?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
As a trace element, selenium can cause serious harm to organisms when the concentration is too high. Coal-fired power plants are the main source of man-made selenium emissions. How to control the selenium pollution of coal-fired power plants to realize the renewable selenium and the sustainability of coal has not attracted enough attention from the whole world. This paper outlines the conversion and occurrence of selenium in coal-fired power plants. A small part of the selenium produced by combustion can be removed by selective catalytic reduction (SCR) and electrostatic precipitator (ESP) after the gas phase undergoes physical condensation and chemical adsorption to combine with the particulate matter in the flue gas.Because the chemical precipitation method has poor selenium removal effect, the remaining part enters the flue gas desulfurization absorption tower and can be enriched in the desulfurization slurry. The occurrence situation and conversion pathway of selenium in desulfurization slurry are introduced subsequently, the research progress of selenium removal from wet desulfurization wastewater is reviewed from three aspects: physics, biology and chemistry. We believe that the coupling application of oxidation-reduction potential (ORP) and pH can optimize selenium removal in the desulfurization system by improving the oxidation control. As a technology for wet desulfurization system to treat selenium pollution, it has a good development prospect in near future.
Implications: Selenium is a trace element present in coal. It is not only of great significance to the life activities of organisms, but also a kind of rare resource. As the most important source of man-made emissions, coal-fired power plants will cause waste of selenium resources and selenium pollution in the surrounding environment. In this study, the occurrence, conversion and control of selenium in coal-fired power plants were systematically sorted out and analyzed. It is helpful for scholars to study the selenium transformation process more deeply. It is of great significance for policy formulation of recommended control technologies and emission limits. It is of great value for the formulation of recommended control technology and the in-depth study of the selenium transformation process.
Introduction
Selenium exists in soil, water and air as a trace element in nature. It is not only important for biological life activities, but also a rare resource with strategic significance Chang et al. (Citation2019a). Selenium can play a positive and important role in organisms only in a narrow concentration range. The Food and Nutrition Board of the US National Research Council established an estimated safe and adequate daily dietary intake for humans is 11–280 Hadrup and Ravn-Haren (Citation2020). In drinking water, World Health Organization provisional guideline for Se was set at 40
Santos et al. (Citation2015a). When the concentration is too high, it will cause harm to organisms and induce serious selenium-related diseases Zhang et al. (Citation2020a). Selenium has also been identified by the European Commission as an important raw material for strategic low-carbon energy technology. It has application prospects in photovoltaic applications and has a medium risk of bottleneck Qin et al. (Citation2017). Since selenium pollution occurs at a lower concentration than other pollution situations, the treatment of selenium pollution has not attracted enough attentions Dodig and Čepelak (Citation2004). For example,many countries such as the United States, China, Japan, Ireland and Indiahavegroundwater selenium pollution Liu et al. (Citation2020). The highest concentration in parts of India is 4475
Malhotra, Pal, and Pal (Citation2020). At the same time, selenium will bioaccumulate in higher nutritional level organisms through the food chain, which will cause concerns about long-term environmental impact. In recent years, people’s strong interest in the effects of selenium in cancer Vinceti et al. (Citation2018), genetics Shu et al. (Citation2020), health Park et al. (Citation2020), technology, renewable energy, and sustainable energy Chang et al. Citation2019b) has also increased the attention of selenium pollution Lenz and Lens (Citation2009) , , , , .
Table 1. Comparison of studies on removing selenium by membrane treatment
Table 2. Comparison of studies on selenium removal in wetlands
Table 3. Comparison of studies on selenium removal in bioreactor
Table 4. Comparison of the adsorption capacity of that of different adsorbents
Table 5. Comparison of the removal capacity of that of different ZVI
The source of selenium in the environment can be divided into natural sources and man-made sources Long, Zhang, and Luo (Citation2019). Natural sources mainly include soil and dust, bioaccumulation and volcanic eruptions Tian et al. (Citation2010). Man-made sources mainly include coal combustion Li and Fan (Citation2008), mineral mining, and metal and petroleum processing Cordoba and Staicu (Citation2018), of which coal combustion accounts for more than 50% of man-made sources Han et al. (Citation2021).
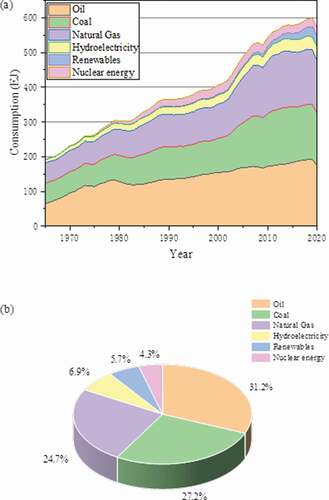
To achieve the global emissions targets set by the United Nations Framework Convention on Climate Change. The rapid development of global new energy to support the transition to a low-carbon future. In 2020, the economic turmoil caused by COVID-19 has affected the global energy market. As shown in , global energy demand fell by 4.5%, of which coal consumption fell by 4.2% compared to 2019, accounting for 27.2% of the total energy, and the total production was 7741.6 million tons Company (Citation1990). Although the proportion of thermal power generation is gradually decreasing, as a simple and reliable resource, coal is still the most secure energy component. In the future, the focus will be on improving the efficiency of coal utilization, the degree of cleanliness and the recycling of other high-value resources. Therefore, under the condition that the energy composition is relatively stable, the optimization of the existing technology has also attracted more attention from government agencies and researchers. In 2020, the Steam Electric Power Generating Effluent Limitations Guidelines and Standards revised by United States Environmental Protection Agency (US EPA) set limits on the discharge of desulfurization wastewater. The minimum requirement for selenium is the maximum daily limit of 70 . The State Council of China published the 14th Five-Year Plan (2021–2025) also put forward requirements for strengthening the coordinated control of pollutants George et al. (Citation2020). In the existing the air pollution control devices (APCDs) system, the main process and principle are based on the removal of dust,
,
. The removal of selenium by the system can only play a partial synergistic effect.Unauthorized discharge of selenium-containing pollutants will change the environmental concentration of selenium in a local area, which will destroy the ecosystem.Therefore, targeted treatment of selenium pollution in coal-fired power plants is necessary.
Figure 1. (a) Consumption trend of energy sources during the period of 1965–2020, and (b) the share of energy sources in world primary energy consumption in 2020.
This paper first describes the distribution and conversion of selenium in coal-fired power plants from two parts: the gas phase and the liquid phase.Then, the existing WFGD system selenium pollution control methods are reviewed and prospected from the three parts of physics, biology and chemistry. It is believed that the control of selenium pollution should not increase the complexity of coal-fired power plants with an independent system. It is found that the use of oxidation-reduction potential (ORP) and pH coupling technology can not only be used as a technology for wet desulfurization system to control selenium pollution, but also has a good application prospect for the regeneration of selenium resources.
Distribution and transformation of selenium
Current status of selenium emissions from coal-fired power plants
The average content of selenium in coal is about 2.5 mg/kg. When coal is consumed in large quantities, coal-fired power plants are the main source of man-made selenium emissions Tang et al. (Citation2012). The general concentration of selenium in the desulfurization wastewater is 1–10 mg/L Santos et al. (Citation2015b). If it is discharged without proper treatment, it will cause waste of selenium resources and pollution of the surrounding environment of the power plant.
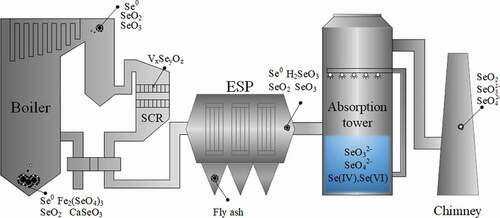
In coal-fired power plants, when selenium from coal combustion is processed and discharged by the APCDs system, each system unit will actively capture or passively block the combustion products of selenium due to differences in environmental factors and operating principles. The process of occurrence and transformation in coal-fired power plants is shown in .
In the process, selective catalytic reduction (SCR) and electrostatic precipitator (ESP) can partially remove selenide in flue gas. This is the result of no adjustments to the removal of selenium. It is also very difficult to further improve the removal efficiency of selenium in the flue gas so that the emissions of coal-fired power plants will not cause selenium pollution to the surrounding environment and ecology. The main difficulty lies in the low concentration of selenium in the flue gas, the short residence time of each processing unit and the enrichment of selenium by the WFGD system Zhong et al. (Citation2011).
While wet desulfurization achieves the removal of SO2, the by-product gypsum can be sold as a construction raw material to generate certain economic value or landfill Li et al. (Citation2015). Toreduce the economic and environmental costs of maintaining the water balance of the WFGD system, part of the water in the gypsum slurry is directly returned to the absorption tower after passing through the cyclone, the remaining part is dehydrated by a vacuum belt conveyor to form desulfurization wastewater, then returned to the absorption tower after chemical precipitation. It is also for this reason that after the selenium in the flue gas enters the wet desulfurization system, the phase, valence and structure changes occur, the removal efficiency of Se by the chemical precipitation method is difficult to exceed 50% Cheng et al. (Citation2009). This causes the enrichment of selenium in the desulfurization system into the unit where selenium occurs in the most complex and concentrated form in coal-fired power plants Weng et al. (Citation2021). If this process is not properly treated, it will reduce the quality of the plaster and endanger the surrounding environment Córdoba et al. (Citation2015). But from another aspect, the desulfurization system is also the most suitable part for Se ocontrol.
Changes in Se form in flue gas
During the combustion process, the selenium in the coal volatilizes into gaseous and
Huang et al. (Citation2004). As the temperature of the flue gas decreases, a small part of the gaseous
and
combine with the particulate matter in the flue gas through physical condensation and chemical adsorption Senior et al. (Citation2010), which is mainly affected by the calcium mineral content and the types of iron oxides (
) Ma et al. (Citation2020b). In this process,
may react with
to form a stable compound calcium selenite
Agnihotri et al. (Citation1998):
Or reacts with
to form
Huang et al. (Citation2020b):
At the same time, most of them still exist in gaseous form. Since the particle size of fly ash is smaller than that of bottom ash, it can provide and
with a larger specific surface area, so most of the gaseous
and
combined with particulate matter are condensed and adsorbed on fly ash Córdoba et al. (Citation2012). The bottom ash is discharged from the bottom of the boiler, the fly ash enters the subsequent APCDs along with the flue gas bottom ash Huang et al. (Citation2020a).
When the flue gas temperature is cooled to 430–475°C, the volatilized HCl reacts with and
in the flue gas to generate
. In this process,
can play a similar role to
and inhibit the formation of
:
catalyst is the most used catalyst for the selective catalytic reduction (SCR) denitration in coal-fired power plants. When the flue gas temperature is higher than 250°C,
and
in the flue gas can react with the catalyst and
in the
process Liu et al. (Citation2019):
This will reduce the redox performance of SCR and the concentration of acidic sites, and even generate unreducible product to inhibit SCR activity. However, since the Se concentration in the flue gas is extremely low, so the effect on the SCR catalyst is relatively low.
After the flue gas containing fly ash passes through the SCR and air preheater, the temperature of the flue gas will be further reduced.The gaseous and
in the flue gas will easily combine with fine particles, so after entering Electrostatic precipitators (ESP), more than 30% of Se can be captured by trapping fine particles.
Changes in the form of selenium in the liquid phase
Wet desulfurization is the mainstream removal process for coal-fired power plants, wet limestone-gypsum desulfurization has become the most common
control technology for coal-fired power plants due to its economic and stability advantages. The main component
in limestone reacts with
in the flue gas to form
. The whole desulfurization process can be divided into two parts: acid-base reaction and oxidation reaction. When the pH is in the range of 4.5–5.5, the main acid-base reaction is Córdoba (Citation2017):
When the pH is higher than 5.5, the main acid-base reaction is:
The oxidation part is forced to oxidize by passing in air to oxidize the and
in the slurry to
, ensure that the
content in the discharged gypsum slurry exceeds 90%. The main reaction is:
After treatment with APCDs, only less than 3% of selenium in the flue gas continues to be discharged in gaseous form. in the flue gas reacts with
in the flue gas and
in the absorption tower to form
, which is captured by the absorption tower:
However, the volatilization of chlorine in the flue gas will affect the form of selenium, and the produced can oxidize
to
. The oxidized
reacts with
to form
after entering the absorption tower:
However, most of is still produced by oxidation of
generated after
enters the absorption tower:
To reduce water consumption in the WFGD system, part of the gypsum slurry filtrate will be directly reused after passing through the cyclone, and the remaining part will be reused after chemical precipitation treatment. In this way, most of the selenium in the slurry is re-transmitted to the absorption tower to continue to participate in the reaction, only a small part of the selenium is discharged from the WFGD system due to the production of solid products or the water contained in the gypsum Lin et al. (Citation2020).
In the process of selenium enrichment in the absorption tower, the presence of ORP and other active substances will also lead to an increase in selenium species and a complex morphological structure Alvarez-Ayuso, Querol, and Tomas (Citation2006). At the same time, because sulfur and selenium are elements of the same main group and have similar chemical properties Akiho et al. (Citation2013), the type and content of sulfur in the absorption tower are abundant, there is a certain amount of selenium-sulfur compounds Guo et al. (Citation2017). It is possible that selenium completely replaces sulfur or selenium replaces part of the sulfur to form a stable structure with the remaining sulfur.
The pH of the slurry will determine whether the and
in the absorption tower will undergo decomposition reactions, resulting in
,
and
,
.The decomposition products can react with incompletely oxidized bisulfite
in the slurry Ball and Milne (Citation1995):
Among them, contains two isomers, one is S-bonded isomer-
, the other is easier to produce O-bonded isomer-
. Since sulfur is more electronegative than selenium, some isomers that form O-bonded isomers will slowly transform into S-bonded isomers.
How to remove selenium
In the past few decades when selenium pollution was proposed, some technologies for treating selenium-containing wastewater have been developed and applied. Treatment technology can be divided into physical, biological and chemical.
Physical Method
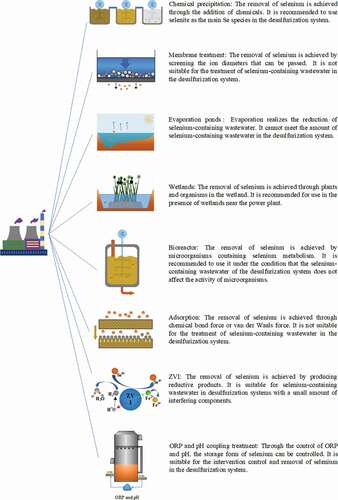
Membrane treatment
Membrane treatment is determined by the pore size of the membrane. Membrane treatment to remove selenium in water is not only used in the field of drinking water and general wastewater Zeeshan et al. (Citation2020), the combined use of chemical precipitation and reverse osmosis (RO) is also one of USEPA’s recommended desulfurization wastewater treatment methods.
In the treatment method of using membrane to remove selenium from water, RO and NF are usually selected according to the particle size and electronegativity of selenite () and selenate (
) Richards, Richards, and SchäFer (Citation2011). With the reduction of membrane material costs and the improvement of membrane performance, membrane processes such as RO and NF have been successfully used to remove low-concentration selenium in water bodies such as drinking water and municipal wastewater. However, when treating wastewater with many types of impurities and high concentration of pollutants, solid deposition on the surface of the membrane or growth of microorganisms on the surface of the membrane will block the membrane and reduce efficiency. Membrane systems often require pretreatment to remove excess TSS and organic matter to prevent fouling and clogging in industrial applications, but this greatly increases the complexity of the system.In addition to the above-mentioned shortcomings, the disposal of concentrated waste liquid or brine after membrane treatment is also a problem that has to be considered. Therefore, membrane treatment for selenium-containing wastewater in WFGD systems has good applicability at present and in the future. However, it is more reasonable to use it as a component unit of the processing system in conjunction with other technologies.
Evaporation ponds
The evaporation pond uses solar radiation as the main energy source to reduce the amount of selenium-containing wastewater. After evaporating the water, the total amount of selenium in the water decreases, but it does not eliminate the impact of selenium pollution on the surrounding ecology Gao et al. (Citation2003). The study on the evaporation pond concluded that the main reason for the decrease in the total amount of selenium in the evaporation pond water was the adsorption of selenium on the sediment particles. Among them, 80% Gao et al. (Citation2000) are organically associated selenium and elemental selenium.
The use of evaporation ponds to remove selenium compounds in wastewater is mainly used in agricultural wastewater Ryu, Gao, and Tanji (Citation2011). The low pollutant concentration of agricultural wastewater and the low cost of agricultural water determine that the evaporation pond is highly competitive in areas with high agricultural utilization.Although the evaporation pond technology cannot quickly remove selenium pollutants, its simple and low input characteristics can be used as a feasible solution under special conditions. As the selenium-containing wastewater treatment technology of the WFGD system, the applicability of the evaporation pond treatment is poor at present and worse in the future.
Biological method
Wetlands
In the environment, selenium mainly exists in five forms Zhao et al. (Citation2020): selenate ; selenite
; elemental Se
; selenides
and organic forms of Se. Among them, elemental Se is the most stable and usually exists in sediments. Selenium mainly exists in the form of more flexible selenate and selenite in natural waters and polluted waters. There is a selenium cycle in nature, researchers hope to take advantage of this natural process by studying the selenium removal mode of wetlands Lin and Terry (Citation2003).
The main mechanism of selenium removal in constructed wetlands is Martin et al. (Citation2018):
Selenate
is reduced to selenite
which is adsorbed into sediment particles;
The selenium oxide anion is dissimilated and reduced to the element selenium
to form a precipitate.
The selenium oxide anion is reduced to the insoluble selenide
.
It volatilizes into the atmosphere through the assimilation and reduction of methylated microorganisms and plants.
Removal of selenium pollutants in water through wetlands has the advantages of large water treatment volume, fewer operators, no additional chemicals, and underground wetlands that can even operate in cold weather Salhani, Boulyga, and Stengel (Citation2003). It has been successfully applied to the treatment of agricultural wastewater and drinking water Amweg, Stuart, and Weston (Citation2003). In this process, most of the pollutants in the water can be treated, so wetlands treatment is a comprehensive treatment technology. However, the removal of selenium pollutants in wastewater by wetlands is a natural process, which mainly relies on biological transformation Bhattacharya, Sarkar, and Das (Citation2003), evaporation and deposition Zhu and Banuelos (Citation2017) within the site, the treatment time is long. Wetlands covers a large area, and the conversion efficiency of inorganic selenium to organic selenium is highly correlated with biological abundance, so wetlands processing requires strict geographical conditions. If used for the treatment of selenium-containing wastewater in the WFGD system, wetlands can be used as a natural landscape while completing low-concentration selenium pollution treatment, but it is still necessary to consider whether it is toxic to aquatic organisms and birds in the wetland, and whether the concentration of selenium in sediments and groundwater is long-term increase Naftz et al. (Citation2005). Wetlands is a suitable technology for treating selenium-containing wastewater in the WFGD system only under specific conditions, it does not have high promotion significance.
Bioreactor
Researchers have discovered many microorganisms that can tolerate and transform selenium oxide anions, and they try to conduct in-depth research on the ecological mechanism of action Tan et al. (Citation2016). Researchers have discovered many microorganisms that can tolerate and transform selenium oxide anions, and they try to conduct in-depth research on the ecological mechanism of action. There are three ways of selenium metabolism in most microorganisms Nancharaiah and Lens (Citation2015):
Dissimilative reduction of selenium oxide anion to elemental selenium
Zhang et al. (Citation2020b);
Dissimilatory reduction of selenium oxide anion to elemental selenium
;
Assimilation and reduction of selenium oxide anion to selenoprotein.
The microorganisms living in the sediments can undergo the above process under hypoxic environment Lenz et al. (Citation2008). Because of the high reactivity of , not only anaerobic microorganisms can reduce it, but also aerobic microorganisms can complete the reduction Wang et al. (Citation2018). The higher valence of
has fewer microorganisms that can degrade it, it is more difficult to be reduced to
Nakajima et al. (Citation2013) than
.
With the deepening of research, from the previous discovery that only anaerobic microorganisms can reduce selenate to the discovery of aerobic microorganisms reducing selenate in recent years, there are more and more microorganisms that can be used to treat selenium pollution (Blum et al. Citation2001; Schilling et al. Citation2020; Xia et al. Citation2018; Zahir, Zhang, and Frankenberger Citation2003). At the same time, a variety of bioreactors have also been developed and applied, and the conversion efficiency has been improved by changing the culture conditions Tan et al. (Citation2018). Such as ABMet®, Envirogen fluidized bed reactor, Methanogenic UASB reactor, Upflow fungal pelleted reactor, Packed-bed medium-reactor, Aerobic jar fermenter and Aerobic jar fermenter.
Since the treatment of selenium pollutants by Bioreactor has been proven to remove selenium to a low level in engineering applications, it is currently considered to be one of the most available technologies in many fields, including coal-fired power plants.There are one or more of the following deficiencies in the use of different Bioreacto:r
Pre-treatment is required to meet the strict concentration requirements of suspended solids and other harmful substances to microorganisms in the treated wastewater.
To prevent short circuit and degassing of the flow, it is necessary to regularly use backwash water to remove excess microorganisms.
Because of the requirement of residence time, a larger area is required.
To ensure the survival of microorganisms, additional carbon sources need to be added.
Biological sludge needs to be dewatered and landfilled.
Biological residues containing elemental selenium need to be handled carefully.
In general, Bioreactor is an excellent technology for treating WFGD selenium-containing desulfurization wastewater, it has the potential for improvement in the future.
Chemical method
Chemical precipitation
In the desulfurization wastewater treatment industry, chemical precipitation is the most commonly used method because of its reliability and low price. For this method, the wastewater flows through three series tanks for neutralization, precipitation, and flocculation. This method is also called “three-linked tanks” Zou et al. (Citation2020). It can remove most heavy metals (such as mercury, lead, chromium) and suspended solids. In terms of selenium pollution control, because of the low reactivity of selenate, the removal effect of chemical precipitation on selenate is weak. Selenite has higher reactivity and can be removed with higher efficiency. Zhong et al. (Citation2011) Therefore, chemical precipitation treatment of selenium-containing wastewater in the WFGD system is a simple treatment method for high selenite. In other cases, since the effect of removing selenium is unsatisfactory, it is more suitable as a pretreatment.
Adsorption
Adsorption is a technology widely used in industry. The adsorbent interacts with the adsorbate by intermolecular chemical bonding or van der Waals force. This process is not only easy to implement, but also includes the advantages of high efficiency and low price. Studies have shown that selenium can be adsorbed on metal oxides and hydroxides, but the adsorption effect is affected by the type of selenide, of which has the best adsorption effect Tan et al. (Citation2019).
The adsorption treatment of drinking water and other purified water has been widely and maturely applied. The adsorption capacity of the adsorbent is closely related to its own specific surface area, the clean environment can ensure the work efficiency and service life of the adsorbent. The technology of using adsorption to treat WFGD selenium-containing wastewater is still more at the research level. It can be seen from the research in recent years that the adsorption capacity of the new adsorbent has been significantly improved, but the difficulty of preparing the adsorbent has also increased. Moreover, the practical problem facing researchers is how to ensure the adsorption capacity of the adsorbent as much as possible in the process of treating actual WFGD selenium-containing wastewater. If too much pretreatment of WFGD wastewater is needed, the easy-to-implement feature of adsorption technology will be lost.If simple pretreatment or no pretreatment conditions can achieve the removal of selenium pollution in wastewater, the adsorption technology is more suitable for the treatment of WFGD selenium-containing wastewater.
Zero-valent iron (ZVI)
In recent years, people have been paying attention to Zero-valent iron (ZVI), which is a cheap and environmental friendly material used to eliminate heavy metals and metalloids in groundwater and drinking water Okonji et al. (Citation2020). The corrosion of ZVI surface is a spontaneous reaction. This process will generate and hydrogen as electron donors to reduce pollutants. Corrosion products can also be used as reactive components to eliminate contaminant components. These ingredients include primary products (
), secondary products (
-hydroxide) and tertiary products (
-hydroxide) Huang et al. (Citation2013). The main mechanisms of ZVI to remove pollutants include adsorption, co-precipitation and size exclusion.
As a popular water treatment technology, ZVI has been applied in small-scale engineering in the process of treating WFGD selenium-containing wastewater Zhang et al. (Citation2019). Both and
can play a reducing role in the process, and
can play a role in adsorption.Since the form of iron has a great influence on the treatment capacity, the control of pH is the key to ensure the reduction processs. However, there is no selectivity in this process, the interference of dissolved oxygen and other anions will cause additional consumption of ZVI.In general, ZVI is a WFGD selenium-containing wastewater treatment technology that is simple to put into use, but has high requirements for later operation.
Oxidation-reduction potential(ORP)coupled with pH treatment
Oxidation-Reduction Potential (ORP) as a parameter to evaluate the redox properties of water environment has been applied in drinking water, metal corrosion and metallurgy industries.In the WFGD system, the entire oxidation process is closely related to the redox properties of the slurry system, ORP can reflect the changes in the concentration of various ions in the entire slurry system Ma et al. (Citation2020a). The common ORP of wet desulfurization slurry ranges from 100 mV to 300 mV. However, because there is no strict ORP monitoring and implementation standard for desulfurization slurry, there is a big gap in ORP of desulfurization slurry from different power plants, the situation of lower than 100 mV and higher than 500 mV also frequently occurs.Afterthe flue gas enters the absorption tower, the selenium in the flue gas will also undergo a series of valence changes, there is a corresponding relationship between selenium in different valence states and ORP.Potential–pH diagram is an effective method to study the law of selenium change Lai et al. (Citation2013). The reaction in the solution can be expressed by the following general formula:
When the temperature and pressure do not change, the Gibbs free energy change in the above formula can be expressed as:
For =1,
, and bring in
and
at the same time, it can be transformed into:
where:
a, b, c Stoichiometric number.
m number involved in the reaction.
n Electronic number involved in the reaction.
T Temperature (K).
R Molar gas constant (8.314
at 298 K).
F Faraday constant (96,485
in general).
Standard Gibbs free energy at 298 K (
).
Standard molar reactive Gibbs free energy (
).
Standard molar Gibbs energy of formation (
).
Electrode potential (V).
Standard electrode potential (V).
The transformed formula can establish the relationship between potential energy and pH, which can increase the scope of application.Under the condition of constant temperature, R and F are determined. Therefore, after obtaining the standard Gibbs free energy or standard potential
of the reaction participating substances, the relationship between the corresponding potential and pH can be calculated by the above equation, and the relationship between the potential and pH can be drawn based on this equation Nakamaru and Altansuvd (Citation2014).
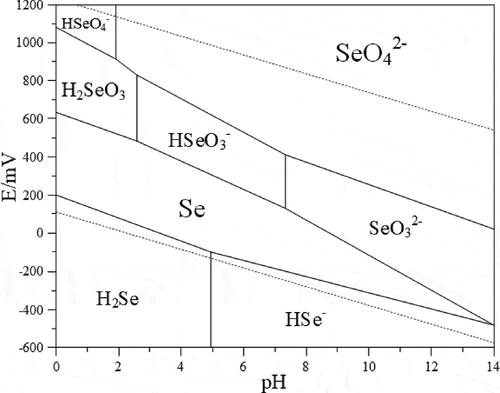
By consulting the thermodynamic data and , the corresponding standard electromotive force
and pH can be obtained. shows the potential – pH diagram of
system at 298 K.
The complexity of the WFGD system is higher than that of the system. Existing studies have not accurately explained the morphological transformation of selenium in the WFGD system.However, the successful establishment and application of the
system can provide a direction for the optimization of the WFGD system.
After the selenium in the flue gas enters the absorption tower, it will diffuse from the gas phase to the liquid phase. Under different redox potentials and pH conditions, it will become different forms. Among them, most of the valence states of selenium are and
. The wet desulfurization slurry contains a large number of species. In addition to these two main valence states, many other low-content selenides are also produced.Therefore, to grasp the thermodynamic law of selenide in the wet desulfurization system, the thermodynamic behavior of the selenium form change in the
system can be understood through the establishment of the potential pH diagram, and on this basis, the selenium in the desulfurization system In-depth research on occurrence and transformation. In the wet desulfurization process, the reaction after the flue gas enters the absorption tower is mainly divided into two parts: acid-base reaction and oxidation-reduction reaction. The acid-base level and oxidation-reduction potential in the slurry environment are both important basis for understanding and controlling the desulfurization process. Because of the outstanding contribution of acid-base adjustment to
absorption and
dissociation, pH has long been a necessary control parameter for desulfurization systems, but ORP as an indicator of the oxidation process has not received enough attention in FGD field. ORP and pH are closely related and can influence each other. Under the condition of not reducing the desulfurization efficiency and limestone dissociation efficiency, this treatment method can understand and control the oxidation-reduction status of the absorption tower slurry through ORP. This allows proper intervention before the formation of complex selenium compounds, avoiding the formation of too many selenide species and complicating the subsequent treatment process, and contributing to the regeneration and utilization of selenium resources. This is a source governance strategy with high application value.
Summary
The above eight selenium pollution treatment technologies have been proved to be able to achieve the removal of selenium pollutants in the process of theoretical research. As there are differences in application conditions in actual projects due to differences in technology, when used as a selenium pollutant removal program for coal-fired power plants, it is necessary to combine the actual conditions of coal-fired power plants to select technical routes. shows the comprehensive evaluation of eight selenium pollution treatment technologies.
Challenges of treating selenium with ORP
Conversion of selenium in WFGD
There are many species in wet desulfurization slurry, so is selenide. Part of the selenium in the flue gas is transferred to the slurry system, the remaining part remains in the gas phase; A small part of the selenium transferred to the liquid phase reacts with other substances to change into a solid state and is discharged with the gypsum slurry. Most selenium undergoes valence changes and remains in the desulfurization slurry. The possible selenium forms and production mechanisms in the whole process are not perfect. At present, only some of the main selenium forms are understood, further research on the conversion of selenium in WFGD is needed.
How does ORP accurately reflect the form of selenium in the WFGD system
As a comprehensive measurement index, ORP reflects the comprehensive redox properties of the measurement environment. There are many factors that can affect the WFGD system in such a complex system. The main shortcoming at present is that the mechanism of the selenium conversion process needs to be further improved. It is necessary to establish a proper relationship between ORP and selenium forms in WFGD.
How to regulate ORP in WFGD
During the operation of the WFGD system, various factors have different degrees of influence on ORP, there are parameters that can be easily adjusted and factors that are not easy to change. It is necessary to find out the factors suitable for adjusting the ORP of the WFGD system, such as the flow rate of the oxidation fan, to control the type and form of substances in the system by controlling the slurry ORP.
Regeneration of selenium resources
The regeneration of selenium resources through the oxidation-reduction potential (ORP) and pH coupling technology is a new way to increase the sustainability of coal. However, after achieving the selenium valence state control target in the WFGD system, a specific regeneration process is needed to utilize the selenium resources in the existing WFGD system. It is hoped that in this way, under the global low-carbon development trend, the use value of coal will be improved.
Conclusion
(1) As a trace element, selenium exists in nature at a very low concentration, mainly in five forms: selenate ; selenite
; elemental Se
; selenides
and organic forms of Se. However, as a typical man-made emission source, coal-fired power plants emit inorganic selenium compounds mainly composed of selenite
and selenite
to the surroundings. This will result in excessive selenium concentration in the surrounding environment and waste of selenium resources. This selenium will also act on other organisms through the food chain to induce serious selenium-related diseases.
(2) In coal-fired power plants, selenium in coal volatilizes into gaseous and
after combustion. A small part is combined with the particulate matter in the flue gas, most of them are still in gaseous form and enter the subsequent APCDs together with the flue gas. Selenium can react weakly with the catalyst in the SCR unit, more than 30% is captured together with the particulate matter in the ESP. After entering the absorption tower, only less than 3% of selenium continues to be discharged in gaseous form, and the remaining part is mainly in the form of selenite
and selenite
in the slurry, desulfurization wastewater and gypsum.
(3) At present, the treatment methods for selenium pollution are mainly divided into three categories: physical, biological and chemical. With the development of membrane materials, membrane treatment technology has a better application prospect than evaporation pond technology. Evaporation ponds are only suitable for application conditions where the concentration of selenium pollutants is low, the amount of waste water is small, and the economy is pursued.But the best way to use membrane treatment technology is still as part of the treatment system. Wetland and bioreactor are the current mainstream treatment technologies. Through biological treatment, the highly toxic inorganic selenium can be harmless and recycled, which is a process that strengthens natural circulation. However, the survival stability of microorganisms, sludge treatment and floor space are still the main difficulties restricting its development. Adsorption and ZVI as chemical treatment methods have the advantages of significant effects and simple operation, but as long-term or continuous selenium pollutant control technologies, they have the problem of insufficient treatment depth.
(4) ORP has great potential as a tool for selenium pollution control and selenium resource regeneration in wet desulfurization systems. Adding ORP to the WFGD control system can intervene in time before the formation of complex selenium pollutants. At the same time, it also has the advantages of reducing the difficulty of governance, low cost and strong coupling. However, there are still many challenges. It is necessary to explain the conversion of selenium in WFGD from the generation mechanism, so as to realize the accurate judgment of the existence form of selenium in the WFGD system through ORP. Finally, appropriate ORP regulatory factors must be selected to achieve the ultimate goal of intervening selenide conversion in the WFGD system. Finally, we must choose a suitable resource technology to achieve the ultimate goal of regeneration of selenium resources in coal.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Shuangchen Ma
Shuangchen Ma, professor, coal–fired pollution control chemistry.
Fang Xu
Fang Xu, Ph.D. candidate, pollution control chemistry.
Dao Qiu
Dao Qiu, engineer. Shuaijun Fan, studying master.
Ruimin Wang
Ruimin Wang, engineer.
Yang Li
Yang Li, studying master.
Xiangyang Chen
Xiangyang Chen, studying master.
References
- Agnihotri, R., S. Chauk, S. Mahuli, and L. S. Fan. 1998. Selenium removal using Ca-Based sorbents: Reaction kinetics. Environmental Science and Technology 32 (12):1841–46. doi:https://doi.org/10.1021/es971119j.
- Akiho, H., S. Ito, H. Matsuda, and T. Yoshioka. 2013. Elucidation of the mechanism of reaction between S2O82–, Selenite and Mn2+ in Aqueous Solution and Limestone-Gypsum FGD Liquor. Environmental Science & Technology 47 (19):11311–17. doi:https://doi.org/10.1021/es3042302.
- Alvarez-Ayuso, E., X. Querol, and A. Tomas. 2006. Environmental impact of a coal combustion-desulphurisation plant: Abatement capacity of desulphurisation process and environmental characterisation of combustion by-products. Chemosphere 65 (11):2009–17. doi:https://doi.org/10.1016/j.chemosphere.2006.06.070.
- Amweg, E. L., D. L. Stuart, and D. P. Weston. 2003. Comparative bioavailability of selenium to aquatic organisms after biological treatment of agricultural drainage water. Aquatic Toxicology 63 (1):13–25. doi:https://doi.org/10.1016/S0166-445X(02)00110-8.
- Bajaj, M., S. Schmidt, and J. Winter. 2012. Formation of Se (0) Nanoparticles by Duganella sp. andAgrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microbial Cell Factories 11 (1):64–64. doi:https://doi.org/10.1186/1475-2859-11-64.
- Ball, S., and J. Milne. 1995. Studies on the interaction of selenite and selenium with sulfur donors. Part 3. Sulfite. Canadian Journal of Chemistry 73 (5):716–24. doi:https://doi.org/10.1139/v95-091.
- Bhattacharya, B., S. K. Sarkar, and R. Das. 2003. Seasonal variations and inherent variability of selenium in marine biota of a tropical wetland ecosystem: Implications for bioindicator species. Ecological Indicators 2 (4):367–75. doi:https://doi.org/10.1016/S1470-160X(03)00006-2.
- Blum, J. S., J. F. Stolz, A. Oren, and R. S. Oremland. 2001. Selenihalanaerobacter shriftii gen. nov., sp. nov., a halophilic anaerobe from Dead Sea sediments that respires selenate. Archives of Microbiology 175 (3):208–19. doi:https://doi.org/10.1007/s002030100257.
- Chan, Y. T., Y. T. Liu, Y. M. Tzou, W. H. Kuan, R. R. Chang, and M. K. Wang. 2018. Kinetics and equilibrium adsorption study of selenium oxyanions onto Al/Si and Fe/Si coprecipitates. Chemosphere 198:59–67. doi:https://doi.org/10.1016/j.chemosphere.2018.01.110.
- Chang, C., R. Yin, X. Wang, S. Shao, C. Chen, and H. Zhang. 2019a. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Science of the Total Environment 669:83–90. doi:https://doi.org/10.1016/j.scitotenv.2019.02.451.
- Chang, L., J. Yang, Y. Zhao, H. Liu, J. Zhang, and C. Zheng. 2019b. Behavior and fate of As, Se, and Cd in an ultra-low emission coal-fired power plant. Journal of Cleaner Production 209:722–30. doi:https://doi.org/10.1016/j.jclepro.2018.10.270.
- Cheng, C.-M., P. Hack, P. Chu, Y.-N. Chang, T.-Y. Lin, C.-S. Ko, P.-H. Chiang, -C.-C. He, Y.-M. Lai, and W.-P. Pan. 2009. Partitioning of Mercury, Arsenic, Selenium, Boron, and Chloride in a Full-Scale Coal Combustion Process Equipped with Selective Catalytic Reduction, Electrostatic Precipitation, and Flue Gas Desulfurization Systems. Energy & Fuels 23 (10):4805–16. doi:https://doi.org/10.1021/ef900293u.
- Company, B. P. 1990. Statistical review of world energy. London, England, British Petroleum Company, 1990.
- Córdoba, P., I. Castro, M. Maroto-Valer, and X. Querol. 2015. The potential leaching and mobilization of trace elements from FGD-gypsum of a coal-fired power plant under water re-circulation conditions. Journal of Environmental Sciences 32:72–80. doi:https://doi.org/10.1016/j.jes.2014.11.009.
- Cordoba, P., and L. C. Staicu. 2018. Flue gas desulfurization effluents: An unexploited selenium resource. Fuel 223:268–76.
- Córdoba, P., R. Ochoa-Gonzalez, O. Font, M. Izquierdo, X. Querol, C. Leiva, M. A. López-Antón, M. Díaz-Somoano, M. Rosa Martinez-Tarazona, C. Fernandez, et al. 2012. Partitioning of trace inorganic elements in a coal-fired power plant equipped with a wet Flue Gas Desulphurisation system. Fuel 92 (1):145–57. doi:https://doi.org/10.1016/j.fuel.2011.07.025.
- Córdoba, P. 2017. Partitioning and speciation of selenium in wet limestone flue gas desulphurisation systems: A review. Fuel 202:184–95. doi:https://doi.org/10.1016/j.fuel.2017.04.015.
- Das, S., M. Jim Hendry, and J. Essilfie-Dughan. 2013. Adsorption of selenate onto ferrihydrite, goethite, and lepidocrocite under neutral pH conditions. Applied Geochemistry 28:185–93. doi:https://doi.org/10.1016/j.apgeochem.2012.10.026.
- Dodig, S., and I. Čepelak. 2004. The facts and controversies about selenium. Acta Pharmaceutica (Zagreb, Croatia) 54 (4):261–76.
- Fernandez-Llamosas, H., L. Castro, M. L. Blazquez, E. Diaz, and M. Carmona. 2017. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Scientific Reports 7 (1):16046. doi:https://doi.org/10.1038/s41598-017-16252-1.
- Gao, S., K. K. Tanji, D. W. Peters, and M. J. Herbel. 2000. Water selenium speciation and sediment fractionation in a California flow-through wetland system. Journal of Environmental Quality 29 (4):1275–83. doi:https://doi.org/10.2134/jeq2000.00472425002900040034x.
- Gao, S., K. K. Tanji, Z. Q. Lin, N. Terry, D. W. Peters, and S. Removal. 2003. Mass balance in a constructed flow-through wetland system. Journal of Environmental Quality 32:4. doi:https://doi.org/10.2134/jeq2003.1557.
- George, A., B. Shen, D. Kang, J. Yang, and J. Luo. 2020. Emission control strategies of hazardous trace elements from coal-fired power plants in China. Journal of Environmental Sciences 93:66–90. doi:https://doi.org/10.1016/j.jes.2020.02.025.
- Gui, M., J. K. Papp, A. S. Colburn, N. D. Meeks, B. Weaver, I. Wilf, and D. Bhattacharyya. 2015. Engineered iron/iron oxide functionalized membranes for selenium and other toxic metal removal from power plant scrubber water. Journal of Membrane Science 488:79–91. doi:https://doi.org/10.1016/j.memsci.2015.03.089.
- Guo, X. Y., X. U. Run-Ze, Q. H. Tian, and L. I. Dong. 2017. Hydrogen peroxide catalytic reduction of selenate by sulfur dioxide and formation mechanism of selenium. The Chinese Journal of Nonferrous Metals 27:2370–2378. doi:https://doi.org/10.19476/j.ysxb.1004.0609.2017.11.24 .
- Hadrup, N., and G. Ravn-Haren. 2020. Acute human toxicity and mortality after selenium ingestion: A review. Journal of Trace Elements in Medicine and Biology 58:126435. doi:https://doi.org/10.1016/j.jtemb.2019.126435.
- Han, D., Q. Wu, S. Wang, L. Xu, L. Duan, M. Wen, G. Li, Z. Li, Y. Tang, and K. Liu. 2021. Distribution and emissions of trace elements in coal-fired power plants after ultra-low emission retrofitting. Science of the Total Environment 754:142285 . doi:https://doi.org/10.1016/j.scitotenv.2020.142285.
- He, Y., J. Liu, G. Han, and T.-S. Chung. 2018. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. Journal of Membrane Science 555:299–306. doi:https://doi.org/10.1016/j.memsci.2018.03.055.
- He, Y., Y. P. Tang, and T. S. Chung. 2016. Concurrent removal of selenium and arsenic from water using Polyhedral Oligomeric Silsesquioxane (POSS)–Polyamide Thin-Film Nanocomposite Nanofiltration Membranes. Industrial & Engineering Chemistry Research 55 (50):12929–38. doi:https://doi.org/10.1021/acs.iecr.6b04272.
- Hong, S. H., F. N. Lyonga, J. K. Kang, E. J. Seo, C. G. Lee, S. Jeong, S. G. Hong, and S. J. Park. 2020. Synthesis of Fe-impregnated biochar from food waste for Selenium() removal from aqueous solution through adsorption: Process optimization and assessment. Chemosphere 252:126475. doi:https://doi.org/10.1016/j.chemosphere.2020.126475.
- Huang, Y. H., P. K. Peddi, C. Tang, H. Zeng, and X. Teng. 2013. Hybrid zero-valent iron process for removing heavy metals and nitrate from flue-gas-desulfurization wastewater. Separation & Purification Technology 118 (Complete):690–98. doi:https://doi.org/10.1016/j.seppur.2013.07.009.
- Huang, Y., B. Jin, Z. Zhong, R. Xiao, Z. Tang, and H. Ren. 2004. Trace elements (Mn, Cr, Pb, Se, Zn, Cd and Hg) in emissions from a pulverized coal boiler. Fuel Processing Technology 86 (1):23–32. doi:https://doi.org/10.1016/j.fuproc.2003.10.022.
- Huang, Y., H. Gong, H. Hu, B. Fu, B. Yuan, S. Li, G. Luo, and H. Yao. 2020a. Migration and emission behavior of arsenic and selenium in a circulating fluidized bed power plant burning arsenic/selenium-enriched coal. Chemosphere 263:127920. doi:https://doi.org/10.1016/j.chemosphere.2020.127920.
- Huang, Y., H. Hu, H. Gong, H. Xing, B. Yuan, B. Fu, A. Li, and H. Yao. 2020b. Mechanism study of selenium retention by iron minerals during coal combustion. Proceedings of the Combustion Institute 38(3): 4189–4197. doi:https://doi.org/10.1016/j.proci.2020.08.006 .
- Johnson, P. I., R. M. Gersberg, M. Rigby, and S. Roy. 2009. The fate of selenium in the Imperial and Brawley constructed wetlands in the Imperial Valley (California). Ecological Engineering 35 (5):908–13. doi:https://doi.org/10.1016/j.ecoleng.2008.12.020.
- Jones, C. P., P. R. Grossl, M. C. Amacher, J. L. Boettinger, A. R. Jacobson, and J. R. Lawley. 2017. Selenium and salt mobilization in wetland and arid upland soils of Pariette Draw, Utah (USA). Geoderma 305:363–73.
- Jones, C. P. Amacher, M. C. Grossl, P. R. Jacobson, A. R. 2020. Selenium mass balance and flux in water of Pariette Wetlands, Utah (USA). Applied Geochemistry, 113. doi:https://doi.org/10.1016/j.geoderma.2017.06.028.
- Lai, Y., C. Han, C. Yan, F. Liu, J. Li, and Y. Liu. 2013. Thermodynamic analysis on metal selenides electrodeposition. Journal of Alloys and Compounds 557:40–46. doi:https://doi.org/10.1016/j.jallcom.2012.12.150.
- Lenz, M., E. V. Hullebusch, G. Hommes, P. Corvini, and P. Lens. 2008. Selenate removal in methanogenic and sulfate-reducing upflow anaerobic sludge bed reactors. Water Research 42 (8–9):2184–94. doi:https://doi.org/10.1016/j.watres.2007.11.031.
- Lenz, M., and P. N. Lens. 2009. The essential toxin: The changing perception of selenium in environmental sciences. Science of the Total Environment 407 (12):3620–33. doi:https://doi.org/10.1016/j.scitotenv.2008.07.056.
- Li, F., and L. S. Fan. 2008. Clean coal conversion processes – Progress and challenges. Energy & Environmental Science 1 (2):248–67. doi:https://doi.org/10.1039/b809218b.
- Li, J., X. Zhuang, C. Leiva, A. Cornejo, O. Font, X. Querol, N. Moeno, C. Arenas, and C. Fernandez-Pereira. 2015. Potential utilization of FGD gypsum and fly ash from a Chinese power plant for manufacturing fire-resistant panels. Construction & Building Materials 95:910–21. doi:https://doi.org/10.1016/j.conbuildmat.2015.07.183.
- Li, Y., X. Guo, H. Dong, X. Luo, X. Guan, X. Zhang, and X. Xia. 2018. Selenite removal from groundwater by zero-valent iron (ZVI) in combination with oxidants. Chemical Engineering Journal 345:432–40. doi:https://doi.org/10.1016/j.cej.2018.03.187.
- Lin, J., N. Chen, R. Feng, M. J. Nilges, and Y. Pan. 2020. Sequestration of Selenite and Selenate in Gypsum (CaSO42H2O): Insights from Single-Crystal EPR and Synchrotron XAS Study. Environmental Science and Technology 3169-3180:54.
- Lin, Z.-Q., and N. Terry. 2003. Selenium Removal by Constructed Wetlands: Quantitative Importance of Biological Volatilization in the Treatment of Selenium-Laden Agricultural Drainage Water. Environmental Science & Technology 37 (3):606–15. doi:https://doi.org/10.1021/es0260216.
- Liu, H., X. Wang, B. Zhang, Z. Han, W. Wang, Q. Chi, J. Zhou, L. Nie, S. Xu, D. Liu, et al. 2020. Concentration and distribution of selenium in soils of mainland China, and implications for human health. Journal of Geochemical Exploration 220:106654. doi:https://doi.org/10.1016/j.gexplo.2020.106654 .
- Liu, Z., J. Han, L. Zhao, Y.-W. Wu, H.-X. Wang, X.-Q. Pei, M.-X. Xu, Q. Lu, and Y.-P. Yang. 2019. Effects of Se and SeO2 on the denitrification performance of V2O5-WO3/TiO2 SCR catalyst. Applied Catalysis A: General 587:117263. doi:https://doi.org/10.1016/j.apcata.2019.117263.
- Long, J., S. Zhang, and K. Luo. 2019. Selenium in Chinese coal gangue: Distribution, availability, and recommendations. Resources, Conservation and Recycling 149:140–50. doi:https://doi.org/10.1016/j.resconrec.2019.05.039.
- Ma, S. C., F. Xu, D. Li, S. Fan, D. Xu, and C. Ma. 2020a. ORP as slurry oxidation index and model modification in wet desulfurization system. Journal of the Air & Waste Management Association 70 (8):765–74.
- Ma, T., Y. Huang, S. Deng, B. Fu, G. Luo, J. Wang, H. Hu, C. Yuan, and H. Yao. 2020b. The relationship between selenium retention and fine particles removal during coal combustion. Fuel 265:116859. doi:https://doi.org/10.1016/j.fuel.2019.116859.
- Malhotra, M., M. Pal, and P. Pal. 2020. A response surface optimized nanofiltration-based system for efficient removal of selenium from drinking Water. Journal of Water Process Engineering 33: 101007. doi:https://doi.org/10.1016/j.jwpe.2019.101007.
- Martin, A. J., C. Fraser, S. Simpson, N. Belzile, Y. W. Chen, J. London, and D. Wallschlager. 2018. Hydrological and biogeochemical controls governing the speciation and accumulation of selenium in a wetland influenced by mine drainage. Environmental Toxicology and Chemistry 37 (7):1824–38. doi:https://doi.org/10.1002/etc.4123.
- Martínez, M., J. Giménez, J. de Pablo, M. Rovira, and L. Duro. 2006. Sorption of selenium(IV) and selenium(VI) onto magnetite. Applied Surface Science 252 (10):3767–73. doi:https://doi.org/10.1016/j.apsusc.2005.05.067.
- Naftz, D. L., J. Yahnke, J. Miller, and S. Noyes. 2005. Selenium mobilization during a flood experiment in a contaminated wetland: Stewart Lake Waterfowl Management Area, Utah. Applied Geochemistry 20 (3):569–85. doi:https://doi.org/10.1016/j.apgeochem.2004.09.009.
- Nakajima, T., R. Kamito, H. Takanashi, A. Ohki, and M. Lenz. 2013. Reduction of Selenate from Simulated Wet Flue Gas Desulfurization Wastewater Using Photocatalyst and Microorganism.Journal of Water and Environment Technology 11 (5):419–27. doi:https://doi.org/10.2965/jwet.2013.419.
- Nakamaru, Y. M., and J. Altansuvd. 2014. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 111:366–71. doi:https://doi.org/10.1016/j.chemosphere.2014.04.024.
- Nancharaiah, Y. V., and P. N. Lens. 2015. Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79 (1):61–80.
- Okonji, S. O., J. A. Dominic, D. Pernitsky, and G. Achari. 2020. Removal and recovery of selenium species from wastewater: Adsorption kinetics and co-precipitation mechanisms. Journal of Water Process Engineering 38:101666 . doi:https://doi.org/10.1016/j.jwpe.2020.101666.
- Olegario, J. T., N. Yee, M. Miller, J. Sczepaniak, and B. Manning. 2009. Reduction of Se(VI) to Se(-II) by zerovalent iron nanoparticle suspensions. Journal of Nanoparticle Research 12 (6):2057–68. doi:https://doi.org/10.1007/s11051-009-9764-1.
- Park, K. C., Y. Kwon, Y. Lee, D. K. Kim, Y. Jang, and S. Lee. 2020. Low selenium levels are associated with decreased bone mineral densities. Journal of Trace Elements in Medicine and Biology 61:126534. doi:https://doi.org/10.1016/j.jtemb.2020.126534.
- Peak, D. 2006. Adsorption mechanisms of selenium oxyanions at the aluminum oxide/water interface. Journal of Colloid and Interface Science 303 (2):337–45. doi:https://doi.org/10.1016/j.jcis.2006.08.014.
- Pollard, J., J. Cizdziel, K. Stave, and M. Reid. 2007. Selenium concentrations in water and plant tissues of a newly formed arid wetland in Las Vegas, Nevada. Environmental Monitoring and Assessment 135 (1–3):447–57. doi:https://doi.org/10.1007/s10661-007-9664-8.
- Qin, J., G. Qiu, J. Jian, H. Zhou, L. Yang, A. Charnas, D. Y. Zemlyanov, C. Y. Xu, X. Xu, W. Wu, et al. 2017. Controlled Growth of a Large-Size 2D Selenium Nanosheet and Its Electronic and Optoelectronic Applications. ACS Nano 11 (10):10222–29. doi:https://doi.org/10.1021/acsnano.7b04786.
- Richards, L. A., B. S. Richards, and A. I. Schäfer. 2011. Renewable energy powered membrane technology: Salt and inorganic contaminant removal by nanofiltration/reverse osmosis. Journal of Membrane Science 369 (1–2):188–95.
- Rovira, M., J. Gimenez, M. Martinez, X. Martinez-Llado, J. de Pablo, V. Marti, and L. Duro. 2008. Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. Journal of Hazardous Materials 150 (2):279–84. doi:https://doi.org/10.1016/j.jhazmat.2007.04.098.
- Ryu, J.-H., S. Gao, and K. K. Tanji. 2011. Accumulation and speciation of selenium in evaporation basins in California, USA. Journal of Geochemical Exploration 110 (2):216–24. doi:https://doi.org/10.1016/j.gexplo.2011.05.011.
- Salhani, N., S. F. Boulyga, and E. Stengel. 2003. Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland. Chemosphere 50 (8):967–73. doi:https://doi.org/10.1016/S0045-6535(02)00607-0.
- Santos, S., G. Ungureanu, B. Rui, and C. Botelho. 2015b. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Science of the Total Environment 521–522:246–60.
- Santos, S., G. Ungureanu, R. Boaventura, and C. Botelho. 2015a. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Science of the Total Environment 521-522:246–60.
- Schilling, K., A. Basu, C. Wanner, R. A. Sanford, C. Pallud, T. M. Johnson, and P. R. D. Mason. 2020. Mass-dependent selenium isotopic fractionation during microbial reduction of seleno-oxyanions by phylogenetically diverse bacteria. Geochimica et Cosmochimica Acta 276:274–88. doi:https://doi.org/10.1016/j.gca.2020.02.036.
- Senior, C., B. V. Otten, J. Wendt, and A. Sarofim. 2010. Modeling the behavior of selenium in Pulverized-Coal Combustion systems. Combustion & Flame 157 (11):2095–105. doi:https://doi.org/10.1016/j.combustflame.2010.05.004.
- Shu, Y., M. Wu, S. Yang, Y. Wang, and H. Li. 2020. Association of dietary selenium intake with telomere length in middle-aged and older adults. Clinical Nutrition 39 (10):3086–91. doi:https://doi.org/10.1016/j.clnu.2020.01.014.
- Tan, L. C., Y. V. Nancharaiah, S. Lu, E. D. van Hullebusch, R. Gerlach, and P. N. L. Lens. 2018. Biological treatment of selenium-laden wastewater containing nitrate and sulfate in an upflow anaerobic sludge bed reactor at pH 5.0. Chemosphere 211:684–93.
- Tan, G., Y. Mao, H. Wang, M. Junaid, and N. Xu. 2019. Comparison of biochar- and activated carbon-supported zerovalent iron for the removal of Se(IV) and Se(VI): Influence of pH, ionic strength, and natural organic matter. Environmental Science and Pollution Research 26 (21):21609–18.
- Tan, L. C., Y. V. Nancharaiah, E. D. van Hullebusch, and P. N. L. Lens. 2016. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnology Advances 34 (5):886–907. doi:https://doi.org/10.1016/j.biotechadv.2016.05.005.
- Tang, C., Y. H. Huang, H. Zeng, and Z. Zhang. 2014a. Promotion effect of Mn2+ and Co2+ on selenate reduction by zero-valent iron. Chemical Engineering Journal 244:97–104. doi:https://doi.org/10.1016/j.cej.2014.01.059.
- Tang, C., Y. H. Huang, H. Zeng, and Z. Zhang. 2014b. Reductive removal of selenate by zero-valent iron: The roles of aqueous Fe(2+) and corrosion products, and selenate removal mechanisms. Water Research 67:166–74. doi:https://doi.org/10.1016/j.watres.2014.09.016.
- Tang, Q., G. Liu, Z. Yan, and R. Sun. 2012. Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at Huainan, Anhui, China. Fuel 95:334–39. doi:https://doi.org/10.1016/j.fuel.2011.12.052.
- Tian, H. Z., Y. Wang, Z. G. Xue, K. Cheng, Y. P. Qu, F. H. Chai, and J. M. Hao. 2010. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in China, 1980–2007. Atmospheric Chemistry and Physics 10 (23):11905–19. doi:https://doi.org/10.5194/acp-10-11905-2010.
- Vinceti, M., M. Vicentini, L. A. Wise, C. Sacchettini, C. Malagoli, P. Ballotari, T. Filippini, M. Malavolti, and P. G. Rossi. 2018. Cancer incidence following long-term consumption of drinking water with high inorganic selenium content. Science of the Total Environment 635:390–96. doi:https://doi.org/10.1016/j.scitotenv.2018.04.097.
- Wang, Y., X. Shu, Q. Zhou, T. Fan, T. Wang, X. Chen, M. Li, Y. Ma, J. Ni, J. Hou, et al. 2018. Selenite Reduction and the Biogenesis of Selenium Nanoparticles by Alcaligenesfaecalis Se03 Isolated from the Gut of Monochamus alternatus (Coleoptera: Cerambycidae). Int J Mol Sci 19:9.
- Weng, Q., Y. Gong, X. Tian, Y. Zhuo, S. Wang, P. Hu, and T. Lyu. 2021. The Distribution and Conversion of Selenite and Selenate with the Bubbling of Simulated flue gas in Simulated WFGD Slurry. Journal of Hazardous Materials 416:125823. doi:https://doi.org/10.1016/j.jhazmat.2021.125823 .
- Xia, X., S. Wu, N. Li, D. Wang, S. Zheng, and G. Wang. 2018. Novel bacterial selenite reductase CsrF responsible for Se(IV) and Cr(VI) reduction that produces nanoparticles in Alishewanella sp. WH16-1. Journal of Hazardous Materials 342:499–509. doi:https://doi.org/10.1016/j.jhazmat.2017.08.051.
- Yiqiang, Z., W. Juanfang, A. Chris, and W. T. Frankenberger. 2005. Removal of Selenate from Water by Zerovalent Iron. Journal of Environmental Quality 34 (2):487. doi:https://doi.org/10.2134/jeq2005.0487.
- Zahir, Z., Y. Zhang, and W. T. Frankenberger. 2003. Fate of Selenate Metabolized by Enterobacter taylorae Isolated from Rice Straw. Journal of Agricultural & Food Chemistry 51 (12):3609–13. doi:https://doi.org/10.1021/jf0300442.
- Zeeshan, M. H., R. U. Khan, M. Shafiq, and A. Sabir. 2020. Polyamide intercalated nanofiltration membrane modified with biofunctionalized core shell composite for efficient removal of Arsenic and Selenium from wastewater. Journal of Water Process Engineering 34:101175. doi:https://doi.org/10.1016/j.jwpe.2020.101175.
- Zhao, Q., J. C. Huang, S. He, and W. Zhou. 2020. Enhancement of a constructed wetland water treatment system for selenium removal. Science of the Total Environment 714:136741.
- Zhang, L., H. Song, Y. Guo, B. Fan, Y. Huang, X. Mao, K. Liang, Z. Hu, X. Sun, Y. Fang, et al. 2020a. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017-2019): Is current selenium-rich agro-food safe enough? Journal of Hazardous Materials 398:123224. doi:https://doi.org/10.1016/j.jhazmat.2020.123224.
- Zhang, N., D. Gang, and L. S. Lin. 2010. Adsorptive Removal of Parts per Million Level Selenate Using Iron-Coated GAC Adsorbents. Journal of Environmental Engineering 136 (10):1089–95. doi:https://doi.org/10.1061/(ASCE)EE.1943-7870.0000245.
- Zhang, W., H. Oswal, J. Renew, K. Ellison, and C. H. Huang. 2019. Removal of heavy metals by aged zero-valent iron from flue-gas-desulfurization brine under high salt and temperature conditions. Journal of Hazardous Materials 373:572–79. doi:https://doi.org/10.1016/j.jhazmat.2019.03.117.
- Zhang, X., W. Y. Fan, M. C. Yao, C. W. Yang, and G. P. Sheng. 2020b. Redox state of microbial extracellular polymeric substances regulates reduction of selenite to elemental selenium accompanying with enhancing microbial detoxification in aquatic environments. Water Research 172:115538. doi:https://doi.org/10.1016/j.watres.2020.115538.
- Zhong, L., Y. Cao, W. Li, K. Xie, and W. P. Pan. 2011. Selenium speciation in flue desulfurization residues. Journal of Environmental Sciences 23 (1):171–76.
- Zhu, H., and G. Banuelos. 2017. Evaluation of two hybrid poplar clones as constructed wetland plant species for treating saline water high in boron and selenium, or waters only high in boron. Journal of Hazardous Materials 333:319–28. doi:https://doi.org/10.1016/j.jhazmat.2017.03.041.
- Zou, R., H. Zhang, G. Luo, C. Fang, M. Shi, H. Hu, X. Li, and H. Yao. 2020. Selenium migration behaviors in wet flue gas desulfurization slurry and an in-situ treatment approach. Chemical Engineering Journal 385:123891. doi:https://doi.org/10.1016/j.cej.2019.123891.