ABSTRACT
The landfill leachate is considered a toxic effluent composed of recalcitrant contaminants that requires innovative alternatives for its decontamination. Coupling between advanced oxidation processes (AOPs) and aerobic biological treatments are highlighted in this research. Therefore, a bibliographic review of the research made from 2010 to 2021 was developed. These combined alternatives were applied in leachates, and it is oriented toward the analysis of knowledge gaps, trends, and future proposals of the treatment combined that contribute to researchers who wish to work on the subject. These kinds of treatments were chosen due to a bibliometric analysis made. Also, the information was searched in several scientific database. This work was found to be unpublished, as no reviews were found so far that agglomerate studies of coupling between photocatalytic and aerobic biological processes to treat leachates. Besides, AOPs are ideal for treating wastewater of complex composition, however, when it is used as the only treatment, they are usually unprofitable, which justifies their coupling with biological treatments. Subsequently, it was determined that the knowledge main gap is the lack of documentation of treatment costs, which makes it difficult to implement on a real scale. In addition to this, the couplings trends are toward doping with metallic and nonmetallic ions of the catalyst used in the photocatalytic process to improve the efficiency of these. Finally, future research should work on finding alternatives that allow the optimization of the resources used in the combined systems and on promoting the recovery of existing products in the leachate.
Implications: Leachates generate several environmental impacts due to their toxic composition. Even when coupling between heterogeneous photocatalysis and biologic treatment can solve them, issues like cost analysis and the scaling-up factor have not been developed, and futures researchers should work on that. Besides, the trend founded in almost all investigations was the catalyst doping with metals and nonmetals ions, particularly when they use TiO2 because it gives the possibility of improving efficiencies just with a structural variation. Finally, these treatment combinations require more analyses and comparison of their remotion over emerging pollutants and their performance with new designs.
Introduction
From the degradation and percolation of the waste deposited in the sanitary landfills and due to the effect of their contact with rainwater, a residual liquid called leachate is generated. This liquid is characterized by high levels of toxic and recalcitrant compounds (Mukherjee et al. Citation2015; Ruiz-Delgado et al. Citation2020) making it difficult to treat efficiently and economically (Luo et al. Citation2020). Its composition is usually determined by factors such as the geographic area in which the landfill is located, the type of waste disposed of at that site (Mavakala et al. Citation2016), and the age of the landfill. The last mentioned is a determining factor at the moment to evaluate the degree of contamination (Costa, Alfaia, and Campos Citation2019). According to (Caroline Baettker et al. Citation2020; Sackey, Kočí, and van Gestel Citation2020), it is possible to make predictions about the composition that leachate could have simply by analyzing a series of factors in which it is generated. There are even studies such as the one carried out by (Bhatt et al. Citation2017), which are oriented toward making predictions that allow knowing BOD5 and COD values from mathematical models.
(Vaccari, Tudor, and Vinti Citation2019) found that in areas with high levels of rainfall there is a higher degree of metal concentration and solubility of leachate contaminants. Similarly, they conclude that the degree of pollution of this residual liquid shows a higher concentration in the dumpsites than in the landfills, caused by the lack of operational controls. Likewise, it was found that leachates contain higher concentrations of organic material in equatorial zones than in warm temperate zones and in landfills whose operation exceeded 10 years. In addition, (Somani et al. Citation2019) and (Zegzouti et al. Citation2019), who compare different samples of young leachates and mature leachates, conclude that the last mentioned have lower concentrations of contaminants in terms of COD and biochemical oxygen demand (BOD5).
On the other hand, the consumption of products used by the population which ends their useful life in a landfill is the main factor that determines the composition of it. This is confirmed by (Moody and Townsend Citation2017), who noted that landfills that dispose of urban solid waste contain higher concentrations of organic material in terms of BOD5 and TOC, while for landfills where industrial waste are disposed, the composition of this leachate is based on metals and some anions. (Ishchenko Citation2019) related concentrations of heavy metals in leachates to the type of waste that generates it, founding that in landfills where toxic wastes were disposed, higher values of metals were reported, which increases the toxicity of the leachate (Gupta and Paulraj Citation2017) and at the same time, it restricts the type of treatment to be selected for decontamination.
Another key factor when making this selection is biodegradability, which especially conditions the application of biological treatments. Studies have shown that the value of this parameter is inversely proportional to the age of the leachate and it is in a range below 0.3 (Castillo-Suárez et al. Citation2019; Chen et al. Citation2019a; Ghahrchi and Rezaee Citation2020) which is often calculated from the relationship between BOD5 and COD. However, (Corsino et al. Citation2020) proposed that the value of BOD5 in the leachate could suffer alterations associated with its toxicity, so it is validated the calculation of Biodegradability from the TOC/COD ratio, where the value is directly proportional to the age of the leachate (Chou et al. Citation2015).
Also, according to the report of “Global Perspective on Waste Management” published by the United Nations, between 7 and 10 billion tons of solid waste are generated in the world every year, if it is considered that the volume of leachate generated per unit of waste mass is around 25% (Youcai Citation2018) this would mean that each year more than 2 billion liters of leachate are generated in the world. Hence, this requires significant attention, due to if there is a failure in the control of them, this problem can affect water sources near to the waste disposal site (Yan et al. Citation2015; Vahabian et al. Citation2019) with substances that are toxic to health (Swati, Vijay, and Ghosh Citation2018). According to the last, (Dullius et al. Citation2020; Maiti et al. Citation2016; Mishra et al. Citation2019) analyzed groundwater and surface water samples from around the landfills, and in further physicochemical analyses, high concentrations of heavy metals (Pb, Fe), as well as chlorides and dissolved solids, were found.
According to (Przydatek and Kanownik Citation2019; Talalaj and Biedka Citation2016), a water source contaminated with leachate negatively impacts organisms that come into contact with it such as algae, plants, invertebrates, or mammals. This observation is supported by (Khalil et al. Citation2018), who made a crossing between human cell cultures and leachate-contaminated water, and demonstrate drastic damage to the DNA with the capacity to affect human morphology and be a precursor to diseases such as cancer (Annangi et al. Citation2016; Izah, Chakrabarty, and Srivastav Citation2016; Yaghmaien et al. Citation2019), so it is of vital importance to radically avoid contamination of water sources and the food chain with the residual liquid under study.
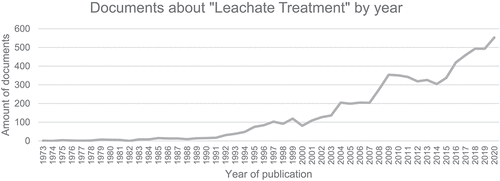
Regarding leachate treatment and according to the information available on the Web of Science, approximately 598 articles were published worldwide during 2019, which represents 293 more than those published in 2010, and China is the country that made the greatest contribution to this topic during this period. In addition, according to information consulted in Scopus, it was found that the first article related to leachate treatment was published in 1973, (Thornton and Blanc Citation1973) who conducted leachate decontamination from a chemical process, reaching high removals in suspended solids and color but low efficiency in terms of organic and inorganic material, this concluded that new treatment alternatives should be developed that would allow a general removal of the contamination present in the residual liquid. Below, the traceability and increase in the number of annual publications related to leachate treatment are graphically represented in .
The development of leachate treatment technologies is a challenge for the researchers, generated by the wide range of contaminants in the leachate, and whose aim is to return this residual liquid to the environment without affecting the environment, regulating the cost and efficiency of the treatment applied. The highlights of some biological and physicochemical treatments are shown in .
Table 1. Comparison of leachate treatment alternatives.
According to , is possible to conclude that all alternatives have a thing for highlighting. However, biological treatments cannot be the first best solution because the toxic composition of leachate may inhibit the microorganisms. Also, physicochemical treatment properties can get better the biodegradability of leachate, allowing an effluent optimum for coupling with biological treatments. A relevant gap found was the lack of research that compare several treatments with the same experimental conditions and carry out analysis with different inlet compositions. Also, few papers include financial analysis of the treatments.
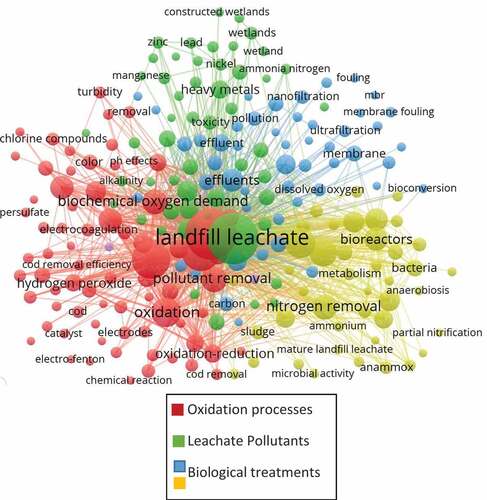
To find out which treatments are the most used for decontamination of landfill leachate, and trying to determine which ones can be combined, and which ones have achieved the greatest relevance from 2010 to the present, the VOSVIEWER tool is used, the goal of it is the creation of a correlation map shown in for oriented the manuscript toward a possible coupled able to remove toxic and emerging pollutants.
According to , it is possible to determine that, in relation to treatment of coupled leachates, since 2010 the key words most frequently present in scientific papers are “landfill leachate treatment” with an occurrence of 403 papers and “Chemical Oxygen Demand” with an occurrence of 231 papers, which in turn appear connected with the topics “bioreactors” (162 papers) and the key word “oxidation” (134 papers), which are also connected and show a direct relationship with biodegradation (55 articles), hydrogen peroxide (47 articles) and with specific treatments both biological and membrane bioreactors (37 articles) and activated sludge (37 articles) represented mostly by the color blue and gold, as well as with specific advanced oxidation processes such as hydrogen peroxide (47 articles), fenton reaction (31 articles), ozone (29 articles) represented mostly by the color red. It follows then that these treatments are the ones commonly used in a coupled way for the removal of contaminants present in leachates.
Besides, it is possible to conclude that methodologies for optimization processes like exergy analysis have not been used for researchers because this keyword was not correlated in papers consulted in . The last represents a gap of knowledge in leachate treatments and allows that futures researchers deepen in this new insight for getting better investigation results. Also, the main parameters analyzed in the bibliography consulted were ammonia (139 articles), toxicity (31 ones), heavy metals (51 ones) and organic matter (37 ones), then futures research should be oriented to evaluate these parameters of landfill leachate.
This bibliographic review wants to deep into the results getting in the correlation map shows in , where two alternatives (AOP-Biological treatment), which have been trending in the last years, can be related to each other, analyzing the researcher’s methodologies applied, and trying to achieve new perspectives of them. Taking into account that leachate compositions are dynamically random, no conventional and innovative treatment techniques such as advanced oxidation processes are needed to offer alternatives to conventional systems currently in use (Kamaruddin et al. Citation2015), directed toward the elimination of emerging contaminants and strong chemical structures, which according to (Nika et al. Citation2020), exceed traditional alternatives, biological treatments and some of greater complexity such as reverse osmosis without being removed, but thanks to analyses made by (Park et al. Citation2020), it is demonstrated that by applying a process of photocatalysis with TiO2/UV, the removal of these contaminants is obtained by the action of the OH radicals which are generated. Another benefit of these combinations is associated with the increase of 70% in the biodegradability of the leachates after the chemical process, which reduces their complexity and allows an ideal influence to be treated by a process of less complexity such as the biological ones. (da Costa et al. Citation2018). As an example of the last, (Chen et al. Citation2019b; Gomes et al. Citation2019b) employing these combination achieved high removals of ammonia nitrogen, heavy metals, and organic matter represented in the COD and BOD5. Publications in which AOP and biological treatment are used together in leachate are shown in .
Table 2. Publications in which AOP and biological treatment are used together in leachate.
Analyzing , is possible to conclude that the main cost of the advanced oxidation process was energy consumption, mainly when UV-lamps were used. The last is so strongly related to the photoelectron alternative, which also has problems with the amount of the reagents used to achieve optimal conditions. For biological treatments, according to , MBR can be the best option in the landfill leachate decontamination field. However, researchers did not include an alternative to treat the membrane waste generated in the process, knowing that although they remove pollutants due to the microorganism action, membranes after their useful life will be toxic and hazardous waste. Finally, sludge activated can be effective in the purification of young leachates because these have high biodegradability. However, this one should not be used for medium-aged or mature leachate, because these inhibit the microorganisms, getting the lowest efficiencies.
Successful studies of couplings photocatalytic treatment – biological Process in leachate pollutant removal
(Klauck et al. Citation2017; Mohajeri et al. Citation2019; Mohan et al. Citation2019; Tripathy et al. Citation2019; Zhang et al. Citation2018) applied AOPs for leachate decontamination, achieving removals over 70% in terms of COD, TOC, and some emerging contaminants. However, despite being an attractive alternative due to its effectiveness, AOPs are usually expensive when they are used as the only treatment, which is why it is recommended that they are implemented as a pre-treatment to obtain an effluent with biodegradable properties and notoriously less toxic, which can be coupled with conventional systems such as biological treatments (da Costa et al. Citation2018) or are implemented as polishing processes that remove contaminating agents that have not been eliminated (Zhao et al. Citation2020)
Similarly, (Baiju et al. Citation2018; Cai et al. Citation2020; Kattel et al. Citation2016) demonstrate that the combination of physicochemical and biological processes offers better results than their independent implementation. Among these connections, the one carried out by (Chemlal et al. Citation2014) stands out. They combined a TiO2/UV photocatalytic treatment with activated sludge, obtaining removals of more than 86% for both BOD5 and COD and achieving an increase of more than 60% in the biodegradability of the phototreated effluent, which, considering the cost and efficiency of the treatment, is highly striking, especially due to the use of solar radiation. However, the authors did not establish an escalation factor, which could make it difficult to implement on a real scale. Although there is enough literature related to combinations, especially between advanced oxidation processes followed by biological treatment, there has not been a deepening in the elaboration of works that agglomerate such information and evidence the specific benefits of the different couplings, especially of heterogeneous photocatalysis using TiO2 with biological processes such as activated sludge or membrane bioreactors, which gives opportunity to bibliographic reviews on the subject as the one developed in the current document.
Regarding the order of application of combination treatments, according to (Oulego et al. Citation2016), subjecting a raw leachate sample to a conventional biological process results in an insignificant reduction in parameters such as toxicity and color, and it is then necessary to apply the photocatalytic process as a pre-treatment. However, in some cases the chemical treatment can be applied as a polishing action or a subsequent stage to highly complex biological processes that offer highly treated effluents as is the case developed by (Hassan et al. Citation2017), who applied a heterogeneous photocatalytic treatment after a waste-based bioreactor analog, obtaining 82% COD removal after the use of advanced oxidation using TiO2, proving that by using another catalyst (S2O8), a 62% removal is obtained, but that by increasing the pH of the treatment solution, that is to say, resembling it to a real condition, an efficiency in the elimination of NH4, greater than 90% is achieved with this last oxidant. In addition to this, it should be taken into account that factors such as turbidity could also affect the performance of the above-mentioned AOP, so a pre-treatment is recommended to ensure the reduction of solids and thus to adapt the conditions necessary for the photons to enter the treatment system.
Problems, trends, and challenges of photocatalysis treatments in presence of a biological treatment
Although photocatalysis using TiO2 is indeed considered a treatment capable of oxidizing pollutants with chemically complex structures through reactive oxygen species without emitting any toxic residue in the phototreated effluent (Athanasekou, Likodimos, and Falaras Citation2018), it is also true that the main problem related to the mentioned catalyst is oriented toward its efficiency, where only 7% of the band interval is solar powered (Sanzone et al. Citation2018). According to (Xing et al. Citation2018) it is possible to state that the main challenge faced by future research wishing to improve removal efficiencies using this advanced oxidation process in the presence of solar UV, will be based on the achievement or modification of a broadband catalyst which allows capturing a greater amount of solar radiation. According to (Likodimos Citation2018), this kind of improvement will allow mixing the mass flow, the slow photon transit and the adhesion of porous macro structures, which will finally be beneficial for the electronic properties of the catalyst.
A recent review of the application of photocatalytic treatments for the decontamination of toxic wastewater, in which leachates are included, by (Rueda-Marquez et al. Citation2020), places us in front of certain affirmations that evidence the state of this alternative at the present, this emphasizes that despite being an effective treatment, the intellectual and bibliographical production in the subject is quite limited compared to other processes of advanced oxidation as the Fenton. Also, the works must shorten the existing gap, especially in the scale of application of the treatments, since most of them are developed at laboratory scale and the costs and feasibility of implementing the designs at real scale are not included. Also, it is necessary that the works shorten the existing gap, especially in the scale of application of the treatments, since most of them are developed at laboratory scale and the costs and feasibility of implementing the designs at real scale are not included. Additionally, the pH of the influent becomes a challenge to optimize, because it unusually presents the acidity conditions required by the AOPs to achieve efficiencies above 70% in the removal of contaminants (Boczkaj and Fernandes Citation2017; Casado Citation2019). Also, when the researchers work with natural pH, usually the treatments time increase considerably, as in the case of (Wang et al. Citation2021), who developed their analysis with a natural leachate pH of 8.8, and the optimal conditions were getting after 9 hours which considering topics like the energy cost, or the reagents cost can difficult the treatment sustainability, being remarkable the innovation in future treatments that are developed in a pH close to the real but without affecting the time and cost of the same.
On the other hand, the current research tries to develop an analysis of the possible gaps in knowledge, limitations, problems, challenges, and opportunities based on the results and the scientific progress found in numerous publications on advanced oxidation processes (AOP) couplings, especially TiO2/UV heterogeneous photocatalysis with aerobic biological treatments, which have been used to remove contaminants from leachates coming from sanitary landfills. It also analyzes predictions and aspects that should be emphasized in future research on the subject, oriented toward the conservation and preservation of water, looking for a way that once the leachate is treated, an effluent with optimal conditions can be generated to offer the possibility of reusing and minimizing the environmental impact commonly caused. Furthermore, some opportunities offered by leachate treatment are discussed, such as the recovery of substances as metals and minerals or energy efficiency alternatives that have been applied and that are striking in meeting the challenges of sustainable development proposed by the current and future population.
Discussion
According to the review carried out in this article, the coupling of advanced oxidation processes with biological treatment for the treatment of leachates is not usual; but its proper implementation would allow the reconversion, adaptation, or redesign of existing treatment systems, in some cases considered obsolete, to treat leachates from sanitary landfills with significantly improved efficiency. The methods usually studied individually for the treatment of leachate frequently include biological, activated carbon adsorption, chemical oxidation, membranes, physicochemical, among others. Chemical oxidation by itself has shown good results in the elimination of specific pollutants that are difficult to treat, but the problem with its implementation alone is its high cost of operation. Although biological treatment can decompose several highly relevant pollutants, it is necessary to first convert initially persistent organic compounds into biodegradable intermediates, because if raw leachate is exposing to this kind of treatment, its pollutants removal efficiency will be so low, as the case of (Wiszniowski et al. Citation2006) who applied sludge activated over raw leachate, getting a COD remotion under 20%. But after a photocatalytic coupled (UVc/TiO2), the COD remotion increase more than 70%. Hence, advanced chemical oxidation represents a solution. In this way, the combination of chemical and biological oxidation, although integrating several techniques, can drastically reduce the consumption of oxidation agents, and their combined cost would be significantly below that of other disposal options such as incineration. In addition, if the recycling of water and other valuable components is achieved, not only would the cost be reduced, but also the overall effect on the environment
“Catalyst doping” as the keycard for getting better results
Understanding that the main problem found in heterogeneous photocatalysis is the bandgap, a lot of researchers have been developed their analysis in presence of metal and nonmetal ions, for improving their pollutant removal efficiencies and for getting better treatment costs. A clear example of the last was the recent coupling done by (Yasmin et al. Citation2020), who developed a photocatalytic treatment using TiO2/Ag as a catalyst in the presence of UV radiation combined with a batch biological process worked with an inoculum using the “Candida Tropicalis” strain. To obtain better results, they used a process to optimize variables such as catalyst concentration, pH levels, and reaction time, which allowed them to conclude that at acidic pH and with a test time of fewer than three hours, more than 69% of parameters such as COD and TOC are removed, while the biodegradability of the leachate increases significantly, making it wastewater suitable for continuing with a biological process. Once the coupling is applied, the treatment efficiency improves significantly, reaching 90% COD removal and 84% TOC. They also proved that by using recycled TiO2/Ag, removal percentages higher than 60% are achieved. What is striking about the mentioned alternative, in addition to the evidenced results that validate its usefulness, is that the necessary irradiation to reach such percentages is much lower compared to another advanced oxidation alternative such as photo-electrooxidation, which requires approximately ranges close to 380 W*cm2 evidenced in the studies made by (Müller et al. Citation2015) (Klauck et al. Citation2017).
Similarly, this treatment highlights the application of metal ions combined with TiO2, since higher removals are achieved from a chemical modification of the catalyst, which in turn reduces the reaction times of the treatments, reducing operating costs and making it feasible to implement the photocatalytic process on a real scale. Despite all the benefits mentioned, it is possible to detail that in order to achieve large removals in the photocatalytic process, it is necessary to reduce the pH being validated by (Arshad et al. Citation2020; Kaabeche et al. Citation2019) which at a real scale could mean a problem related to the increase in the operating cost of the combined system, but at the same time it becomes an opportunity to develop knowledge based on the design of new alternatives that allow the omission of this process of acidification of the phototreatment solution.
Another combination of treatment applied to leachates was done by (Elleuch et al. Citation2020), who coupled a biological treatment using kefir grains with an advanced photocatalytic oxidation process employing nanoparticles of the TiO2 catalyst, which is doped with ions of a metal (Ag) achieving post-docking removals of 96% and 98% in terms of COD and TOC. The notable aspect of the research is that by obtaining an effluent with much reduced polluting characteristics, there is an opportunity to reuse the treated water, which is then a valid option oriented toward the sustainability of the water resource. However, it would have been interesting to know the effect of treatment when subjected to different concentrations of leachate, since as is well known the composition of this waste liquid is highly variable concerning the type of waste deposited in the landfill.
About the doping of the catalyst with metallic ions used in the works mentioned above (in both cases with Ag), it is possible to conclude that making this chemical modification increases the antibacterial and photocatalytic activity, data experimentally verified by (Ali et al. Citation2018). Also (Mohan Reddy and Devaraju Citation2019) determined that it is possible to increase the reaction kinetics with a simple addition of silver (Ag) to the structure of the catalyst. A similar case was made by (de Matos Rodrigues et al. Citation2019), who by combining cerium oxide with TiO2 managed to generate a heterostructure that facilitates the removal of emerging contaminants in a photocatalysis process. Another analysis in which they apply this variation was done by (Rivero et al. Citation2020) who by structurally combining TiO2-rGO obtained photocatalytic degradations of different emerging contaminants much higher than when using unmodified TiO2. According to (Barkul et al. Citation2017), doping with metals to the catalyst generates thermal instability and these materials can reduce photocatalytic activity since it could simulate hole traps.
Subsequently, there are also numerous investigations in which work has been done with nonmetallic dopants such as iodine (Zhang et al. Citation2014b), nitrogen (Barkul et al. Citation2016; Mohamed et al. Citation2015), boron (Dozzi et al. Citation2014), fluorine (Dozzi and Selli Citation2016) and some combinations between these elements (Hamilton et al. Citation2014), which demonstrates the versatility of the TiO2 catalyst and provides insight into the direction of researchers implementing these treatment alternatives (Fagan et al. Citation2016; Parangi and Mishra Citation2019). However, it is possible to conclude that an in-depth comparison is needed to clarify which would be the ideal doping for the catalyst applied specifically to the treatment of leachates, since until now there has been frequent work on decontamination of industrial and textiles effluents (Al-Mamun et al. Citation2019; Janitabar-Darzi Citation2014; Kanan et al. Citation2020; Sahoo, Gupta, and Pillai Citation2012) while in the few studies on leachates doping has been applied with metal ions.
Similarly, future research should determine the ranges of contamination that the residual liquid entering the photocatalytic system could have, which allows the simulation of real conditions and thus facilitate the real scale of the treatments. (Azadi, Karimi-Jashni, and Javadpour Citation2018) use a simulation based on genetic algorithms and artificial intelligence to find out the removal efficiencies when leachates are subjected to treatment with metal-ion doped TiO2, which offers an additional alternative that would optimize the resources spent in the experimental phases and which in turn would allow the incidence of the variables in the treatment to be calculated as a percentage. However, the most important challenge for futures researchers should be to work with catalysts and metallic or nonmetallic ions that are not toxic to the microorganisms that carry out the coupled biological processes but rather contribute to their development. shows a brief analysis of TiO2 doping that has been making in recent years.
Table 3. Doping catalyst applied in toxic wastewater.
Critical analysis of coupled alternatives for treating toxic wastewater like leachates and some views toward the future of them
Besides of the solution developed in the last section, which is the way that relevant amount of researches are following and in the futures investigation they will optimize, the study developed for (Sinha et al. Citation2018) mention that the separation and recovery of the catalyst is currently a problem of photocatalytic process couplings. This becomes a beneficial opportunity for this type of alternatives, since if it is solved, the cost of the treatment will decrease. It is recommended for future research to develop couplings capable of retaining and giving opportunity for reuse of the catalyst without affecting its efficiency. Recently, several researchers have worked on alternatives that allow the separation of the catalyst and extend its useful life in the treatment system, within which it has been discovered that the use of the photocatalytic process combined with membrane systems could provide beneficial results to the mentioned problems.
The above is justified in the work conducted by (Doruk, Yatmaz, and Dizge Citation2016), who based on a hybrid design of TiO2 membrane immersed in the photoreactor proved to be useful concerning the recovery and reuse of the catalyst, however, it does not exceed 55% of removal in terms of COD. Another research in which the use of membranes with photocatalytic reactors is integrated is carried out by (Deveci et al. Citation2016), who coupled a TiO2/UV photocatalytic reactor with a membrane biological system obtaining COD efficiencies of 93%, which emits an effluent with characteristics suitable for their re-use. The latest proves that comparing AOP coupled to MBR with other couplings treatments like electrocoagulation with biofiltration (Le, Dang, and Tran Citation2021) the efficiency for removing pollutants measured in COD is around 20% higher. Nevertheless, the main difficulty of this alternative is associated with the high costs of implementing this coupling, but if the removal time, the source of UV radiation and the complexity of the reactors used are adjusted (Pan et al. Citation2019), it could become the system that would offer the greatest benefits in the treatment of leachate in the future.
Subsequently, some researchers in their studies apply a coupling between biological processes in batch with a TiO2-based photocatalytic ozonation treatment for the removal of contaminants in chemically complex industrial wastewater with the presence of metals and recalcitrant contaminants similar to the composition of the leachates (Chávez et al. Citation2019). The authors demonstrate that making a dilution with domestic wastewater increases the biodegradability and reduces the concentrations of contaminants, in turn, it could provide an environmentally friendly solution for untreated domestic effluents. It is also proven that developing an advanced oxidation process in the presence of TiO2 and solar radiation approximately doubles the effectiveness of doing so without these conditions. Another important event that the authors determined is that after the reuse of the catalyst used, removal efficiencies of approximately 67–74% are obtained, validating the importance of the coupling used. Despite the findings, there is no evaluation of the operational costs that such couplings would require on a full scale, making it difficult for this alternative to be implemented by other authors.
Concerning the costs involved in the couplings, (Paździor, Bilińska, and Ledakowicz Citation2019) analyzed various combinations between biological processes and advanced oxidation processes applied to industrial effluents of a textile nature and, although it is true that they are not leached, could be similar due to their toxic characteristics. The authors conclude that electrical energy consumption represents the main demand for resources, especially in relation to the consumption of lamps used in advanced oxidation processes. (Koh et al. Citation2004), developed an analysis with 4 kinds of lamps with different sets up and electricity amount consumption, where the best remotion result was getting with the low-pressure mercury lamps with a wavelength of 254 nm but a consumption of more than 80 kW per m3, and making complex its use on a real scale, because it will not be suitable for treating total leachate volume produced in landfills. Therefore, it is necessary to implement environmentally friendly alternatives and different methodologies to optimize treatment costs. According to (Marcelino et al. Citation2015), the use of solar radiation in advanced oxidation processes promotes sustainable development and in turn reduces the pollutant load of effluents with low biodegradability. This is validated by (Spasiano et al. Citation2015), who evaluate the performance of different reactors that operate with solar radiation highlighting that the Composite Parabolic Collectors (CPC) present advantages over other designs such as non-concentrating collectors or cylindrical parabolic collectors since their geometry allows the capture of direct and diffuse sunlight entering the reactor serving as an alternative for the treatment of toxic effluents such as leachates (Silva et al. Citation2013) and concluding that the use of heterogeneous solar photocatalysis with TiO2 could be considered as an innovative system capable of generating a positive impact on the environment. Despite its usefulness, there are several challenges for future research, such as detailed economic analysis of the system, including the cost of maintenance and reactor construction (Díez, Sanromán, and Pazos Citation2019), increasing quantum efficiency, and guaranteeing the reliability of the system in the medium and long term (Malato et al. Citation2016). Other materials that are more economical but do not affect the uniform distribution of sunlight in the reactor or the efficiency of treatment should also be tested.
Another alternative that has been developed in recent years to promote energy efficiency in wastewater treatment is exergetic analyses, which, based on thermodynamic balances, identify the factors and components of the processes involved that consume the most energy, facilitating the development of corrective actions that favor treatment. (Fitzsimons et al. Citation2016; Różycki and Banaś Citation2018; Tumen Ozdil and Tantekin Citation2016; Vakalis et al. Citation2017), have applied these exergetic analyses in wastewater treatment, however these analyses are not very frequent, then it is an innovative proposal for future authors to include in their experimental designs due to with this alternative researchers can know the real work and performance of the treatments.
Also according to (Rodrigues, Madeira, and Boaventura Citation2014), the consumption of reagents is considered of high economic impact to the systems, related to the nutrients necessary to execute the biological process adequately and to the solutions used in the modification of the pH especially in the chemical process. Similarly, the economic analysis of combined treatments made by other authors is not very frequent because most of them are executed on a laboratory scale and do not include it within their objectives. However, when comparing some advanced oxidation processes, it is possible to conclude that catalytic solar oxidation (2 USD per m3) (Costa et al. Citation2013) is cheaper than other alternatives such as Fenton (9.6 USD per m3) (Cortez et al. Citation2011) and O3/UV (11.7 USD per m3) (Venkatesh et al. Citation2015). These values are mostly calculated by applying the equation proposed by (Bolton et al. Citation2001) which multiplies the kW consumed during the AOP by the time of treatment in relation to the volume of wastewater treated and the variation in the concentration of the pollutant, so it is possible to deduce that by not using energy in the generation of UV radiation the monetary cost decreases significantly (Cardoso, Bessegato, and Boldrin Zanoni Citation2016). Factors such as maintenance, human resources, reaction rate, the nature of the wastewater, the flow to be treated, the process residues and the configuration of the reactors complement the economic analysis of combined treatment (Bilińska, Gmurek, and Ledakowicz Citation2016; Buthiyappan, Abdul Aziz, and Wan Daud Citation2015; Jaafarzadeh et al. Citation2018), which means that the chosen systems must be easy to operate, If, instead of a user-friendly biological system, a highly trained personnel is selected, this could affect the operational cost of the system, making it difficult to implement on a full scale. Despite this, according to (Starling et al. Citation2017), if a treated effluent has the properties to be reused in a company, the economic benefit would be outstanding, creating the need for the coupled system to offer such guarantees, which after a detailed cost-benefit analysis could mean the recovery of the investment made in the treatment and in turn reduce the environmental impact generated, in this case of study by the landfill.
Knowing the different problems that society faces in relation to the depletion of resources especially associated with the consumption of fossil fuels and starting from the fact that the coupling for leachate treatment worked out in the current research is developed from solar radiation, an interesting proposal would be the production of renewable photovoltaic energy while the residual liquid is decontaminated. This topic was also mentioned by (Dewil et al. Citation2017; Izumi Citation2015), concluding that more studies are needed to work on the subject, especially on the separation of semiconductor loads and the calculation of the reaction kinetics, costs and power generation capacity that the system could offer. Alternatives like exergy analysis would contribute to the solution of this problematic.
Another problem that researchers have not developed into their studies is the remotion of emerging pollutants which are in Landfills leachates like antibiotics, Per- and Polyfluoroalkyl Substances- PFASs (Tian et al. Citation2018), bisphenol S -BPS (Fang et al. Citation2020), N-butyl benzensulfonamide (Eggen, Moeder, and Arukwe Citation2010) and according to (Xie et al. Citation2020), these pollutants can degrade the health of people, especially the endocrine system. Hence, keeping in mind these kind of pollutants cannot be removed with conventional treatments like adsorption or some biological process (Zhang et al. Citation2020), It is necessary the use of alternatives like the coupled of heterogeneous photocatalysis and non-conventional biological processes like membrane bioreactors, which according to (Tolosana-Moranchel et al. Citation2019) and (Sui et al. Citation2017), It is possible to get the highest remotion level with each one of them mainly for the intervention of the hydroxyl radical (Wang and Xu Citation2012) and the decontamination action of the membranes. The chief gap of knowledge is that there is much information that proves the usefulness of these treatments, but there are not enough papers that evaluate the performance of both alternatives over emerging pollutants when they are coupled, and also almost all research calculated the treatment efficiency in terms of COD and BOD5, but they did not analyze these kinds of pollutants. Then, this is an opportunity for futures researchers who want to carry out works that include emerging pollutants and the combination between an Advanced Oxidation Process and a Biological Process.
To the end of this section, it is so important that futures research include in their analysis the topic mentioned in section 2.1., through the “doping catalyst” the cost, sustainability, and energy demand of treatments can be reduced. If the efficiency of AOP is improved, the energy demand used in the aeration process will be less than develop without doping because the process requires more reaction time. Also, this is strongly related to sustainability, according to , removal efficiency was more than 95%, eliminating recalcitrant and emerging pollutants, things that conventional treatment cannot get. In addition, it contributes to the sustainable development goals, especially goal number 11. Sustainable cities and communities, improving common processes of a city as a leachate treatment generated by municipal waste disposal.
Recovery of valuable products from leachate treatment for improving sustainability
Finally, getting to know the leachates in addition to the pollutant load, have a rich composition in nutrients, which if applied treatments correctly could be recovered. Considering the above (Podder, Reinhart, and Goel Citation2020) show that by combining a physical treatment and an aerobic biological process, it is possible to recover 77% of phosphorus while eliminating 80% of BOD5, however parameters such as COD obtain degradations between 27 and 58%, and if it is taken to a real scale would require a subsequent treatment that reduces environmental impacts and allows to comply the dumping regulations. It would also be interesting to perform these tests on leachates with higher physicochemical parameters and thus facilitate the use of these full-scale studies. Similarly, (Chang et al. Citation2019) achieved a recovery of more than 73% of nitrogen in landfill leachate using a membrane photo-bioreactor while reducing the toxicity of the residual liquid, a value similar to that calculated by (Liu, Novak, and He Citation2020), who by coupling a biological treatment and an advanced oxidation process achieve a recovery rate between 30 and 50 kg of nitrogen per m3. The recovered nutrients have the potential to be used in microbial resynthesis and targeted to economic sectors such as agriculture or animal feed production (Matassa et al. Citation2015; Xu et al. Citation2017).
Also, landfill leachates contain different metals such as iron, copper, zinc, and lead, which can also be recovered and used if certain treatment techniques are applied (Wu et al. Citation2015). The researchers (Zhao, Guo, and Qu Citation2014) worked a photoelectrocatalytic oxidation from TiO2 in leachates, reaching a recovery of more than 84% of copper ions but the high energy consumption becomes the main disadvantage found. According to (Iskander et al. Citation2016), the main problem in metal recovery lies in the low concentrations of metals in some leachates, which in turn are influenced by factors such as the age of the landfill and the characterization of the waste that is disposed there. Additionally, some researchers have been developing alternatives that offer the possibility of recovering energy from treated leachate like the case of (Damiano, Jambeck, and Ringelberg Citation2014) who use a biological fuel cell for leachate treatment with 74% BOD5 removal and small energy generation.
Through some studies, the authors (Gu et al. Citation2019), indicate that three paths allow the generation of bioenergy derived from leachate treatment. Firstly, there is the production of bioenergy from methane produced by anaerobic treatment systems such as UASB reactors and anaerobic membrane bioreactors (Luo et al. Citation2015; Xie et al. Citation2014). The second alternative is related to the production of biohydrogen, which is considered a clean energy source (Anwar et al. Citation2019), however the most notorious limitation is associated with the amount of phosphorus in the leachate which conditions the generation of hydrogen and therefore the efficiency of the process. A third alternative is the use of microbial fuel cells, which have the capacity to convert chemical energy into electrical energy from the metabolism of microorganisms (Hassan et al. Citation2018), this alternative has been replicated by other authors like (Elmaadawy et al. Citation2020; Tao et al. Citation2014; Zhang et al. Citation2014a), nonetheless its main challenge is to achieve the scaling up of this treatment to real life. In general, it is possible to determine that the main problem associated with energy recovery from leachate treatment is that bibliographic production is very limited and so far no studies have been carried out to demonstrate the real application of this recovered energy, making it an attractive option for the future, since as with nutrients and everything that can be recovered from this residual liquid, it could be considered as an added value since it would not only allow the decontamination of the leachate but would also contribute to the environmental and economic sustainability of the treatments applied.
Conclusion
From the information gathered, it is concluded that there are no published review articles that bring together research on combined treatments of heterogeneous photocatalysis and aerobic biological reactors, applied to the decontamination of landfill leachate. Therefore, the work carried out is pioneering and can guide future research on the subject, since it brings together several studies worldwide, analyzing the knowledge gaps, problems, trends, and projections that impact the development of these alternatives. Likewise, the coupling between AOP and biological treatment can contribute to the achievement of sustainable development goal number 11 “Sustainable cities and communities,” since they allow reducing the environmental impact generated in the disposal of municipal waste.
Besides, it has been proven that TiO2 is the most widely used catalyst in photocatalysis processes, especially due to its nontoxic characteristics, cost, and capacity to generate OH radicals. However, the main problem found is associated with its band interval, since only 7% of it is solar powered. Therefore, the trend in most recent research to understand this issue is to make chemical modifications on the catalyst with the addition of metallic and nonmetallic ions trying to improve the treatment efficiency, but there is a relevant lack of information associated with the behavior of this wastewater during the biological treatment since not all the doping would be beneficial because they could generate the inhibition or death of the microorganisms intervening in the process.
Finally, the goal of all treatments is sustainability and environmental benefit. According to the last, couplings between AOP and membrane bioreactors allow removing more than 90% of pollutant’s leachate such as Heavy metals, COD, TOC, and refractory pollutants. This is amazing, considering the complex level of this kind of wastewater. However, it was found that most research omits the scaling up the factor of combination treatments, evidencing a knowledge gap, making it difficult to apply the treatments. Also, it was found that energy consumption is the main cost of the combination between heterogeneous photocatalysis and aerobic biological reactors, so the development of these in the presence of solar radiation would solve the problem significantly. Also, it was highlighted the importance of apply exergetic analysis, when researchers used it, they could optimize their treatments related to the reduction of resources and reagents.
Future perspectives
Future works that apply the mentioned coupling should emphasize the recovery of minerals like phosphorus or nitrogen, which would contribute to the sustainability of the environment, since from a residual liquid as polluting as the leachates raw materials could be generated to be used in the manufacture of fertilizers or other products of diverse sectors of the economy. The researchers can try couplings of AOP with MBR, and due to the knowledge gap analyzed in the review, it is recommended that for future research the operational costs and projections are published.
Besides, almost all research was done by acidic pH conditions, which is far from the reality since the leachate presents values close to neutrality, thus requiring pH regulating substances, which in turn increase costs. However, this allows for future projects to work on achieving the structural modification of TiO2 in such a way that it maintains its capacity to generate hydroxyl radicals during the treatment process but developed at near-real pH. Other catalysts that offer removal efficiencies greater than 70%, among which Sulfur derivatives could be found, and it should be tested and compared with TiO2 to get the optimum catalyst.
As demonstrated by several researchers, leachates contain a great variety of heavy metals, so the future of the treatment of these should not only focus on removing them, but on the contrary should be oriented toward the maximum possibility of recovery of the existing metals, which would generate an important valorization of the alternatives proposed.
Futures researchers should develop analysis about the removal of emerging pollutants that are in landfill leachate through Heterogeneous Photocatalysis coupled to Biological Treatments due to this topic has not been deepened, and these agents can affect the health of people.
Finally, futures research on the subject should apply methodologies that allow the optimization of resources, in which the implementation of exergetic analyses would stand out, and it would be possible to identify the inefficiencies and losses of exergy existing in the treatment system, it leads to making decisions concerning the reduction in the consumption of resources, favoring the rates of sustainability and costs of the treatments.
Credit authorship contribution statement
This work was planned, executed, and discussed by all authors in equal contribution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Salvador Villamizar
Salvador Villamizar. Civil Engineering Ph.D. Universidad del Norte, Barranquilla – Colombia. Water and wastewater treatment emphasis.
Aymer Maturana Cordoba
Aymer Maturana Cordoba. Ph.D., Thermal engineer, fluids, and biofuels - Universidad de São Paulo. Universidad del Norte Professor, Barranquilla – Colombia. Water and wastewater treatments emphasis.
Joseph Soto
Joseph Soto-Verjel. Civil Engineering Ph.D. Universidad del Norte, Barranquilla – Colombia. Water and wastewater treatment emphasis.
References
- Afzal, M., M. Arslan, J. A. Müller, G. Shabir, E. Islam, R. Tahseen, M. Anwar-ul-Haq, A. J. Hashmat, S. Iqbal, and Q. M. Khan. 2019. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nat. Sustain. 2 (9):863–71. doi:10.1038/s41893-019-0350-y.
- Aghbashlo, M., M. Tabatabaei, H. Jazini, and H. S. Ghaziaskar. 2018. Exergoeconomic and exergoenvironmental co-optimization of continuous fuel additives (acetins) synthesis from glycerol esterification with acetic acid using Amberlyst 36 catalyst. Energy Convers. Manage. 165:183–94. doi:10.1016/j.enconman.2018.03.054.
- Ahmed, F. N., and C. Q. Lan. 2012. Treatment of landfill leachate using membrane bioreactors: A review. Desalination 287:41–54. doi:10.1016/j.desal.2011.12.012.
- Al-Mamun, M. R., S. Kader, M. S. Islam, and M. Z. H. Khan. 2019. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 7 (5):103248. doi:10.1016/j.jece.2019.103248.
- Ali, T., A. Ahmed, U. Alam, I. Uddin, P. Tripathi, and M. Muneer. 2018. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 212:325–35. doi:10.1016/j.matchemphys.2018.03.052.
- Aloui, F., F. Fki, S. Loukil, and S. Sayadi. 2009. Application of combined membrane biological reactor and electro-oxidation processes for the treatment of landfill leachates. Water Sci. Technol. 60 (3):605–14. doi:10.2166/wst.2009.377.
- Annangi, B., S. Bonassi, R. Marcos, and A. Hernández. 2016. Biomonitoring of humans exposed to arsenic, chromium, nickel, vanadium, and complex mixtures of metals by using the micronucleus test in lymphocytes. Mutation Res. Rev. Mutation Res. 770:140–61. doi:10.1016/j.mrrev.2016.03.003.
- Anwar, M., S. Lou, L. Chen, H. Li, and Z. Hu. 2019. Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour. Technol. 292:121972. doi:10.1016/j.biortech.2019.121972.
- Arshad, R., T. H. Bokhari, T. Javed, I. A. Bhatti, S. Rasheed, M. Iqbal, A. Nazir, S. Naz, M. I. Khan, M. K. K. Khosa,et al. 2020. Degradation product distribution of Reactive Red-147 dye treated by UV/H2O2/TiO2 advanced oxidation process. Journal of Materials Research and Technology. doi:10.1016/j.jmrt.2020.01.062.
- Athanasekou, C. P., V. Likodimos, and P. Falaras. 2018. Recent developments of TiO2 photocatalysis involving advanced oxidation and reduction reactions in water. J. Environ. Chem. Eng. 6 (6):7386–94. doi:10.1016/j.jece.2018.07.026.
- Azadi, S., A. Karimi-Jashni, and S. Javadpour. 2018. Modeling and optimization of photocatalytic treatment of landfill leachate using tungsten-doped TiO2 nano-photocatalysts: Application of artificial neural network and genetic algorithm. Process Safety Environ. Protection 117:267–77. doi:10.1016/j.psep.2018.03.038.
- Azadi, S., A. Karimi-Jashni, S. Javadpour, and H. Amiri. 2020. Photocatalytic treatment of landfill leachate: A comparison between N-, P-, and N-P-type TiO2 nanoparticles. Environ. Technol. Innovation 19:100985. doi:10.1016/j.eti.2020.100985.
- Baiju, A., R. Gandhimathi, S. T. Ramesh, and P. V. Nidheesh. 2018. Combined heterogeneous electro-fenton and biological process for the treatment of stabilized landfill leachate. J. Environ. Manage. 210:328–37. doi:10.1016/j.jenvman.2018.01.019.
- Barkul, R. P., V. B. Koli, V. B. Shewale, M. K. Patil, and S. D. Delekar. 2016. Visible active nanocrystalline N-doped anatase TiO2 particles for photocatalytic mineralization studies. Mater. Chem. Phys. 173:42–51. doi:10.1016/j.matchemphys.2016.01.035.
- Barkul, R. P., M. K. Patil, S. M. Patil, V. B. Shevale, and S. D. Delekar. 2017. Sunlight-assisted photocatalytic degradation of textile effluent and Rhodamine B by using iodine doped TiO2 nanoparticles. J. Photochem. Photobiol. A 349:138–47. doi:10.1016/j.jphotochem.2017.09.011.
- Bhatt, A. H., R. V. Karanjekar, S. Altouqi, M. L. Sattler, M. D. S. Hossain, and V. P. Chen. 2017. Estimating landfill leachate BOD and COD based on rainfall, ambient temperature, and waste composition: Exploration of a Mars statistical approach. Environ. Technol. Innovation 8:1–16. doi:10.1016/j.eti.2017.03.003.
- Bilińska, L., M. Gmurek, and S. Ledakowicz. 2016. Comparison between industrial and simulated textile wastewater treatment by AOPs – Biodegradability, toxicity and cost assessment. Chem. Eng. J. 306:550–59. doi:10.1016/j.cej.2016.07.100.
- Boczkaj, G., and A. Fernandes. 2017. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 320:608–33. doi:10.1016/j.cej.2017.03.084.
- Bolton, J. R., K. G. Bircher, W. Tumas, and C. A. Tolman. 2001. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC technical report). Pure Appl. Chem. 73 (4):627–37. doi:10.1351/pac200173040627.
- Buthiyappan, A., A. R. Abdul Aziz, and W. M. A. Wan Daud. 2015. Degradation performance and cost implication of UV-integrated advanced oxidation processes for wastewater treatments. Rev. Chem. Eng. 31 (3). doi: 10.1515/revce-2014-0039.
- Cai, Q. Q., M. Y. Wu, R. Li, S. H. Deng, B. C. Y. Lee, S. L. Ong, and J. Y. Hu. 2020. Potential of combined advanced oxidation – Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies. Chem. Eng. J. 389:123419. doi:10.1016/j.cej.2019.123419.
- Cardoso, J. C., G. G. Bessegato, and M. V. Boldrin Zanoni. 2016. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 98:39–46. doi:10.1016/j.watres.2016.04.004.
- Carney Almroth, B., J. Cartine, C. Jönander, M. Karlsson, J. Langlois, M. Lindström, J. Lundin, N. Melander, A. Pesqueda, I. Rahmqvist, et al. 2021. Assessing the effects of textile leachates in fish using multiple testing methods: From gene expression to behavior. Ecotoxicol. Environ. Saf. 207:111523. doi:10.1016/j.ecoenv.2020.111523.
- Caroline Baettker, E., C. Kozak, H. G. Knapik, and M. M. Aisse. 2020. Applicability of conventional and non-conventional parameters for municipal landfill leachate characterization. Chemosphere 251:126414. doi:10.1016/j.chemosphere.2020.126414.
- Casado, J. 2019. Towards industrial implementation of electro-fenton and derived technologies for wastewater treatment: A review. J. Environ. Chem. Eng. 7 (1):102823. doi:10.1016/j.jece.2018.102823.
- Castillo-Suárez, L. A., V. Lugo-Lugo, I. Linares-Hernández, V. Martínez-Miranda, M. Esparza-Soto, and M. D. L. Á. Mier-Quiroga. 2019. Biodegradability index enhancement of landfill leachates using a Solar Galvanic-Fenton and Galvanic-Fenton system coupled to an anaerobic–aerobic bioreactor. Solar Energy 188:989–1001. doi:10.1016/j.solener.2019.07.010.
- Chang, H., Q. Fu, N. Zhong, X. Yang, X. Quan, S. Li, J. Fu, and C. Xiao. 2019. Microalgal lipids production and nutrients recovery from landfill leachate using membrane photobioreactor. Bioresour. Technol. 277:18–26. doi:10.1016/j.biortech.2019.01.027.
- Chaouki, Z., M. Hadri, M. Nawdali, M. Benzina, and H. Zaitan. 2021. Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP). Sci. Afr. 12:e00721. doi:10.1016/j.sciaf.2021.e00721.
- Chávez, A. M., O. Gimeno, A. Rey, G. Pliego, A. L. Oropesa, P. M. Álvarez, and F. J. Beltrán. 2019. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 361:89–98. doi:10.1016/j.cej.2018.12.064.
- Chemlal, R., L. Azzouz, R. Kernani, N. Abdi, H. Lounici, H. Grib, N. Mameri, and N. Drouiche. 2014. Combination of advanced oxidation and biological processes for the landfill leachate treatment. Ecol. Eng. 73:281–89. doi:10.1016/j.ecoleng.2014.09.043.
- Chen, W., Z. Gu, P. Wen, and Q. Li. 2019a. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 217:411–22. doi:10.1016/j.chemosphere.2018.11.002.
- Chen, W., A. Zhang, G. Jiang, and Q. Li. 2019b. Transformation and degradation mechanism of landfill leachates in a combined process of SAARB and ozonation. Waste Manage. 85:283–94. doi:10.1016/j.wasman.2018.12.038.
- Chen, W., Z. Gu, G. Ran, and Q. Li. 2021. Application of membrane separation technology in the treatment of leachate in China: A review. Waste Manage. 121:127–40. doi:10.1016/j.wasman.2020.12.002.
- Chou, Y.-C., S.-L. Lo, J. Kuo, and C.-J. Yeh. 2015. Microwave-enhanced persulfate oxidation to treat mature landfill leachate. J. Hazard. Mater. 284:83–91. doi:10.1016/j.jhazmat.2014.10.043.
- Colombo, A., A. N. Módenes, D. E. Góes Trigueros, S. I. Giordani da Costa, F. H. Borba, and F. R. Espinoza-Quiñones. 2019. Treatment of sanitary landfill leachate by the combination of photo-fenton and biological processes. J. Clean. Prod. 214:145–53. doi:10.1016/j.jclepro.2018.12.310.
- Corsino, S. F., M. Capodici, D. Di Trapani, M. Torregrossa, and G. Viviani. 2020. Assessment of landfill leachate biodegradability and treatability by means of allochthonous and autochthonous biomasses. N. Biotechnol. 55:91–97. doi:10.1016/j.nbt.2019.10.007.
- Cortez, S., P. Teixeira, R. Oliveira, and M. Mota. 2011. Evaluation of fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J. Environ. Manage. 92 (3):749–55. doi:10.1016/j.jenvman.2010.10.035.
- Costa, M. P. D., J. V. S. Pancotto, M. A. K. De Alcântara, A. S. Cavalcanti, O. L. C. Guimarães, and H. J. Izário Filho. 2013. Combination of sunlight irradiated oxidative processes for landfill leachate: Heterogeneous catalysis (TiO2) versus homogeneous catalysis (H2O2). Ambiente Agua. 8 (1). doi: 10.4136/ambi-agua.1063.
- Costa, A. M., R. G. D. S. M. Alfaia, and J. C. Campos. 2019. Landfill leachate treatment in Brazil – An overview. In Journal of environmental management, Vol. 232, 110–16. Academic Press. doi:10.1016/j.jenvman.2018.11.006.
- da Costa, F. M., S. D. A. Daflon, D. M. Bila, F. V. da Fonseca, and J. C. Campos. 2018. Evaluation of the biodegradability and toxicity of landfill leachates after pretreatment using advanced oxidative processes. Waste Manage. 76:606–13. doi:10.1016/j.wasman.2018.02.030.
- Damiano, L., J. R. Jambeck, and D. B. Ringelberg. 2014. Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl. Biochem. Biotechnol. 173 (2):472–85. doi:10.1007/s12010-014-0854-x.
- de Albuquerque, E. M., E. Pozzi, I. K. Sakamoto, and P. Jurandyr. 2018. Treatability of landfill leachate combined with sanitary sewage in an activated sludge system. J. Water Process Eng. 23:119–28. doi:10.1016/j.jwpe.2018.03.011.
- de Carluccio, M., A. Fiorentino, and L. Rizzo. 2020. Multi-barrier treatment of mature landfill leachate: Effect of fenton oxidation and air stripping on activated sludge process and cost analysis. J. Environ. Chem. Eng. 8 (5):104444. doi:10.1016/j.jece.2020.104444.
- de Matos Rodrigues, M. H., P. A. Rodrigues de Sousa, K. C. M. Borges, L. de Melo Coelho, R. de Fátima Gonçalves, M. D. Teodoro, F. Vilella da Motta, R. Maribondo do Nascimento, and M. G. Júnior. 2019. Enhanced degradation of the antibiotic sulfamethoxazole by heterogeneous photocatalysis using Ce0,8Gd0,2O2-δ/TiO2 particles. J. Alloys Compd. 808:151711. doi:10.1016/j.jallcom.2019.151711.
- Dereli, R. K., E. Clifford, and E. Casey. 2020. Co-treatment of leachate in municipal wastewater treatment plants: Critical issues and emerging technologies. Crit. Rev. Environ. Sci. Technol. 1–50. doi:10.1080/10643389.2020.1745014.
- Deveci, E. Ü., N. Dizge, H. C. Yatmaz, and Y. Aytepe. 2016. Integrated process of fungal membrane bioreactor and photocatalytic membrane reactor for the treatment of industrial textile wastewater. Biochem. Eng. J. 105:420–27. doi:10.1016/j.bej.2015.10.016.
- Dewil, R., D. Mantzavinos, I. Poulios, and M. A. Rodrigo. 2017. New perspectives for advanced oxidation processes. J. Environ. Manage. 195:93–99. doi:10.1016/j.jenvman.2017.04.010.
- Díez, A. M., M. A. Sanromán, and M. Pazos. 2019. New approaches on the agrochemicals degradation by UV oxidation processes. Chem. Eng. J. 376:120026. doi:10.1016/j.cej.2018.09.187.
- Doruk, N., H. C. Yatmaz, and N. Dizge. 2016. Degradation efficiency of textile and wood processing industry wastewater by photocatalytic process using in situ ultrafiltration membrane. Clean 44 (3):224–31. doi:10.1002/clen.201400203.
- Dozzi, M. V., L. Artiglia, G. Granozzi, B. Ohtani, and E. Selli. 2014. Photocatalytic activity vs structural features of titanium dioxide materials singly doped or codoped with fluorine and boron. J. Phys. Chem. C 118 (44):25579–89. doi:10.1021/jp5084696.
- Dozzi, M. V., and E. Selli. 2016. Effects of the calcination temperature on the photoactivity of B- and F-doped or codoped TiO2 in formic acid degradation. Mater. Sci. Semiconductor Process. 42:36–39. doi:10.1016/j.mssp.2015.08.007.
- Dullius, T., F. R. Espinoza-Quiñones, P. L. Obregón, A. N. Módenes, I. Ribeiro, and A. R. de Pauli. 2020. An assessment of landfill leachate influences on water quality in the Boi Piguá river basin (Paraná, Brazil) using the TXRF technique. Environ. Earth Sci. 79 (5):95. doi:10.1007/s12665-020-8834-7.
- Eggen, T., M. Moeder, and A. Arukwe. 2010. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci.Total Environ. 408 (21):5147–57. doi:10.1016/j.scitotenv.2010.07.049.
- El Mrabet, I., M. Benzina, H. Valdés, and H. Zaitan. 2020. Treatment of landfill leachates from Fez city (Morocco) using a sequence of aerobic and fenton processes. Sci. Afr. 8:e00434. doi:10.1016/j.sciaf.2020.e00434.
- Elleuch, L., M. Messaoud, K. Djebali, M. Attafi, Y. Cherni, M. Kasmi, A. Elaoud, I. Trabelsi, and A. Chatti. 2020. A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J. Hazard. Mater. 382:121119. doi:10.1016/j.jhazmat.2019.121119.
- Elmaadawy, K., J. Hu, S. Guo, H. Hou, J. Xu, D. Wang, T. Liang, J. Yang, S. Liang, K. Xiao, et al. 2020. Enhanced treatment of landfill leachate with cathodic algal biofilm and oxygen-consuming unit in a hybrid microbial fuel cell system. Bioresour. Technol. 310:123420. doi:10.1016/j.biortech.2020.123420.
- Fagan, R., D. E. McCormack, D. D. Dionysiou, and S. C. Pillai. 2016. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semiconductor Process. 42:2–14. doi:10.1016/j.mssp.2015.07.052.
- Fang, Z., Y. Gao, X. Wu, X. Xu, A. K. Sarmah, N. Bolan, B. Gao, S. M. Shaheen, J. Rinklebe, Y. S. Ok, et al. 2020. A critical review on remediation of bisphenol S (BPS) contaminated water: Efficacy and mechanisms. Crit. Rev. Environ. Sci. Technol. 50 (5):476–522. doi:10.1080/10643389.2019.1629802.
- Feng, H., W. Mao, Y. Li, X. Wang, and S. Chen. 2021. Characterization of dissolved organic matter during the O3-based advanced oxidation of mature landfill leachate with and without biological pre-treatment and operating cost analysis. Chemosphere 271:129810. doi:10.1016/j.chemosphere.2021.129810.
- Ferraz, F. M., and Q. Yuan. 2020. Performance of oat hulls activated carbon for COD and color removal from landfill leachate. J. Water Process Eng. 33:101040. doi:10.1016/j.jwpe.2019.101040.
- Fitzsimons, L., M. Horrigan, G. McNamara, E. Doherty, T. Phelan, B. Corcoran, Y. Delauré, and E. Clifford. 2016. Assessing the thermodynamic performance of Irish municipal wastewater treatment plants using exergy analysis: A potential benchmarking approach. J. Clean. Prod. 131:387–98. doi:10.1016/j.jclepro.2016.05.016.
- Franco, D. S. P., F. A. Duarte, N. P. G. Salau, and G. L. Dotto. 2020. Analysis of indium (III) adsorption from leachates of LCD screens using artificial neural networks (ANN) and adaptive neuro-fuzzy inference systems (ANIFS). J. Hazard. Mater. 384:121137. doi:10.1016/j.jhazmat.2019.121137.
- Gautam, P., S. Kumar, and S. Lokhandwala. 2019. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: A critical review. J. Clean. Prod. 237:117639. doi:10.1016/j.jclepro.2019.117639.
- Ghahrchi, M., and A. Rezaee. 2020. Electro-catalytic ozonation for improving the biodegradability of mature landfill leachate. J. Environ. Manage. 254:109811. doi:10.1016/j.jenvman.2019.109811.
- Giannakis, S., K.-Y. A. Lin, and F. Ghanbari. 2021. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 406:127083. doi:10.1016/j.cej.2020.127083.
- Gomes, A. I., S. G. S. Santos, T. F. C. V. Silva, R. A. R. Boaventura, and V. J. P. Vilar. 2019b. Treatment train for mature landfill leachates: Optimization studies. Sci. Total Environ. 673:470–79. doi:10.1016/j.scitotenv.2019.04.027.
- Gu, N., J. Liu, J. Ye, N. Chang, and -Y.-Y. Li. 2019. Bioenergy, ammonia and humic substances recovery from municipal solid waste leachate: A review and process integration. Bioresour. Technol. 293:122159. doi:10.1016/j.biortech.2019.122159.
- Gupta, A., and R. Paulraj. 2017. Leachate composition and toxicity assessment: An integrated approach correlating physicochemical parameters and toxicity of leachates from MSW landfill in Delhi. Environ. Technol. 38 (13–14):1599–605. doi:10.1080/09593330.2016.1238515.
- Hamilton, J. W. J., J. A. Byrne, P. S. M. Dunlop, D. D. Dionysiou, M. Pelaez, K. O’Shea, D. Synnott, and S. C. Pillai. 2014. Evaluating the mechanism of visible light activity for N,F-TiO 2 using photoelectrochemistry. J. Phys. Chem. C 118 (23):12206–15. doi:10.1021/jp4120964.
- Han, M., X. Duan, G. Cao, S. Zhu, and S.-H. Ho. 2020. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Safety Environ. Protection 139:230–40. doi:10.1016/j.psep.2020.04.046.
- Haranaka-Funai, D., F. Didier, J. Giménez, P. Marco, S. Esplugas, and A. Machulek-Junior. 2017. Photocatalytic treatment of valproic acid sodium salt with TiO 2 in different experimental devices: An economic and energetic comparison. Chem. Eng. J. 327:656–65. doi:10.1016/j.cej.2017.06.148.
- Hassan, M., Y. Zhao, and B. Xie. 2016. Employing TiO2 photocatalysis to deal with landfill leachate: Current status and development. In Chemical engineering journal, Vol. 285, 264–75. Elsevier. doi:10.1016/j.cej.2015.09.093.
- Hassan, M., X. Wang, F. Wang, D. Wu, A. Hussain, and B. Xie. 2017. Coupling ARB-based biological and photochemical (UV/TiO 2 and UV/S 2 O 8 2−) techniques to deal with sanitary landfill leachate. Waste Manage. 63:292–98. doi:10.1016/j.wasman.2016.09.003.
- Hassan, M., H. Wei, H. Qiu, Y. Su, S. W. H. Jaafry, L. Zhan, and B. Xie. 2018. Power generation and pollutants removal from landfill leachate in microbial fuel cell: Variation and influence of anodic microbiomes. Bioresour. Technol. 247:434–42. doi:10.1016/j.biortech.2017.09.124.
- Hu, L., G. Zeng, G. Chen, H. Dong, Y. Liu, J. Wan, A. Chen, Z. Guo, M. Yan, H. Wu, et al. 2016. Treatment of landfill leachate using immobilized Phanerochaete chrysosporium loaded with nitrogen-doped TiO 2 nanoparticles. J. Hazard. Mater. 301:106–18. doi:10.1016/j.jhazmat.2015.08.060.
- Ishchenko, V. 2019. Heavy metals in municipal waste: The content and leaching ability by waste fraction. J. Environ. Sci. Health Part A 54 (14):1448–56. doi:10.1080/10934529.2019.1655369.
- Iskander, S. M., B. Brazil, J. T. Novak, and Z. He. 2016. Resource recovery from landfill leachate using bioelectrochemical systems: Opportunities, challenges, and perspectives. Bioresour. Technol. 201:347–54. doi:10.1016/j.biortech.2015.11.051.
- Ismail, S., M. Nasr, E. Abdelrazek, H. M. Awad, S. Zhaof, F. Meng, and A. Tawfik. 2020. Techno-economic feasibility of energy-saving self-aerated sponge tower combined with up-flow anaerobic sludge blanket reactor for treatment of hazardous landfill leachate. J. Water Process Eng. 37:101415. doi:10.1016/j.jwpe.2020.101415.
- Izah, S. C., N. Chakrabarty, and A. L. Srivastav. 2016. A review on heavy metal concentration in potable water sources in Nigeria: Human health effects and mitigating measures. Exposure Health 8 (2):285–304. doi:10.1007/s12403-016-0195-9.
- Izumi, Y. 2015. Recent advances (2012–2015) in the photocatalytic conversion of carbon dioxide to fuels using solar energy: Feasibilty for a new energy, 1–46. doi: 10.1021/bk-2015-1194.ch001.
- Jaafarzadeh, N., A. Takdastan, S. Jorfi, F. Ghanbari, M. Ahmadi, and G. Barzegar. 2018. The performance study on ultrasonic/Fe 3 O 4 /H 2 O 2 for degradation of azo dye and real textile wastewater treatment. J. Mol. Liq. 256:462–70. doi:10.1016/j.molliq.2018.02.047.
- Janitabar-Darzi, S. 2014. Structural and photocatalytic activity of mesoporous N-Doped TiO 2 with band-to-band visible light absorption ability. Particulate Sci. Technol. 32 (5):506–11. doi:10.1080/02726351.2014.920443.
- Kaabeche, O. N. E. H., R. Zouaghi, S. Boukhedoua, S. Bendjabeur, and T. Sehili. 2019. A Comparative Study on Photocatalytic Degradation of Pyridinium – Based Ionic Liquid by TiO2 and ZnO in Aqueous Solution. International Journal of Chemical Reactor Engineering 17 (9). doi:10.1515/ijcre-2018-0253.
- Kamaruddin, M. A., M. S. Yusoff, H. A. Aziz, and Y.-T. Hung. 2015. Sustainable treatment of landfill leachate. Appl. Water Sci. 5 (2):113–26. doi:10.1007/s13201-014-0177-7.
- Kamyab, B., H. Zilouei, and B. Rahmanian. 2019. Investigation of the effect of hydraulic retention time on anaerobic digestion of potato leachate in two-stage mixed-UASB system. Biomass Bioenergy 130:105383. doi:10.1016/j.biombioe.2019.105383.
- Kanan, S., M. A. Moyet, R. B. Arthur, and H. H. Patterson. 2020. Recent advances on TiO 2 -based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. 62 (1):1–65. doi:10.1080/01614940.2019.1613323.
- Kattel, E., A. Kivi, K. Klein, T. Tenno, N. Dulova, and M. Trapido. 2016. Hazardous waste landfill leachate treatment by combined chemical and biological techniques. Desalination Water Treatment 57 (28):13236–45. doi:10.1080/19443994.2015.1057539.
- Khalil, C., C. Al Hageh, S. Korfali, and R. S. Khnayzer. 2018. Municipal leachates health risks: Chemical and cytotoxicity assessment from regulated and unregulated municipal dumpsites in Lebanon. Chemosphere 208:1–13. doi:10.1016/j.chemosphere.2018.05.151.
- Klauck, C. R., A. Giacobbo, C. G. Altenhofen, L. B. Silva, A. Meneguzzi, A. M. Bernardes, and M. A. S. Rodrigues. 2017. Toxicity elimination of landfill leachate by hybrid processing of advanced oxidation process and adsorption. Environ. Technol. Innovation 8:246–55. doi:10.1016/j.eti.2017.07.006.
- Klauson, D., A. Kivi, E. Kattel, K. Klein, M. Viisimaa, J. Bolobajev, S. Velling, A. Goi, T. Tenno, and M. Trapido. 2015. Combined processes for wastewater purification: Treatment of a typical landfill leachate with a combination of chemical and biological oxidation processes. J. Chem. Technol. Biotechnol. 90 (8):1527–36. doi:10.1002/jctb.4484.
- Koh, I.-O., X. Chen-Hamacher, K. Hicke, and W. Thiemann. 2004. Leachate treatment by the combination of photochemical oxidation with biological process. J. Photochem. Photobiol. A 162 (2–3):261–71. doi:10.1016/j.nainr.2003.08.011.
- Le, T. S., N. M. Dang, and D. T. Tran. 2021. Performance of coupling electrocoagulation and biofiltration processes for the treatment of leachate from the largest landfill in Hanoi, Vietnam: Impact of operating conditions. Sep. Purif. Technol. 255:117677. doi:10.1016/j.seppur.2020.117677.
- Li, J., C. He, T. Tian, Z. Liu, Z. Gu, G. Zhang, and W. Wang. 2020. UASB-modified Bardenpho process for enhancing bio-treatment efficiency of leachate from a municipal solid waste incineration plant. Waste Manage. 102:97–105. doi:10.1016/j.wasman.2019.10.028.
- Liang, J., J. Wang, K. Song, X. Wang, K. Yu, and C. Liang. 2020. Enhanced photocatalytic activities of Nd-doped TiO2 under visible light using a facile sol-gel method. J. Rare Earths 38 (2):148–56. doi:10.1016/j.jre.2019.07.008.
- Likodimos, V. 2018. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B 230:269–303. doi:10.1016/j.apcatb.2018.02.039.
- Liu, Z., R. Liu, and F. Nie. 2017. MFPAC coagulation and aged refuse adsorption pretreatment of landfill leachate. Chinese J. Environ. Eng. 11 (1):393–400. doi:10.12030/j.cjee.201509024.
- Liu, X., J. T. Novak, and Z. He. 2020. Synergistically coupling membrane electrochemical reactor with fenton process to enhance landfill leachate treatment. Chemosphere 247:125954. doi:10.1016/j.chemosphere.2020.125954.
- Luo, J., G. Qian, J. Liu, and Z. P. Xu. 2015. Anaerobic methanogenesis of fresh leachate from municipal solid waste: A brief review on current progress. Renew. Sustain. Energy Rev. 49:21–28. doi:10.1016/j.rser.2015.04.053.
- Luo, H., Y. Zeng, Y. Cheng, D. He, and X. Pan. 2020. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. In Science of the total environment, Vol. 703, 135468. Elsevier B.V. doi:10.1016/j.scitotenv.2019.135468.
- Maiti, S. K., S. De, T. Hazra, A. Debsarkar, and A. Dutta. 2016. Characterization of leachate and its impact on surface and groundwater quality of a closed dumpsite – A case study at Dhapa, Kolkata, India. Procedia Environ. Sci. 35:391–99. doi:10.1016/j.proenv.2016.07.019.
- Malato, S., M. I. Maldonado, P. Fernández-Ibáñez, I. Oller, I. Polo, and R. Sánchez-Moreno. 2016. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the plataforma Solar de Almeria. Mater. Sci. Semiconductor Process. 42:15–23. doi:10.1016/j.mssp.2015.07.017.
- Marcelino, R. B. P., M. T. A. Queiroz, C. C. Amorim, M. M. D. Leão, and F. F. Brites-Nóbrega. 2015. Solar energy for wastewater treatment: Review of international technologies and their applicability in Brazil. Environ.l Sci. Pollution Res. 22 (2):762–73. doi:10.1007/s11356-014-3033-2.
- Matassa, S., D. J. Batstone, T. Hülsen, J. Schnoor, and W. Verstraete. 2015. Can direct conversion of used nitrogen to new feed and protein help feed the world? Environ. Sci. Technol. 49 (9):5247–54. doi:10.1021/es505432w.
- Mavakala, B. K., S. Le Faucheur, C. K. Mulaji, A. Laffite, N. Devarajan, E. M. Biey, G. Giuliani, J. P. Otamonga, P. Kabatusuila, P. T. Mpiana, et al. 2016. Leachates draining from controlled municipal solid waste landfill: Detailed geochemical characterization and toxicity tests. Waste Manage. 55:238–48. doi:10.1016/j.wasman.2016.04.028.
- Mira Anuar, N., and C.-M. Chan. 2020. Adsorption of Escherichia coli from landfill leachate using dredged marine soils as geosorbent: The influence of temperature. Mater. Today 31:278–81. doi:10.1016/j.matpr.2020.06.007.
- Mishra, S., D. Tiwary, A. Ohri, and A. K. Agnihotri. 2019. Impact of municipal solid waste landfill leachate on groundwater quality in Varanasi, India. Groundwate Sustain. Dev. 9:100230. doi:10.1016/j.gsd.2019.100230.
- Mohajeri, S., A. A. Hamidi, M. H. Isa, and M. A. Zahed. 2019. Landfill leachate treatment through electro-fenton oxidation. Pollution 5 (1):199–209. doi:10.22059/poll.2018.249210.364.
- Mohamed, M. A., W. N. W. Salleh, J. Jaafar, A. F. Ismail, and N. A. M. Nor. 2015. Photodegradation of phenol by N-Doped TiO2 anatase/rutile nanorods assembled microsphere under UV and visible light irradiation. Mater. Chem. Phys. 162:113–23. doi:10.1016/j.matchemphys.2015.05.033.
- Mohammad-pajooh, E., A. E. Turcios, G. Cuff, D. Weichgrebe, K.-H. Rosenwinkel, M. D. Vedenyapina, and L. R. Sharifullina. 2018. Removal of inert COD and trace metals from stabilized landfill leachate by granular activated carbon (GAC) adsorption. J. Environ. Manage. 228:189–96. doi:10.1016/j.jenvman.2018.09.020.
- Mohan, S., H. Mamane, D. Avisar, I. Gozlan, A. Kaplan, and G. Dayalan. 2019. Treatment of diethyl phthalate leached from plastic products in municipal solid waste using an ozone-based advanced oxidation process. Materials 12 (24):4119. doi:10.3390/ma12244119.
- Mohan Reddy, K., and J. Devaraju. 2019. Kinetics of photo fenton process and Ag-TiO2 photocatalyst under UV-light. Mater. Today 17:235–38. doi:10.1016/j.matpr.2019.06.424.
- Moody, C. M., and T. G. Townsend. 2017. A comparison of landfill leachates based on waste composition. Waste Manage. 63:267–74. doi:10.1016/j.wasman.2016.09.020.
- Mukherjee, S., S. Mukhopadhyay, M. A. Hashim, and B. Sen Gupta. 2015. Contemporary environmental issues of landfill leachate: Assessment and remedies. Crit. Rev. Environ. Sci. Technol. 45 (5):472–590. doi:10.1080/10643389.2013.876524.
- Müller, G. T., A. Giacobbo, E. A. dos Santos Chiaramonte, M. A. S. Rodrigues, A. Meneguzzi, and A. M. Bernardes. 2015. The effect of sanitary landfill leachate aging on the biological treatment and assessment of photoelectrooxidation as a pre-treatment process. Waste Management 36:177–83. doi:10.1016/j.wasman.2014.10.024.
- Nazia, S., N. Sahu, V. Jegatheesan, S. K. Bhargava, and S. Sridhar. 2021. Integration of ultrafiltration membrane process with chemical coagulation for proficient treatment of old industrial landfill leachate. Chem. Eng. J. 412:128598. doi:10.1016/j.cej.2021.128598.
- Nika, M. C., K. Ntaiou, K. Elytis, V. S. Thomaidi, G. Gatidou, O. I. Kalantzi, N. S. Thomaidis, and A. S. Stasinakis. 2020. Wide-scope target analysis of emerging contaminants in landfill leachates and risk assessment using risk quotient methodology. J. Hazard. Mater. 394:122493. doi:10.1016/j.jhazmat.2020.122493.
- Oulego, P., S. Collado, A. Laca, and M. Díaz. 2016. Impact of leachate composition on the advanced oxidation treatment. Water Res. 88:389–402. doi:10.1016/j.watres.2015.09.048.
- Pan, Z., C. Song, L. Li, H. Wang, Y. Pan, C. Wang, J. Li, T. Wang, and X. Feng. 2019. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng. J. 376:120909. doi:10.1016/j.cej.2019.01.188.
- Parangi, T., and M. K. Mishra. 2019. Titania nanoparticles as modified photocatalysts: A review on design and development. Comments Inorganic Chem. 39 (2):90–126. doi:10.1080/02603594.2019.1592751.
- Parde, D., A. Patwa, A. Shukla, R. Vijay, D. J. Killedar, and R. Kumar. 2021. A review of constructed wetland on type, treatment and technology of wastewater. Environ. Technol. Innovation 21:101261. doi:10.1016/j.eti.2020.101261.
- Park, Y.-K., -H.-H. Ha, Y. H. Yu, B.-J. Kim, H.-J. Bang, H. Lee, and S.-C. Jung. 2020. The photocatalytic destruction of cimetidine using microwave-assisted TiO2 photocatalysts hybrid system. J. Hazard. Mater. 391:122568. doi:10.1016/j.jhazmat.2020.122568.
- Paździor, K., L. Bilińska, and S. Ledakowicz. 2019. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 376:120597. doi:10.1016/j.cej.2018.12.057.
- Pieus Thanikkal, M., and S. Pooppana Antony. 2021. Integrated electro-fenton and membrane bioreactor system for matured landfill leachate treatment. J. Hazardous Toxic Radioactive Waste 25 (1):04020058. doi:10.1061/(ASCE)HZ.2153-5515.0000556.
- Podder, A., D. Reinhart, and R. Goel. 2020. Integrated leachate management approach incorporating nutrient recovery and removal. Waste Manage. 102:420–31. doi:10.1016/j.wasman.2019.10.048.
- Przydatek, G., and W. Kanownik. 2019. Impact of small municipal solid waste landfill on groundwater quality. Environ. Monit. Assess. 191 (3):1–14. doi:10.1007/s10661-019-7279-5.
- Ren, X., X. Xu, Y. Xiao, W. Chen, and K. Song. 2019. Effective removal by coagulation of contaminants in concentrated leachate from municipal solid waste incineration power plants. Sci.Total Environ. 685:392–400. doi:10.1016/j.scitotenv.2019.05.392.
- Reshadi, M. A. M., A. Bazargan, and G. McKay. 2020. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci.Total Environ. 731:138863. doi:10.1016/j.scitotenv.2020.138863.
- Rivero, M. J., P. Ribao, B. Gomez-Ruiz, A. Urtiaga, and I. Ortiz. 2020. Comparative performance of TiO2-rGO photocatalyst in the degradation of dichloroacetic and perfluorooctanoic acids. Sep. Purif. Technol. 240:116637. doi:10.1016/j.seppur.2020.116637.
- Rodrigues, C. S. D., L. M. Madeira, and R. A. R. Boaventura. 2014. Synthetic textile dyeing wastewater treatment by integration of advanced oxidation and biological processes – Performance analysis with costs reduction. J. Environ. Chem. Eng. 2 (2):1027–39. doi:10.1016/j.jece.2014.03.019.
- Rojviroon, O., T. Rojviroon, and S. Sirivithayapakorn. 2015. Removal of color and chemical oxygen demand from landfill leachate by photocatalytic process with AC/TiO2. Energy Procedia 79:536–41. doi:10.1016/j.egypro.2015.11.530.
- Różycki, S., and M. Banaś. 2018. Exergy analysis of cavitation pretreatment of sludge. E3S Web Conf. 49:00089. doi:10.1051/e3sconf/20184900089.
- Rueda-Marquez, J. J., I. Levchuk, P. Fernández Ibañez, and M. Sillanpää. 2020. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 258:120694. doi:10.1016/j.jclepro.2020.120694.
- Ruiz-Delgado, A., P. Plaza-Bolaños, I. Oller, S. Malato, and A. Agüera. 2020. Advanced evaluation of landfill leachate treatments by low and high-resolution mass spectrometry focusing on microcontaminant removal. J. Hazard. Mater. 384:121372. doi:10.1016/j.jhazmat.2019.121372.
- Sackey, L. N. A., V. Kočí, and C. A. M. van Gestel. 2020. Ecotoxicological effects on Lemna minor and Daphnia magna of leachates from differently aged landfills of Ghana. Sci.Total Environ. 698:134295. doi:10.1016/j.scitotenv.2019.134295.
- Saeed, T., M. J. Miah, N. Majed, M. K. Alam, and T. Khan. 2021. Effect of effluent recirculation on nutrients and organics removal performance of hybrid constructed wetlands: Landfill leachate treatment. J. Clean. Prod. 282:125427. doi:10.1016/j.jclepro.2020.125427.
- Sahoo, C., A. K. Gupta, and I. M. S. Pillai. 2012. Heterogeneous photocatalysis of real textile wastewater: Evaluation of reaction kinetics and characterization. J. Environ. Sci. Health Part A 47 (13):2109–19. doi:10.1080/10934529.2012.695996.
- Saleem, M., A. Spagni, L. Alibardi, A. Bertucco, and M. C. Lavagnolo. 2018. Assessment of dynamic membrane filtration for biological treatment of old landfill leachate. J. Environ. Manage. 213:27–35. doi:10.1016/j.jenvman.2018.02.057.
- Sanzone, G., M. Zimbone, G. Cacciato, F. Ruffino, R. Carles, V. Privitera, and M. G. Grimaldi. 2018. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 123:394–402. doi:10.1016/j.spmi.2018.09.028.
- Seibert, D., F. Henrique Borba, F. Bueno, J. J. Inticher, A. N. Módenes, F. R. Espinoza-Quiñones, and R. Bergamasco. 2019. Two-stage integrated system photo-electro-fenton and biological oxidation process assessment of sanitary landfill leachate treatment: An intermediate products study. Chem. Eng. J. 372:471–82. doi:10.1016/j.cej.2019.04.162.
- Silva, T. F. C. V., M. E. F. Silva, A. Cristina Cunha-Queda, A. Fonseca, I. Saraiva, R. A. R. Boaventura, and V. J. P. Vilar. 2013. Sanitary landfill leachate treatment using combined solar photo-fenton and biological oxidation processes at pre-industrial scale. Chem. Eng. J. 228:850–66. doi:10.1016/j.cej.2013.05.060.
- Sinha, S., D. Roy, S. Neogi, and S. De. 2018. Application of advanced oxidation process for the treatment of hydrofracked water. Environmental Division 2018 - Core Programming Area at the 2018 AIChE Annual Meeting Pittsburgh, PA, 24–26.
- Somani, M., M. Datta, S. K. Gupta, T. R. Sreekrishnan, and G. V. Ramana. 2019. Comprehensive assessment of the leachate quality and its pollution potential from six municipal waste dumpsites of India. Bioresour. Technol. Rep. 6:198–206. doi:10.1016/j.biteb.2019.03.003.
- Song, J., W. Zhang, J. Gao, X. Hu, C. Zhang, Q. He, F. Yang, H. Wang, X. Wang, and X. Zhan. 2020. A pilot-scale study on the treatment of landfill leachate by a composite biological system under low dissolved oxygen conditions: Performance and microbial community. Bioresour. Technol. 296:122344. doi:10.1016/j.biortech.2019.122344.
- Spasiano, D., R. Marotta, S. Malato, P. Fernandez-Ibañez, and I. Di Somma. 2015. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. In Applied catalysis B: Environmental, Vols. 170–171, 90–123. Elsevier. doi:10.1016/j.apcatb.2014.12.050.
- Starling, M. C. V. M., L. A. S. Castro, R. B. P. Marcelino, M. M. D. Leão, and C. C. Amorim. 2017. Optimized treatment conditions for textile wastewater reuse using photocatalytic processes under UV and visible light sources. Environ.l Sci. Pollution Res. 24 (7):6222–32. doi:10.1007/s11356-016-6157-8.
- Stoyanova, A. M., H. Y. Hitkova, N. K. Ivanova, A. D. Bachvarova-Nedelcheva, R. S. Iordanova, and M. P. Sredkova. 2013. Photocatalytic and antibacterial activity of Fe-doped TiO 2 nanoparticles prepared by nonhydrolytic sol-gel method. Bulgarian Chem. Comm. 45 (4):497–504.
- Sui, Q., W. Zhao, X. Cao, S. Lu, Z. Qiu, X. Gu, and G. Yu. 2017. Pharmaceuticals and personal care products in the leachates from a typical landfill reservoir of municipal solid waste in Shanghai, China: Occurrence and removal by a full-scale membrane bioreactor. J. Hazard. Mater. 323:99–108. doi:10.1016/j.jhazmat.2016.03.047.
- Swati, T. I. S., V. K. Vijay, and P. Ghosh. 2018. Scenario of landfilling in India: Problems, challenges, and recommendations. In Handbook of environmental materials management, 1–16. Springer International Publishing. doi:10.1007/978-3-319-58538-3_167-1.
- Talalaj, I. A., and P. Biedka. 2016. Use of the landfill water pollution index (LWPI) for groundwater quality assessment near the landfill sites. Environ.l Sci. Pollution Res. 23 (24):24601–13. doi:10.1007/s11356-016-7622-0.
- Tao, Q., J. Luo, J. Zhou, S. Zhou, G. Liu, and R. Zhang. 2014. Effect of dissolved oxygen on nitrogen and phosphorus removal and electricity production in microbial fuel cell. Bioresour. Technol. 164:402–07. doi:10.1016/j.biortech.2014.05.002.
- Tejera, J., D. Hermosilla, A. Gascó, R. Miranda, V. Alonso, C. Negro, and Á. Blanco. 2021. Treatment of mature landfill leachate by electrocoagulation followed by fenton or UVA-LED photo-fenton processes. J. Taiwan Inst. Chem. Eng. 119:33–44. doi:10.1016/j.jtice.2021.02.018.
- Thornton, R. J., and F. C. Blanc. 1973. Leachate treatment by coagulation and precipitation. J. Environ. Eng. Div. Proc. ASCE 99 (EE4):535–44.
- Tian, Y., Y. Yao, S. Chang, Z. Zhao, Y. Zhao, X. Yuan, F. Wu, and H. Sun. 2018. Occurrence and phase distribution of neutral and ionizable per- and Polyfluoroalkyl Substances (PFASs) in the atmosphere and plant leaves around landfills: A case study in Tianjin, China. Environ. Sci. Technol. 52 (3):1301–10. doi:10.1021/acs.est.7b05385.
- Tolosana-Moranchel, Á., A. Manassero, M. L. Satuf, O. M. Alfano, J. A. Casas, and A. Bahamonde. 2019. TiO2-rGO photocatalytic degradation of an emerging pollutant: Kinetic modelling and determination of intrinsic kinetic parameters. J. Environ. Chem. Eng. 7 (5):103406. doi:10.1016/j.jece.2019.103406.
- Tripathy, B. K., G. Ramesh, A. Debnath, and M. Kumar. 2019. Mature landfill leachate treatment using sonolytic-persulfate/hydrogen peroxide oxidation: Optimization of process parameters. Ultrason. Sonochem. 54:210–19. doi:10.1016/j.ultsonch.2019.01.036.
- Tsui, T.-H., H. Wu, B. Song, -S.-S. Liu, A. Bhardwaj, and J. W. C. Wong. 2020. Food waste leachate treatment using an Upflow Anaerobic Sludge Bed (UASB): Effect of conductive material dosage under low and high organic loads. Bioresour. Technol. 304:122738. doi:10.1016/j.biortech.2020.122738.
- Tumen Ozdil, N. F., and A. Tantekin. 2016. Exergy and exergoeconomic assessments of an electricity production system in a running wastewater treatment plant. Renew. Energy 97:390–98. doi:10.1016/j.renene.2016.05.039.
- Us Saqib, N., R. Adnan, and I. Shah. 2017. Modifications of pure and Ag doped TiO2 by pre-sulphated and calcination temperature treatments. Res. Chem. Intermediates 43 (11):6571–88. doi:10.1007/s11164-017-3005-5.
- Vaccari, M., T. Tudor, and G. Vinti. 2019. Characteristics of leachate from landfills and dumpsites in Asia, Africa and Latin America: An overview. Waste Manage. 95:416–31. doi:10.1016/j.wasman.2019.06.032.
- Vahabian, M., Y. Hassanzadeh, and S. Marofi. 2019. Assessment of landfill leachate in semi-arid climate and its impact on the groundwater quality case study: Hamedan, Iran. Environ. Monit. Assess. 191 (2):1–19. doi:10.1007/s10661-019-7215-8.
- Vakalis, S., K. Moustakas, D. Malamis, A. Sotiropoulos, and S. Malamis. 2017. Assessing the removal of heavy metals in industrial wastewater by means of chemical exergy. Desalination Water Treatment 91:146–51. doi:10.5004/dwt.2017.20588.
- Venkatesh, S., A. R. Quaff, N. D. Pandey, and K. Venkatesh. 2015. Impact of ozonation on decolorization and mineralization of azo dyes: Biodegradability enhancement, by-products formation, required energy and cost. Ozone 37 (5):420–30. doi:10.1080/01919512.2015.1027810.
- Vymazal, J., Y. Zhao, and Ü. Mander. 2021. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 169:106318. doi:10.1016/j.ecoleng.2021.106318.
- Wang, J. L., and L. J. Xu. 2012. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 42 (3):251–325. doi:10.1080/10643389.2010.507698.
- Wang, Z., Y. Peng, L. Miao, T. Cao, F. Zhang, S. Wang, and J. Han. 2016. Continuous-flow combined process of nitritation and ANAMMOX for treatment of landfill leachate. Bioresour. Technol. 214:514–19. doi:10.1016/j.biortech.2016.04.118.
- Wang, C., X. Sun, H. Shan, H. Zhang, and B. Xi. 2021. Degradation of landfill leachate using UV-TiO2 photocatalysis combination with aged waste reactors. Processes 9 (6):946. doi:10.3390/pr9060946.
- Wiszniowski, J., D. Robert, J. Surmacz-Gorska, K. Miksch, and J.-V. Weber. 2006. Leachate detoxification by combination of biological and TiO2-photocatalytic processes. Water Sci. Technol. 53 (3):181–90. doi:10.2166/wst.2006.091.
- Wu, D., T. Wang, X. Huang, J. Dolfing, and B. Xie. 2015. Perspective of harnessing energy from landfill leachate via microbial fuel cells: Novel biofuels and electrogenic physiologies. Appl. Microbiol. Biotechnol. 99 (19):7827–36. doi:10.1007/s00253-015-6857-x.
- Wu, C., W. Chen, Z. Gu, and Q. Li. 2021. A review of the characteristics of fenton and ozonation systems in landfill leachate treatment. Sci.Total Environ. 762:143131. doi:10.1016/j.scitotenv.2020.143131.
- Xie, Z., Z. Wang, Q. Wang, C. Zhu, and Z. Wu. 2014. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresour. Technol. 161:29–39. doi:10.1016/j.biortech.2014.03.014.
- Xie, W., W. Zhong, B. M. R. Appenzeller, J. Zhang, M. Junaid, and N. Xu. 2020. Nexus between perfluoroalkyl compounds (PFCs) and human thyroid dysfunction: A systematic review evidenced from laboratory investigations and epidemiological studies. Crit. Rev. Environ. Sci. Technol. 1–46. doi:10.1080/10643389.2020.1795052.
- Xing, Z., J. Zhang, J. Cui, J. Yin, T. Zhao, J. Kuang, Z. Xiu, N. Wan, and W. Zhou. 2018. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B 225:452–67. doi:10.1016/j.apcatb.2017.12.005.
- Xu, Y., C. Chen, X. Li, J. Lin, Y. Liao, and Z. Jin. 2017. Recovery of humic substances from leachate nanofiltration concentrate by a two-stage process of tight ultrafiltration membrane. J. Clean. Prod. 161:84–94. doi:10.1016/j.jclepro.2017.05.095.
- Yaghmaien, K., M. Hadei, P. Hopke, S. Gharibzadeh, M. Kermani, M. Yarahmadi, B. Emam, and A. Shahsavani. 2019. Comparative health risk assessment of BTEX exposures from landfills, composting units, and leachate treatment plants. Air Qual. Atmos. Health 12 (4):443–51. doi:10.1007/s11869-019-00669-w.
- Yan, H., I. T. Cousins, C. Zhang, and Q. Zhou. 2015. Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater. Sci. Total Environ. 524–525:23–31. doi:10.1016/j.scitotenv.2015.03.111.
- Yasmin, C., E. Lobna, M. Mouna, D. Kais, K. Mariam, S. Rached, C. Abdelwaheb, and T. Ismail. 2020. New trend of Jebel Chakir landfill leachate pre-treatment by photocatalytic TiO2/Ag nanocomposite prior to fermentation using Candida tropicalis strain. Int. Biodeterior. Biodegradation 146:104829. doi:10.1016/j.ibiod.2019.104829.
- Youcai, Z. 2018. Leachate generation and characteristics. In Pollution control technology for leachate from municipal solid waste, Vols. 1–30. Elsevier. doi:10.1016/b978-0-12-815813-5.00001-2.
- Zegzouti, Y., A. Boutafda, L. El Fels, M. El Hadek, A. Lebrihi, F. Bekkaoui, and M. Hafidi. 2019. Quality and quantity of leachate with different ages and operations in semi-arid climate. Desalination Water Treatment 152:174–84. doi:10.5004/dwt.2019.24017.
- Zhang, G., H. Zhang, Y. Ma, G. Yuan, F. Yang, and R. Zhang. 2014a. Membrane filtration biocathode microbial fuel cell for nitrogen removal and electricity generation. Enzyme Microb. Technol. 60:56–63. doi:10.1016/j.enzmictec.2014.04.005.
- Zhang, L., J. Zhou, J. Li, G. Liu, X. Lin, B. Mao, R. Liu, S. Zhang, and J.-Q. Wang. 2014b. Surface structural reconstruction for optical response in iodine-modified TiO 2 photocatalyst system. J. Phys. Chem. C 118 (25):13726–32. doi:10.1021/jp503966r.
- Zhang, C., M. Jin, J. Tang, and X. Gao. 2018. Removal of cyclic volatile methylsiloxanes in effluents from treated landfill leachate by electrochemical oxidation. J. Mater. Cycles Waste Manage. 20 (1):690–94. doi:10.1007/s10163-016-0554-4.
- Zhang, T., Y. Liu, Y. Rao, X. Li, D. Yuan, S. Tang, and Q. Zhao. 2020. Enhanced photocatalytic activity of TiO2 with acetylene black and persulfate for degradation of tetracycline hydrochloride under visible light. Chem. Eng. J. 384:123350. doi:10.1016/j.cej.2019.123350.
- Zhao, X., L. Guo, and J. Qu. 2014. Photoelectrocatalytic oxidation of Cu-EDTA complex and electrodeposition recovery of Cu in a continuous tubular photoelectrochemical reactor. Chem. Eng. J. 239:53–59. doi:10.1016/j.cej.2013.10.088.
- Zhao, J., F. Ouyang, Y. Yang, and W. Tang. 2020. Degradation of recalcitrant organics in nanofiltration concentrate from biologically pretreated landfill leachate by ultraviolet-fenton method. Sep. Purif. Technol. 235:116076. doi:10.1016/j.seppur.2019.116076.
- Zhao, C., J. Zhou, Y. Yan, L. Yang, G. Xing, H. Li, P. Wu, M. Wang, and H. Zheng. 2021. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci.Total Environ. 765:142795. doi:10.1016/j.scitotenv.2020.142795.
- Zielińska, M., D. Kulikowska, and M. Stańczak. 2020. Adsorption – Membrane process for treatment of stabilized municipal landfill leachate. Waste Manage. 114:174–82. doi:10.1016/j.wasman.2020.07.011.
- Zolfaghari, M., K. Jardak, P. Drogui, S. K. Brar, G. Buelna, and R. Dubé. 2016. Landfill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J. Environ. Manage. 184:318–26. doi:10.1016/j.jenvman.2016.10.010.