 2010. Dust suppressant composition. U.S. Patent No. 7658862 B2.
- Thompson, J. C., and B. B. He. 2006. Characterization of crude glycerol from biodiesel production from multiple feedstocks. App. Eng. Agric. 22:261–65. doi:https://doi.org/10.13031/2013.20272.
- Travnik, W. A. 1991. State of art dust suppressants/soil stabilizers. In Proceedings of the 42nd Annual Road Builders’ Clinic, Coeur D’Alene, Idaho, March 5–7, 1991.
- U.S. EPA. 2008. Environmental laws applicable to construction and operation of biodiesel production facilities. EPA-907-B-08-001, U.S. EPA, Washington, DC.
- Yan, W., and S. K. Hoekman. 2012. Dust suppression with glycerin from biodiesel production: A review. J. Environ. Prot. 3:218–24. doi:https://doi.org/10.4236/jep.2012.32027.