Introduction
The 2021 Critical Review (CR) on perfluorooctanoate (PFOA), kidney cancer, and testicular cancer (Bartell and Vieira Citation2021) provides an in-depth examination of evidence regarding sources, distribution and health effects for this family of persistent environmental contaminants. PFOA is one of the most widely used and best studied of the per- and polyfluoroalkyl substances (PFAS), a complex class of emerging contaminants with varying chemical properties, and rapidly changing production and use. Despite being phased out of production by major industries, PFOA is persistent in the environment, bioaccumulative in humans, and has been associated with adverse health effects including adenomas or carcinomas in some animal experiments and human studies. PFOA are ubiquitous and most people carry some body burdens of these compounds, therefore the questions of whether they pose a carcinogenic risk and how one determines the level of that risk while weighing the evidence of that risk with respect to its accuracy and applicability are important ones to address, especially since the carcinogenicity of PFOA has been reviewed previously by several different regulatory agencies and researchers, sometimes with contradictory conclusions. This critical review focuses on the potential cancer risks and the methods by which those risks can be quantified. In this review Bartell and Vieira take us through the steps to critically analyze peer reviewed epidemiological studies on PFOA and cancer, evaluate and convert exposures to a common scale based on average serum concentrations over time, and lay the groundwork to eventually derive meta-analytic human cancer risk slope factors. This review was tightly focused and drilled down into the topic with the framework of epidemiology. In this summary, our expert discussants will provide additional perspectives and information to provide more breadth. to the topic. Their appearance as coauthors of this commentary does not necessarily indicate their agreement with the opinions of other discussants. An online supplement contains additional information and illustrations related to this discussion.
John Watson and Judy Chow: Complementary reviews for PFAS
The environmental concerns of PFAS are broad, with a large range of PFAS compounds, human exposure pathways, and adverse health effects. Other review articles, several of which were published since the CR was finalized, are highlighted here. Each of these provides an extensive bibliography that can be used to “drill-down” to greater detail. Buck et al. (Citation2011) provide a comprehensive and understandable introduction to PFAS and a useful list of PFAS acronyms in current use has been compiled by the (ITRC, Citation2020). Sunderland et al. (Citation2019) construct a model of sources exposure pathways and identify diet, dust (mostly house dust), tap water, food packaging, inhalation, and dermal absorption pathways and show widely varying fractions of exposure, with diet being the largest contributor, followed by tap water. Ojo et al. (Citation2021) summarize health effects studies relating various PFAS compound exposures to developmental toxicity, neurotoxicity, reproductive toxicity, genotoxicity, immunotoxicity, cardiovascular toxicity, and endocrine disruption. The detailed analysis of these recent articles is presented, along with additional references in Supplement 1.
Michael Kleinman: PFOA and PFAS: toxicology and mechanistic considerations
Perfluorooctanoic acid (PFOA) and Perfluoroalkyl substances (PFAS), which are used in many commercial applications, are persistent in the environment, do not readily undergo chemical degradation or biotransformation, bioaccumulate and are found, not only in the serum of occupationally exposed workers, but also the blood and tissues of the general population (Kudo and Kawashima Citation2003; Sanchez Garcia et al. Citation2018) including children and adolescents (Duffek et al. Citation2020). Recent studies have suggested that the biological half-life of PFOA in humans is in the range of 2 to 4 years (Li et al. Citation2018a; Worley, Moore, and Tierney et al. Citation2017); in airport employees exposed to contaminated drinking water but then provided with clean water for 2 weeks, clearance was observed, with a general trend of increasing half-life with increasing chain length (Xu et al. Citation2020). PFOS, PFAS and metabolite compounds, detected in blood samples from North Carolina residents, were associated with releases from a fluorochemical manufacturing facility (Kotlarz et al. Citation2020).
PFOA is well-absorbed following oral and inhalation exposure, and to a lesser extent following dermal exposure. Once absorbed in the body, it distributes predominantly to the liver and plasma, and to a lesser extent the kidney and lungs (Kudo and Kawashima Citation2003). PFOA is excreted in both urine and feces. Biological half-life of PFOA is quite different between species and sexes and the difference is due mainly to the difference in renal clearance. In rats, renal clearance of PFOA is regulated by sex hormones, especially testosterone. PFOA is excreted into urine by active tubular secretion, and certain organic anion transporters are though to be responsible for the secretion. Renal clearance rates in humans were reduced compared to rats, which may be due to an absence of active excretion in human kidneys (Harada et al. Citation2005). Fecal excretion is somewhat important in the elimination of PFOA, but in humans renal clearance is more important (Gao et al. Citation2015). There is evidence that PFOA undergoes enterohepatic circulation resulting in reduced amounts of fecal excretion (Kudo and Kawashima Citation2003). Elucidation of the mechanisms of transport in biological systems leads to elimination of this chemical in the human body. PFOA can affect the kidneys and cause impaired clearance of uric acid and a positive association between increased serum PFOA and hyperuricemia and gout (accumulation of uric acid crystals in joints – often the big toe) (Scinicariello et al. Citation2020).
Many PFAS and PFOA compounds degrade to acid forms (PFAAs), environmental exposures are often to mixtures of compounds and toxic potential (potency) can vary across the family of compounds. Relative potency factors (RPFs) of 22 PFAS were determined compared to the potency of PFOA on the liver (Bil et al. Citation2021). In general compounds with fewer than 7 or more than 12 carbon atoms were less potent than PFOA; perfluorononanoic acid (PFNA) had a potency of 10. The obtained RPFs can be applied to measured PFAS quantities, resulting in the sum of PFOA equivalents in a mixture.
PFOA is a peroxisome proliferator (PPAR agonist) and exerts morphological and biochemical effects characteristic of PPAR agonists. These effects, in animal models, include increased beta-oxidation of fatty acids, increases in several cytochrome P-450 (CYP450)-mediated reactions, and inhibition of the secretion of very low-density lipoproteins and cholesterol from the liver (Kennedy et al. Citation2004). However, in humans, serum concentrations of cholesterol correlate positively with exposure to PFOA and PFOS (Andersen et al. Citation2021). The positive association between PFOS/PFAS serum levels and elevated serum total cholesterol may link to obesity and is consistent with toxicology literature findings of disrupted cholesterol metabolism via induced steatosis following PFAS exposure (Jain and Ducatman Citation2019). Exposure to PFOA in early life may be associated with an increased risk for childhood obesity (Liu, Yang, and Wang et al. Citation2018) with attendant consequences if this persists into adulthood.
These compounds may be immunotoxic in humans. Results from the 1999– 2016 National Health and Nutrition Examination Survey (NHANES) study suggest that PFAS exposures may increase susceptibility to persistent infections, particularly among adolescents (Bulka, Avula, and Fry Citation2021). Both PFOS and PFOA stimulate the release of the pro-inflammatory cytokine IL-1beta in human bronchial epithelial cells providing a mechanism linking these compounds to increased risks of acute lung toxicity and of airway infections (Sorli et al. Citation2020).
Xuelian Bai: PFOA and PFAS in the environment: sources, pathways, detection, and exposures
Per- and poly-fluoroalkyl substances (PFAS) consist of more than 4,000 synthetic chemicals that have been manufactured and released into the environment for over 50 years (Lim Citation2019). PFAS are fully or partially fluorinated chain compounds that consist of carbon and fluorine. illustrates some of the different PFAS classes. The unique physical and chemical properties of PFAS – such as extreme thermal and chemical stability, and oil and water repellency – make them ideal for applications such as textile coatings, paper products, food packaging, nonstick cookware, and the aqueous film-forming foams (AFFF) used in firefighting (Brendel, Fetter, and Staude et al. Citation2018). There are environmental risks and human health concerns associated with PFAS because of their long-term persistence and high bioaccumulation in wildlife and humans (Ahrens and Bundschuh Citation2014; Chang et al. Citation2016; Dalahmeh et al. Citation2018; Giesy and Kannan Citation2001; Graber et al. Citation2019; Lim Citation2019; Tomy et al. Citation2004). Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) are currently the most extensively investigated PFAS because they were the most widely used until their phase-out by manufacturers (Brusseau Citation2018). In 2016, a lifetime health advisory of 0.07 µg/L was issued for long-term exposure to both PFOA and PFOS through drinking water (USEPA Citation2016).
Figure 1. PFAS are present as non-polymers and polymers and involve many different chemical formulations
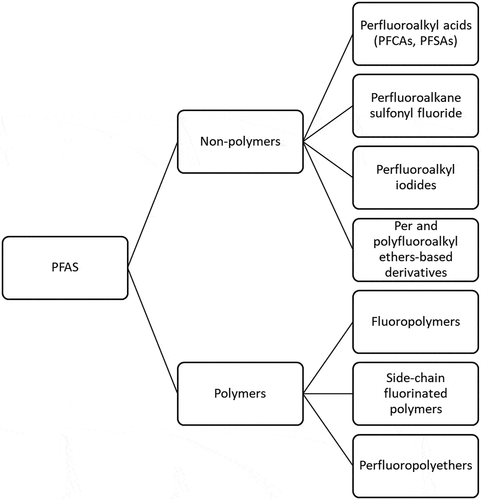
PFAS have been found ubiquitously in wastewater, surface water, soils, sediments, groundwater, and landfill effluents (Banzhaf et al. Citation2017; Dalahmeh et al. Citation2018; Houtz et al. Citation2013). Comparisons of PFAS levels in the influent and effluent of wastewater treatment plants demonstrate that current wastewater treatment processes are not very effective for removing PFAS (Gallen et al. Citation2018). In aquatic environments, short-chain (C < 8) PFAS are more prevalent in surface water and long-chain PFAS are more prevalent in bed sediments (Bai and Son Citation2021). Although some long-chain PFAS may not be detected in water, aquatic organisms may still accumulate PFAS from sediments. Hydrophilic short-chain PFAS have greater impacts on surface waters and hydrophobic long-chain PFAS may accumulate in sediments and present risks to aquatic organisms (Bai and Son Citation2021).
In terrestrial environments, sorption to soils seems to be the primary technique for retaining and mitigating the transport of PFAS to groundwater (Milinovic et al. Citation2015). PFAS sorption to soils involves complex mechanisms and has been shown to be unpredictable due to a wide range of soil properties (Barzen-Hanson et al. Citation2017; Li, Oliver, and Kookana Citation2018b). Sorption processes may be driven by hydrophobic interactions between PFAS and organic carbon, ligand binding through divalent cations, or electrostatic interactions between the functional end groups of PFAS, mineral oxides, and organics in soils (Bräunig et al. Citation2019; Higgins and Luthy Citation2006; Jeon et al. Citation2011; Li, Oliver, and Kookana Citation2018b). In contaminated soils, PFAS also have the potential to accumulate in both plants (Bizkarguenaga et al. Citation2016; Blaine et al. Citation2014; Gobelius, Lewis, and Ahrens Citation2017; Lechner and Knapp Citation2011; Stahl et al. Citation2009; Wen, Li, and Zhang et al. Citation2014) and biota (Houde et al. Citation2011; Navarro et al. Citation2016; Rich et al. Citation2015; Wen et al. Citation2015; Zhao et al. Citation2013). Short-chain PFAS show greater accumulation in plants such as lettuce, tomatoes, and wheat (Blaine et al. Citation2013, Citation2014; Felizeter, McLachlan, and de Voogt Citation2012; Zhao, Fang, and Zhu et al. Citation2014), whereas long-chain PFAS show a greater potential to bioaccumulate in earthworms (Rich et al. Citation2015; Wen et al. Citation2015; Zhao et al. Citation2013). Accumulation in plants and biota provides a possible exposure route for contaminants to be transported from the sources to food webs.
Some PFAS are detected in ambient air, with elevated concentrations observed or expected in urban areas adjacent to emission sources, such as manufacturing facilities, wastewater facilities, fire training facilities, and landfills. PFAS can be present in both the gas and particle phases, as many of them are semi-volatile. Neutral volatile precursor compounds, such as fluorotelomer alcohol (FTOHs), are the dominant PFAS present in the gas phase. However, ionic PFAS – such as PFOA and PFOS with low vapor pressure and high water solubility – favor the particulate phase (Fraser et al. Citation2013). During wastewater treatment, PFAS could be released to the atmosphere by volatilization and aerosol formation (Ahrens et al., Citation2013; Ahrens et al. Citation2011; Tian, Yao, and Chang et al. Citation2018; Weinberg, Dreyer, and Ebinghaus Citation2011) .
Because of PFAS frequent use, wide distribution, continuous release, and resistance to treatment, wastewater and sewage sludge are major sources for PFAS introduction to the environment. PFAS can adsorb on solids during wastewater treatment, and sorption to sludge is the most likely removal process for PFAS during conventional treatment. Although the production of PFOA and PFOS and other long-chain PFAS has decreased, and alternatives have been used over the past several decades, these chemicals are still highly persistent. One likely reason is the degradation of precursors (e.g., FTOHs). The degradation of 8:2 FTOH releases PFOA under aerobic conditions by oxidizing the alcohol to aldehyde before it is oxidized to 8:2 fluorotelomer acid (Dinglasan et al. Citation2004). Other precursors include perfluoroalkyl phosphonates (PAPs), fluorotelomer sulfonate (FTS), perfluoroalkyl sulfonamide-ethanol (FASEs), perfluoroalkyl sulfonamide (FASAs), and perfluoroalkyl sulfonamide-acetic acid (FASAAs) (Dickenson, Pisarenko, and Marti et al. Citation2015).
It is challenging to identify and quantify various PFAS in different environmental matrices, and effective methods are still being developed. Stable, selective, and sensitive sampling and analytical methods need to be developed to improve the detection of PFAS. Critical data and knowledge gaps still exist on fate and transport of PFAS in both agricultural and aquatic ecosystems, especially for newer PFAS. Humans are likely to be exposed to PFAS via contaminated food and water. Thus, understanding the fate and transport pathways is necessary to assess the exposure and risks of PFAS.
Morton Barlaz: potential exposures to PFAS and PFOA from municipal wastes
Approximately 50% of the municipal solid waste (MSW) that is generated in the U.S. is estimated to be disposed in landfills (US EPA Citation2016). MSW includes a number of items that are known to contain poly- and perfluoroalkyl substances (PFAS) including various types of paper, fast food packaging, textiles and carpet (Kim et al. Citation2015; Lang et al. Citation2016; Robel et al. Citation2017). Over 1400 different PFAS were found present in many different products that are ultimately disposed as MSW at the end of their useful life, e.g., climbing rope, cooking and bakeware, leather, as well as textiles, paper and packaging (Gluge et al. Citation2020). Thus, it is not surprising that the presence of PFAS in leachate from landfills that receive MSW is well documented (Benskin et al. Citation2012; Busch et al. Citation2010; Eggen, Moeder, and Arukwe Citation2010; Gallen et al. Citation2017; Huset et al. Citation2011; Lang et al. Citation2017). To assess the significance of landfill leachate as a source of PFAS release to the environment, Lang et al. (Citation2017) conducted a survey of 21 U.S. landfills. For 19 compounds, over half of the landfill leachate samples collected were above the limit of quantification. PFOA and PFOS were both present, and the dominant compound was the 5:3 fluorotelomer carboxylic acid (FTCA). Using data from the survey coupled with an estimate of leachate generation in U.S. landfills by climatic region, it was estimated that 563 to 638 kg of PFASs were released from landfills to wastewater treatment plants (WWTPs) in the U.S. in 2013 (Lang et al. Citation2017).
The presence of PFAS in leachate is important as leachate is typically treated in wastewater treatment plants (WWTPs). WWTPs are not known to attenuate PFOA and PFOS, and previous studies have shown higher effluent PFOA and PFOS concentrations relative to influent concentrations, with the transformation of precursor compounds during biological treatment as the likely explanation (Barlaz, Thelusmond, and Levis et al. Citation2020; Guerra et al. Citation2014; Lindstrom et al. Citation2011; Loganathan et al. Citation2007; Schultz et al. Citation2006). Thus, PFAS-contaminated leachate is a source of PFAS in WWTP effluent. The significance of leachate as a source of PFAS in WWTP effluent will vary as a function of the landfill size and associated leachate production rate, as well as the concentration of PFAS in domestic wastewater and the WWTP effluent flowrate. In ongoing research, we are assessing the significance of landfills as a source of PFAS in WWTP influent (Barlaz, Thelusmond, and Levis et al. Citation2020).
While the presence of PFASs in leachate is well documented, there has been very little work on the release of PFASs to landfill gas (LFG). There are three studies in which PFASs were measured in the ambient air above landfills including studies in Canada, China and Germany (Ahrens et al. Citation2011; Tian, Yao, and Chang et al. Citation2018; Weinberg, Dreyer, and Ebinghaus Citation2011). To date, there are data on air above landfills for 14 total volatile PFASs among five classes including FTOHS, N-Me FASAs, N-EtFASAs, N-Me-FOSEs, N-EtFOSEs, and fluorotelomer acrylates (FTAs) (Ahrens et al., Citation2013; Ahrens et al. Citation2011; Tian, Yao, and Chang et al. Citation2018; Weinberg, Dreyer, and Ebinghaus Citation2011). In a collaboration between North Carolina State and Oregon State Universities, we are currently measuring PFAS in landfill gas from about 25 landfills across the U.S.
Michael A. Abraczinskas: PFAS challenges for environmental regulatory agencies
This critical review of PFAS exposures in humans is timely and deserves considerable attention due to concerns over toxicity and persistence in the environment. This commentary provides the review based on my experience with the state regulatory perspective. As the ability to measure this complex class of chemical compounds increases, regulatory agencies, researchers and the public are learning more about their presence in the environment. With more than 4,000 individual PFAS compounds present in a variety of industrial or consumer products, we are at stage where our ability to measure these compounds far exceeds our understanding of the potential health impacts and our ability to communicate human health risks when the compounds are found in the environment.
From a state or local environmental regulatory agency point of view, addressing regulations for PFAS in the environment is a significant challenge, mainly because there are not federal standards for most PFAS compounds.
From a regulator’s standpoint, what are your options if: 1) you find PFAS to be prevalent in the environment; 2) there are no specific limits for those compounds in environmental regulations; 3) there is sparse knowledge about how they behave in the environment beyond their persistence; and, 4) there has been limited study of their effects on human health and the environment? If those four conditions exist, that’s a really tough spot for a regulatory agency. How do you go about prioritizing, researching, or minimizing impacts? How do you communicate those findings to the public? It can be a significant risk communication challenge.
In a real-world example that the State of North Carolina has been addressing,Footnote1 it has been a multi-media challenge. The first finding was related to significant concentrations of PFAS in surface water. Those concentrations were traced back to an industrial fluoropolymer manufacturing site outfall that drains into the Cape Fear River in southeastern North Carolina. The Cape Fear River serves as the primary source of drinking water for portions of the Wilmington, NC area.
A process water discharge from the outfall to the river was the primary contributor to those high concentrations. Our Department’s Division of Water Resources required the site to discontinue the process waste water discharge in response to the environmental data. Peak PFAS concentrations in the river were significantly reduced after the discharge was severed.
The next challenge involved the finding of significant concentrations of PFAS compounds in ground water wells on the industrial site and later in private residential wells near the facility. As ground water sampling expanded, our Department’s Division of Waste Management highlighted the fact that PFAS concentrations were found upgradient to typical ground water flows at and near the facility, and also on the opposite side of the Cape Fear River. The ground water sampling campaign continued in a manner working from the facility outward to determine the extent of the contamination. Today, PFAS compounds are being found in ground water wells up to 17 miles in the primary downwind direction from the facility and the sampling is still ongoing.
With private wells upgradient found to be contaminated with PFAS compounds unique to this manufacturing operation, the hypothesis was that it was caused by atmospheric deposition. At that point, our Department’s Division of Air Quality turned its attention to acquiring better emissions data from the facility and to collecting ambient air quality monitoring nearby the facility. A complicating factor for this portion of the investigation was the lack of off-the-shelf methods for measuring PFAS in ambient air. Creative measures were taken to design experiments to collect wet deposition samples downwind of the facility. Detailed meteorological forecasts were made to determine where the facility’s plume might be headed during precipitation events, and wet deposition instruments were placed in those downwind locations. That early event-based sampling confirmed the hypothesis and developed a strong causal link between air emissions from the facility and ground water contamination downwind. Later, modeling studies further characterized transport and deposition associated with emissions from this facility.Footnote2
While these data collection efforts were taking place, and the cause-and-effect relationship was materializing between the facility’s emissions and the environmental data, it was recognized that source reduction strategies would provide necessary relief to the public on a much shorter timeline. In this case, both the facility and the agency determined that source control of air emissions was technically feasible.Footnote3
The Department’s efforts to collect quality data (surface water, ground water and air deposition data), and use the scientific method during the investigation, informed the development of a suite of source reduction strategies and remedial actions, despite a lack of specific PFAS standards or regulations. The science and data led to conclusions about the facility that were not disputed, and specific source reduction strategies and remedial actions were formalized in a consent order filed by our Department in Bladen County Superior Court.Footnote4 The order, signed by the agency, the chemical facility’s representatives, and the environmental group’s representatives, was approved by the court in its entirety.
A critical piece of that consent order required the facility to install a thermal oxidizer/scrubber control system that was to achieve a 99.99% PFAS removal efficiency. Thus far, all stack testing that has been conducted by the facility, and reviewed by our Department, has confirmed that the removal efficiency requirement was met. The consent order required further actions by the chemical manufacturer, including, but not limited to:
Achieve maximum reductions in PFAS loading to the Cape Fear River from all sources on an accelerated basis.
Provide permanent drinking water supplies – either in the form of a public waterline connection or whole building filtration system – for those with drinking water wells with GenX above 140 parts per trillion.
Provide under-sink reverse osmosis drinking water systems for well owners with combined concentrations of certain PFAS above 70 parts per trillion or concentrations of certain individual PFAS above 10 parts per trillion.
Sample drinking water wells at least one-quarter mile beyond the closest well that had concentrations of certain PFAS above 10 parts per trillion.
Annually retest wells in a manner sufficient to determine the extent of contamination.
Submit and implement a plan for sampling all process and non-process wastewater and stormwater to identify and measure concentrations of PFAS, including non-targeted analysis to identify PFAS that have not been previously identified.
Notify downstream public water utilities when an event at the facility has the potential to cause a discharge of GenX compounds into the Cape Fear River above 140 parts per trillion.
Implementation of all elements of the consent order continues as of this writing, and the agency continues to hold the facility accountable, review all reports, data and plans that are delivered consistent with the order.
Meanwhile, the agency is also keeping an eye on the horizon regarding other potential PFAS issues while advancing our knowledge base with our colleagues at the North Carolina Department of Health and Human Services and the Secretaries’ Science Advisory Board on the evolving health and risk assessment science of PFAS.
So, where do we stand after many years of working through a situation where a facility’s emissions and discharges resulted in significant PFAS contamination in the environment? There is discussion at the federal level and in other states about the necessity of standards for PFAS compounds and the feasibility of a broader class-based approach for regulating these compounds. Some considerations include the prevalence, persistence, toxicity, bioaccumulation, and mobility of the compounds. Either way, development and implementation of such standards will take time.
This is a significant challenge for environmental regulatory agencies. However, as we have discussed, the collection of quality data and use of the scientific method can be a powerful tool in achieving reduced exposures to these emerging compounds and providing more immediate relief to impacted communities.
Virginia T. Guidry: overall comments
This critical review addresses the associations between PFOA exposure and kidney and testicular cancer. Exposure to per- and polyfluoroalkyl substances (PFAS), including PFOA, is widespread and persistent, with limited understanding of health effects in exposed populations. The rigorous review provided in this paper provides evidence-based public health guidance about PFAS exposures and informs agents of state health departments, such as in North Carolina, responding to PFAS contamination. As an example, the NC Department of Health and Human Services (NCDHHS), Division of Public Health has the following responsibilities:
Provide nonregulatory health guidance
Includes the calculation of a provisional drinking water health goal for GenX
Review new data – review the toxicological and epidemiologic literature on PFAS
Educate the public – translate scientific information for public audiences and provide guidance on actions to protect public health
Engage with researchers – work with researchers studying the health effects of PFAS to help them understand the implications of their research and maximize the benefits to public health in North Carolina
Specifically, this review informs the continued efforts to respond to public concerns about cancers that may be associated with PFAS exposure.
In a recent NCDHHS Cape Fear PFAS Community Survey published online in 2019, 44% of the 1800 survey respondents reported that they are concerned about health impacts of PFAS, and cancer was the leading health concern mentioned. This review helps us respond to those concerns.
Additionally, NCDHHS conducted a review of cancer rates for four counties (Bladen, Brunswick, New Hanover, and Pender) impacted by PFAS contamination in North Carolina. We examined incidence of pancreatic, liver, uterine, testicular, and kidney cancers. Overall, cancer rates in the four counties were similar to state rates. New Hanover County had a higher 20-year rate of testicular cancer during 1996–2015 and a higher 5-year rate of liver cancers during 2006–2010 compared with the state, but rates of both cancers were similar to the state rates during the most recent period (2011–2015).
A strength of this critical review is the combination of the use of Modified Hill’s Criteria for Causation, dose response analysis, and meta-analysis to determine likelihood of causality. The review clearly summarized and thoroughly examined the supporting literature for the weight of evidence they were providing. The reviews conclusion that PFOA is a likely cause of both kidney cancer and testicular cancer in humans is based on a careful and detailed analysis. The findings inform and support efforts to reduce PFOA exposure, as well as exposure to other PFAS, in North Carolina and can be applied more broadly.
The only lament is that this labor intensive process helps to establish the association between just one PFAS and two types of cancer, yet we know that people are exposed to many PFAS that may cause numerous harms. Perhaps we can continue to search for additional computational ways to provide the necessary data and analyses to draw conclusions about the health impacts of exposure to PFAS as a class, and for additional health outcomes, to further drive efforts to reduce exposure or eliminate the use of these chemicals in our consumer products.
Supplemental Material
Download PDF (53.2 KB)Supplemental Material
Download PDF (125.1 KB)Acknowledgment
Mr. Abraczinskas thanks the following for their contributions to the review: Michael Pjetraj, Deputy Director of the NC Division of Air Quality; Michael Scott, Director of the NC Division of Waste Management; Danny Smith, Director of the NC Division of Water Resources; Zaynab Nasif, Public Information Officer of the NC Division of Air Quality; Sharon Martin, Deputy Secretary of Public Affairs of the NC Department of Environmental Quality.
Michael Kleinman and Eric Stevenson than Lisa Bucher for all of her support for the Critical Review Committee and for the Critical Review presentation at the ACE. We also thank Susan Weirman, Sam Altshuler, John Watson and Pat Brush whose comments and insights greatly contributed to the success of the 2021 Critical Review.
Supplemental Material
Supplemental data for this paper can be accessed on the publisher’s website.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Eric D. Stevenson
Eric D. Stevenson, former Director of Meteorology and Measurement, Bay Area Air Quality Management District, San Francisco, CA, and Chair, A&WMA Critical Review Committee.
Michael T. Kleinman
Michael T. Kleinman, Professor, Air Pollution Health Effects Laboratory, Department of Environmental and Occupational Health, University of California, Irvine, CA, and a Past Chair of the A&WMA Critical Review Committee.
Xuelian Bai
Xuelian Bai, Ph.D., Assistant Research Professor, Division of Hydrologic Sciences, Desert Research Institute, Las Vegas, Nv.
Morton Barlaz
Morton Barlaz, Ph.D., P.E., Distinguished University Professor and Head, Department of Civil, Construction, and Environmental Engineering, North Carolina State University, Raleigh, NC.
Michael Abraczinskas
Michael Abraczinskas, Director, North Carolina Department of Environmental Quality, Division of Air Quality, Raleigh, NC.
Virginia Guidry
Virginia Guidry, Ph.D., MPH, Branch Head, Occupational and Environmental Epidemiology, Division of Public Health, Epidemiology Section, North Carolina Department of Health and Human Services Raleigh, NC.
John Watson
John Watson, is a Research Professor of Atmospheric Science at Desert Research Institute, Reno NV.
Judy Chow
Judy Chow, holds the Nazir and Mary Ansari Chair in Entrepreneurialism and Science and is a Research Professor in the Division of Atmospheric Sciences (DAS) at the Desert Research Institute, Reno NV.
Notes
1 NC DEQ GenX Investigation. 2017. https://deq.nc.gov/news/key-issues/genx-investigation.
2 D’Ambro, Emma L.; Murphy, Benjamin N.; Pye, Havala O. T.; Bash, Jesse O.; Bowyer, James; Allen, Chris; Efstathiou, Christos; Gilliam, Robert C.; Reynolds, Lara; Talgo, Kevin. Characterizing the Air Emissions, Transport, and Deposition of Per- and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environmental Science & Technology 2021 55 (2), 862–870.
References
- Ahrens, L., and M. Bundschuh. 2014. “Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review.” Environmental Toxicology and Chemistry 33 ( 9): 1921–1929. https://doi.org/https://doi.org/10.1002/etc.2663.
- Ahrens, L., T. Harner, M. Shoeib, M. Koblizkova, and E. J. Reiner. 2013. “Characterization of two passive air samplers for per- and polyfluoroalkyl substances”. Environ Sci Technol 47 (24): 14024–33. https://doi.org/https://doi.org/10.1021/es4048945. https://www.ncbi.nlm.nih.gov/pubmed/24219299.
- Ahrens, L., M. Shoeib, T. Harner, S. C. Lee, R. Guo, and E. J. Reiner. 2011. “Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere”. Environ Sci Technol 45 (19): 8098–105. https://doi.org/https://doi.org/10.1021/es1036173. https://www.ncbi.nlm.nih.gov/pubmed/21466185.
- Andersen, M. E., B. Hagenbuch, U. Apte, J. C. Corton, T. Fletcher, C. Lau, W. L. Roth, B. Staels, G. L. Vega, H. J. Clewell, 3rd, and M. P. Longnecker. 2021. “Why is elevation of serum cholesterol associated with exposure to perfluoroalkyl substances (PFAS) in humans? A workshop report on potential mechanisms”. Toxicology 459: 152845. https://doi.org/https://doi.org/10.1016/j.tox.2021.152845. https://www.ncbi.nlm.nih.gov/pubmed/34246716.
- Bai, X., and Y. Son. 2021. “Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA”. Sci Total Environ 751: 141622. https://doi.org/https://doi.org/10.1016/j.scitotenv.2020.141622. https://www.ncbi.nlm.nih.gov/pubmed/32871315.
- Banzhaf, S., M. Filipovic, J. Lewis, C. J. Sparrenbom, and R. Barthel. 2017. “A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs)”. Ambio 46 (3): 335–346. https://doi.org/https://doi.org/10.1007/s13280-016-0848-8.
- Barlaz, M. A., J. R. Thelusmond, J. Levis, and N. DeStafano. 2020. “Per- and Polyfluoroalkyl Substances (PFAS) in Landfill Leachate and Municipal Wastewater”. Global Waste Management Symposium, Indian Wells, CA.
- Bartell, S. M., and V. M. Vieira. 2021. “Critical review on PFOA, kidney cancer, and testicular cancer”. J Air Waste Manag Assoc 71 (6): 663–679. https://doi.org/https://doi.org/10.1080/10962247.2021.1909668. https://www.ncbi.nlm.nih.gov/pubmed/33780327.
- Barzen-Hanson, K. A., S. C. Roberts, S. Choyke, K. Oetjen, A. McAlees, N. Riddell, R. McCrindle, P. L. Ferguson, C. P. Higgins, and J. A. Field. 2017. “Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater”. Environmental Science & Technology 51 (4): 2047–2057. https://doi.org/https://doi.org/10.1021/acs.est.6b05843.
- Benskin, J. P., B. Li, M. G. Ikonomou, J. R. Grace, and L. Y. Li. 2012. “Per- and polyfluoroalkyl substances in landfill leachate: patterns, time trends, and sources”. Environ Sci Technol 46 (21): 11532–40. https://doi.org/https://doi.org/10.1021/es302471n. https://www.ncbi.nlm.nih.gov/pubmed/23030600.
- Bil, W., M. Zeilmaker, S. Fragki, J. Lijzen, E. Verbruggen, and B. Bokkers. 2021. “Risk Assessment of Per- and Polyfluoroalkyl Substance Mixtures: A Relative Potency Factor Approach”. Environ Toxicol Chem 40 (3): 859–870. https://doi.org/https://doi.org/10.1002/etc.4835. https://www.ncbi.nlm.nih.gov/pubmed/32729940.
- Bizkarguenaga, E., I. Zabaleta, A. Prieto, L. A. Fernandez, and O. Zuloaga. 2016. “Uptake of 8:2 perfluoroalkyl phosphate diester and its degradation products by carrot and lettuce from compost-amended soil”. Chemosphere 152: 309–317. https://doi.org/https://doi.org/10.1016/j.chemosphere.2016.02.130.
- Blaine, A. C., C. D. Rich, L. S. Hundal, C. Lau, M. A. Mills, K. M. Harris, and C. P. Higgins. 2013. “Uptake of perfluoroalkyl acids into edible crops via land applied biosolids field and greenhouse studies”. Environ Sci Technol 47 (24): 14062–9. https://doi.org/https://doi.org/10.1021/es403094q. http://www.ncbi.nlm.nih.gov/pubmed/24206563.
- Blaine, A. C., C. D. Rich, E. M. Sedlacko, K. C. Hyland, C. Stushnoff, E. R. Dickenson, and C. P. Higgins. 2014. “Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water”. Environ Sci Technol 48 (24): 14361–8. https://doi.org/https://doi.org/10.1021/es504150h. http://www.ncbi.nlm.nih.gov/pubmed/25386873.
- Bräunig, J., C. Baduel, C.M. Barnes, and J.F. Mueller. 2019. “Leaching and bioavailability of selected perfluoroalkyl acids (PFAAs) from soil contaminated by firefighting activities“. Science of the Total Environment 646: 471–479.
- Brendel, S., E. Fetter, C. Staude, L. Vierke, and A. Biegel-Engler. 2018. “Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH”. Environmental Sciences Europe 30. https://doi.org/https://doi.org/10.1186/s12302-018-0134-4.
- Brusseau, M. L. 2018. “Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface”. Science of the Total Environment 613: 176–185. https://doi.org/https://doi.org/10.1016/j.scitotenv.2017.09.065.
- Buck, R.C, J. Franklin, U. Berger, J.M Conder, I.T Cousins, P. de Voogt, A.A. Jensen, K. Kannan, S.A Mabury, and S.P.J van Leeuwen. 2011. “Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins”. Integrated Environmental Assessment and Management 7 (4): 513–541. https://doi.org/https://doi.org/10.1002/ieam.258.
- Bulka, C. M., V. Avula, and R. C. Fry. 2021. “Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: Recent findings from NHANES 1999-2016”. Environ Pollut 275: 116619. https://doi.org/https://doi.org/10.1016/j.envpol.2021.116619. https://www.ncbi.nlm.nih.gov/pubmed/33578314.
- Busch, J., L. Ahrens, R. Sturm, and R. Ebinghaus. 2010. “Polyfluoroalkyl compounds in landfill leachates”. Environ Pollut 158 (5): 1467–71. https://doi.org/https://doi.org/10.1016/j.envpol.2009.12.031. https://www.ncbi.nlm.nih.gov/pubmed/20053490.
- Chang, E. T., H. O. Adami, P. Boffetta, H. J. Wedner, and J. S. Mandel. 2016. “A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans”. Critical Reviews in Toxicology 46 (4): 279–331. https://doi.org/https://doi.org/10.3109/10408444.2015.1122573.
- Dalahmeh, S., S. Tirgani, A. J. Komakech, C. B. Niwagaba, and L. Ahrens. 2018. “Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda”. Science of the Total Environment 631-632: 660–667. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.03.024.
- Dickenson, E., A. Pisarenko, E. Marti, D. Gerrity, and B. J. Vanderford. 2015. Formation of Nitrosamines and Perfluoroalkyl Acids During Ozonation in Water Reuse Applications. (WaterReuse Research Foundation).
- Dinglasan, M. J. A., Y. Ye, E. A. Edwards, and S. A. Mabury. 2004. “Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids”. Environmental Science & Technology 38 (10): 2857–2864. https://doi.org/https://doi.org/10.1021/es0350177.
- Duffek, A., A. Conrad, M. Kolossa-Gehring, R. Lange, E. Rucic, C. Schulte, and J. Wellmitz. 2020. “Per- and polyfluoroalkyl substances in blood plasma - Results of the German Environmental Survey for children and adolescents 2014-2017 (GerES V)”. Int J Hyg Environ Health 228: 113549. https://doi.org/https://doi.org/10.1016/j.ijheh.2020.113549. https://www.ncbi.nlm.nih.gov/pubmed/32502942.
- Eggen, T., M. Moeder, and A. Arukwe. 2010. “Municipal landfill leachates: a significant source for new and emerging pollutants”. Sci Total Environ 408 (21): 5147–57. https://doi.org/https://doi.org/10.1016/j.scitotenv.2010.07.049. https://www.ncbi.nlm.nih.gov/pubmed/20696466.
- Felizeter, S., M. S. McLachlan, and P. de Voogt. 2012. “Uptake of Perfluorinated Alkyl Acids by Hydroponically Grown Lettuce (Lactuca sativa)”. Environmental Science & Technology 46 (21): 11735–11743. https://doi.org/https://doi.org/10.1021/es302398u.
- Fraser, A. J., T. F. Webster, D. J. Watkins, M. J. Strynar, K. Kato, A. M. Calafat, V. M. Vieira, and M. D. McClean. 2013. “Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum”. Environment International 60: 128–136. https://doi.org/https://doi.org/10.1016/j.envint.2013.08.012.
- Gallen, C., D. Drage, G. Eaglesham, S. Grant, M. Bowman, and J. F. Mueller. 2017. “Australia-wide assessment of perfluoroalkyl substances (PFASs) in landfill leachates”. J Hazard Mater 331: 132–141. https://doi.org/https://doi.org/10.1016/j.jhazmat.2017.02.006. https://www.ncbi.nlm.nih.gov/pubmed/28254660.
- Gallen, C., G. Eaglesham, D. Drage, T. H. Nguyen, and J. F. Mueller. 2018. “A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants”. Chemosphere 208: 975–983. https://doi.org/https://doi.org/10.1016/j.chemosphere.2018.06.024. https://www.ncbi.nlm.nih.gov/pubmed/30068041.
- Gao, B., X. He, W. Liu, H. Zhang, N. Saito, and S. Tsuda. 2015. “Distribution of perfluoroalkyl compounds in rats: Indication for using hair as bioindicator of exposure”. J Expo Sci Environ Epidemiol 25 (6): 632–8. https://doi.org/https://doi.org/10.1038/jes.2014.54. https://www.ncbi.nlm.nih.gov/pubmed/25118134.
- Giesy, J. P., and K. Kannan. 2001. “Global distribution of perfluorooctane sulfonate in wildlife”. Environmental Science & Technology 35 (7): 1339–1342. https://doi.org/https://doi.org/10.1021/es001834k.
- Gluge, J., M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier, and Z. Wang. 2020. “An overview of the uses of per- and polyfluoroalkyl substances (PFAS)“. Environ Sci Process Impacts 22 (12): 2345–2373. https://doi.org/https://doi.org/10.1039/d0em00291g. https://www.ncbi.nlm.nih.gov/pubmed/33125022.
- Gobelius, L., J. Lewis, and L. Ahrens. 2017. “Plant Uptake of Per- and Polyfluoroalkyl Substances at a Contaminated Fire Training Facility to Evaluate the Phytoremediation Potential of Various Plant Species”. Environmental Science & Technology 51 (21): 12602–12610. https://doi.org/https://doi.org/10.1021/acs.est.7b02926.
- Graber, J. M., C. Alexander, R. J. Laumbach, K. Black, P. O. Strickland, P. G. Georgopoulos, E. G. Marshall, D. G. Shendell, D. Alderson, Z. Y. Mi, M. Mascari, and C. P. Weisel. 2019. “Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013-2014 NHANES”. Journal of Exposure Science and Environmental Epidemiology 29 (2): 172–182. https://doi.org/https://doi.org/10.1038/s41370-018-0096-z.
- Guerra, P., M. Kim, L. Kinsman, T. Ng, M. Alaee, and S. A. Smyth. 2014. “Parameters affecting the formation of perfluoroalkyl acids during wastewater treatment”. J Hazard Mater 272: 148–54. https://doi.org/https://doi.org/10.1016/j.jhazmat.2014.03.016. https://www.ncbi.nlm.nih.gov/pubmed/24691135.
- Harada, K., K. Inoue, A. Morikawa, T. Yoshinaga, N. Saito, and A. Koizumi. 2005. “Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion”. Environ Res 99 (2): 253–61. https://doi.org/https://doi.org/10.1016/j.envres.2004.12.003. https://www.ncbi.nlm.nih.gov/pubmed/16194675.
- Higgins, C. P., and R. G. Luthy. 2006. “Sorption of perfluorinated surfactants on sediments”. Environmental Science & Technology 40 (23): 7251–7256. https://doi.org/https://doi.org/10.1021/es061000n.
- Houde, M., A. O. De Silva, D. C. G. Muir, and R. J. Letcher. 2011. “Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review PFCs in Aquatic Biota”. Environmental Science & Technology 45 (19): 7962–7973. https://doi.org/https://doi.org/10.1021/es104326w.
- Houtz, E. F., C. P. Higgins, J. A. Field, and D. L. Sedlak. 2013. “Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil“. Environmental Science & Technology 47 (15): 8187–8195. https://doi.org/https://doi.org/10.1021/es4018877.
- Huset, C. A., M. A. Barlaz, D. F. Barofsky, and J. A. Field. 2011. “Quantitative determination of fluorochemicals in municipal landfill leachates”. Chemosphere 82 (10): 1380–6. https://doi.org/https://doi.org/10.1016/j.chemosphere.2010.11.072. https://www.ncbi.nlm.nih.gov/pubmed/21194725.
- ITRC. 2020. PFAS — Per- and Polyfluoroalkyl Substances. Interstate Technology and Regulatory Council (Washington, DC). https://pfas-1.itrcweb.org/, https://pfas-1.itrcweb.org/acronyms/.
- Jain, R. B., and A. Ducatman. 2019. “Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins”. Sci Total Environ 653: 74–81. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.10.362. https://www.ncbi.nlm.nih.gov/pubmed/30408670.
- Jeon, J., K. Kannan, B. J. Lim, K. G. An, and S. D. Kim. 2011. “Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles”. Journal of Environmental Monitoring 13 (6): 1803–1810. https://doi.org/https://doi.org/10.1039/c0em00791a.
- Kennedy, G. L., Jr., J. L. Butenhoff, G. W. Olsen, J. C. O’Connor, A. M. Seacat, R. G. Perkins, L. B. Biegel, S. R. Murphy, and D. G. Farrar. 2004. “The toxicology of perfluorooctanoate”. Crit Rev Toxicol 34 (4): 351–84. https://doi.org/https://doi.org/10.1080/10408440490464705. https://www.ncbi.nlm.nih.gov/pubmed/15328768.
- Kim, M., L. Y. Li, J. R. Grace, J. P. Benskin, and M. G. Ikonomou. 2015. “Compositional effects on leaching of stain-guarded (perfluoroalkyl and polyfluoroalkyl substance-treated) carpet in landfill leachate”. Environ Sci Technol 49 (11): 6564–73. https://doi.org/https://doi.org/10.1021/es505333y. https://www.ncbi.nlm.nih.gov/pubmed/25985932.
- Kotlarz, N., J. McCord, D. Collier, C. S. Lea, M. Strynar, A. B. Lindstrom, A. A. Wilkie, J. Y. Islam, K. Matney, P. Tarte, M. E. Polera, K. Burdette, J. DeWitt, K. May, R. C. Smart, D. R. U. Knappe, and J. A. Hoppin. 2020. “Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina”. Environ Health Perspect 128 (7): 77005. https://doi.org/https://doi.org/10.1289/EHP6837. https://www.ncbi.nlm.nih.gov/pubmed/32697103.
- Kudo, N., and Y. Kawashima. 2003. “Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals”. J Toxicol Sci 28 (2): 49–57. https://doi.org/https://doi.org/10.2131/jts.28.49. https://www.ncbi.nlm.nih.gov/pubmed/12820537.
- Lang, J. R., B. M. Allred, J. A. Field, J. W. Levis, and M. A. Barlaz. 2017. “National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate”. Environ Sci Technol 51 (4): 2197–2205. https://doi.org/https://doi.org/10.1021/acs.est.6b05005. https://www.ncbi.nlm.nih.gov/pubmed/28103667.
- Lang, J. R., B. M. Allred, G. F. Peaslee, J. A. Field, and M. A. Barlaz. 2016. “Release of Per- and Polyfluoroalkyl Substances (PFASs) from Carpet and Clothing in Model Anaerobic Landfill Reactors”. Environ Sci Technol 50 (10): 5024–32. https://doi.org/https://doi.org/10.1021/acs.est.5b06237. https://www.ncbi.nlm.nih.gov/pubmed/27095439.
- Lechner, M., and H. Knapp. 2011. “Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plant and Distribution to the Different Plant Compartments Studied in Cultures of Carrots (Daucus carota ssp Sativus), Potatoes (Solanum tuberosum), and Cucumbers (Cucumis Sativus)”. Journal of Agricultural and Food Chemistry 59 (20): 11011–11018. https://doi.org/https://doi.org/10.1021/jf201355y.
- Li, Y., T. Fletcher, D. Mucs, K. Scott, C. H. Lindh, P. Tallving, and K. Jakobsson. 2018. “Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water”. Occup Environ Med 75 (1): 46–51. https://doi.org/https://doi.org/10.1136/oemed-2017-104651. https://www.ncbi.nlm.nih.gov/pubmed/29133598.
- Li, Y. S., D. P. Oliver, and R. S. Kookana. 2018. “A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs)”. Science of the Total Environment 628–629: 110-120. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.01.167.
- Lim, X. 2019. “The fluorine detectives-Researchers are battling to identify and assess a worrying class of persistent chemicals”. Nature (566): 26–29.
- Lindstrom, A. B., M. J. Strynar, A. D. Delinsky, S. F. Nakayama, L. McMillan, E. L. Libelo, M. Neill, and L. Thomas. 2011. “Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA”. Environ Sci Technol 45 (19): 8015–21. https://doi.org/https://doi.org/10.1021/es1039425. https://www.ncbi.nlm.nih.gov/pubmed/21513287.
- Liu, P., F. Yang, Y. Wang, and Z. Yuan. 2018. “Perfluorooctanoic Acid (PFOA) Exposure in Early Life Increases Risk of Childhood Adiposity: A Meta-Analysis of Prospective Cohort Studies”. Int J Environ Res Public Health 15 (10). https://doi.org/https://doi.org/10.3390/ijerph15102070. https://www.ncbi.nlm.nih.gov/pubmed/30241417.
- Loganathan, B. G., K. S. Sajwan, E. Sinclair, K. Senthil Kumar, and K. Kannan. 2007. “Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia”. Water Res 41 (20): 4611–20. https://doi.org/https://doi.org/10.1016/j.watres.2007.06.045. https://www.ncbi.nlm.nih.gov/pubmed/17632203.
- Milinovic, J., S. Lacorte, M. Vidal, and A. Rigol. 2015. “Sorption behaviour of perfluoroalkyl substances in soils”. Science of the Total Environment 511: 63–71. https://doi.org/https://doi.org/10.1016/j.scitotenv.2014.12.017.
- Navarro, I., A. de la Torre, P. Sanz, J. Pro, G. Carbonell, and M. D. Martinez. 2016. “Bioaccumulation of emerging organic compounds (perfluoroalkyl substances and halogenated flame retardants) by earthworm in biosolid amended soils”. Environmental Research 149: 32–39. https://doi.org/https://doi.org/10.1016/j.envres.2016.05.004.
- Ojo, A.F., C. Peng, and J.C. Ng. 2021. “Assessing the human health risks of per- and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures”. Journal of Hazardous Materials 407. https://doi.org/https://doi.org/10.1016/j.jhazmat.2020.124863.
- Rich, C. D., A. C. Blaine, L. Hundal, and C. P. Higgins. 2015. “Bioaccumulation of Perfluoroalkyl Acids by Earthworms (Eisenia fetida) Exposed to Contaminated Soils”. Environmental Science & Technology 49 (2): 881–888. https://doi.org/https://doi.org/10.1021/es504152d.
- Robel, A. E., K. Marshall, M. Dickinson, D. Lunderberg, C. Butt, G. Peaslee, H. M. Stapleton, and J. A. Field. 2017. “Closing the Mass Balance on Fluorine on Papers and Textiles”. Environ Sci Technol 51 (16): 9022–9032. https://doi.org/https://doi.org/10.1021/acs.est.7b02080. https://www.ncbi.nlm.nih.gov/pubmed/28712295.
- Sanchez Garcia, D., M. Sjodin, M. Hellstrandh, U. Norinder, V. Nikiforova, J. Lindberg, E. Wincent, A. Bergman, I. Cotgreave, and V. Munic Kos. 2018. “Cellular accumulation and lipid binding of perfluorinated alkylated substances (PFASs) - A comparison with lysosomotropic drugs”. Chem Biol Interact 281: 1–10. https://doi.org/https://doi.org/10.1016/j.cbi.2017.12.021. https://www.ncbi.nlm.nih.gov/pubmed/29248446.
- Schultz, M. M., C. P. Higgins, C. A. Huset, R. G. Luthy, D. F. Barofsky, and J. A. Field. 2006. “Fluorochemical mass flows in a municipal wastewater treatment facility”. Environ Sci Technol 40 (23): 7350–7. https://doi.org/https://doi.org/10.1021/es061025m. https://www.ncbi.nlm.nih.gov/pubmed/17180988.
- Scinicariello, F., M. C. Buser, L. Balluz, K. Gehle, H. E. Murray, H. G. Abadin, and R. Attanasio. 2020. “Perfluoroalkyl acids, hyperuricemia and gout in adults: Analyses of NHANES 2009-2014”. Chemosphere 259: 127446. https://doi.org/https://doi.org/10.1016/j.chemosphere.2020.127446. https://www.ncbi.nlm.nih.gov/pubmed/32590180.
- Sorli, J. B., M. Lag, L. Ekeren, J. Perez-Gil, L. S. Haug, E. Da Silva, M. N. Matrod, K. B. Gutzkow, and B. Lindeman. 2020. “Per- and polyfluoroalkyl substances (PFASs) modify lung surfactant function and pro-inflammatory responses in human bronchial epithelial cells”. Toxicol In Vitro 62: 104656. https://doi.org/https://doi.org/10.1016/j.tiv.2019.104656. https://www.ncbi.nlm.nih.gov/pubmed/31536757.
- Stahl, T., J. Heyn, H. Thiele, J. Huther, K. Failing, S. Georgii, and H. Brunn. 2009. “Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants”. Archives of Environmental Contamination and Toxicology 57 (2): 289–298. https://doi.org/https://doi.org/10.1007/s00244-008-9272-9.
- Sunderland, E.M., X.D.C. Hu, C. Dassuncao, A.K. Tokranov, C.C. Wagner, and J.G. Allen. 2019. “A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects“. Journal of Exposure Science and Environmental Epidemiology 29 (2): 131–147. https://doi.org/https://doi.org/10.1038/s41370-018-0094-1.
- Tian, Y., Y. Yao, S. Chang, Z. Zhao, Y. Zhao, X. Yuan, F. Wu, and H. Sun. 2018. “Occurrence and Phase Distribution of Neutral and Ionizable Per- and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China”. Environ Sci Technol 52 (3): 1301–1310. https://doi.org/https://doi.org/10.1021/acs.est.7b05385. https://www.ncbi.nlm.nih.gov/pubmed/29309135.
- Tomy, G. T., W. Budakowski, T. Halldorson, P. A. Helm, G. A. Stern, K. Friesen, K. Pepper, S. A. Tittlemier, and A. T. Fisk. 2004. “Fluorinated organic compounds in an eastern Arctic marine food web”. Environmental Science & Technology 38 (24): 6475–6481. https://doi.org/https://doi.org/10.1021/es049620g.
- US EPA. 2016. “Fact Sheet PFOA & PFOS Drinking Water Health Advisories. (November 2016 EPA 800-F-16-003). U.S. Environmental Protection Agency, Washington, DC.”
- Weinberg, I., A. Dreyer, and R. Ebinghaus. 2011. “Waste water treatment plants as sources of polyfluorinated compounds, polybrominated diphenyl ethers and musk fragrances to ambient air”. Environ Pollut 159 (1): 125–132. https://doi.org/https://doi.org/10.1016/j.envpol.2010.09.023. https://www.ncbi.nlm.nih.gov/pubmed/20951480.
- Wen, B., L. F. Li, H. N. Zhang, Y. B. Ma, X. Q. Shan, and S. Z. Zhang. 2014. “Field study on the uptake and translocation of perfluoroalkyl acids (PFAAs) by wheat (Triticum aestivum L.) grown in biosolids-amended soils”. Environmental Pollution 184: 547–554. https://doi.org/https://doi.org/10.1016/j.envpol.2013.09.040.
- Wen, B., H. N. Zhang, L. F. Li, X. Y. Hu, Y. Liu, X. Q. Shan, and S. Z. Zhang. 2015. “Bioavailability of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in biosolids-amended soils to earthworms (Eisenia fetida)”. Chemosphere 118: 361–366. https://doi.org/https://doi.org/10.1016/j.chemosphere.2014.08.009.
- Worley, R. R., S. M. Moore, B. C. Tierney, X. Ye, A. M. Calafat, S. Campbell, M. B. Woudneh, and J. Fisher. 2017. “Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community”. Environ Int 106: 135–143. https://doi.org/https://doi.org/10.1016/j.envint.2017.06.007. https://www.ncbi.nlm.nih.gov/pubmed/28645013.
- Xu, Y., T. Fletcher, D. Pineda, C. H. Lindh, C. Nilsson, A. Glynn, C. Vogs, K. Norstrom, K. Lilja, K. Jakobsson, and Y. Li. 2020. “Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam“. Environ Health Perspect 128 (7): 77004. https://doi.org/https://doi.org/10.1289/EHP6785. https://www.ncbi.nlm.nih.gov/pubmed/32648786.
- Zhao, S. Y., S. H. Fang, L. Y. Zhu, L. Liu, Z. T. Liu, and Y. H. Zhang. 2014. “Mutual impacts of wheat (Triticum aestivum L.) and earthworms (Eisenia fetida) on the bioavailability of perfluoroalkyl substances (PFASs) in soil”. Environmental Pollution 184: 495–501. https://doi.org/https://doi.org/10.1016/j.envpol.2013.09.032.
- Zhao, S. Y., L. Y. Zhu, L. Liu, Z. T. Liu, and Y. H. Zhang. 2013. “Bioaccumulation of perfluoroalkyl carboxylates (PFCAs) and perfluoroalkane sulfonates (PFSAs) by earthworms (Eisenia fetida) in soil”. Environmental Pollution 179: 45–52. https://doi.org/https://doi.org/10.1016/j.envpol.2013.04.002.
