ABSTRACT
During wastewater treatment, bioaerosols are generated and, can either remain in suspension for several hours or settle on surfaces and workers may be exposed. The presence of pathogens in the air could contribute to an increased frequency of gastrointestinal or respiratory illness amongst workers. Due to harsh winter conditions in Eastern Canada, many of the steps in the wastewater treatment process occur indoors, leading to a greater risk of significant occupational exposure especially if there is inadequate ventilation or a lack of personal protection. This work has used stationary sampling at various indoor wastewater treatment steps both in winter and summer. Bioaerosols were evaluated using both culture and molecular methods along with ventilation characterization. Endotoxins were quantified, as well as total cultivable and gram-negative bacteria and pathogen indicators using qPCR. This study highlights the presence of potential pathogens at all steps in the treatment process, which may represent a potential occupational hazard. Comparisons between summer and winter data suggest that water temperature is an important factor for microbial activity and suggest that increasing the rate of air changes per hour in summer would be beneficial to reduce the concentration of bioaerosols during this time of the year. The screening, grit/FOGs removal and biofiltration were the most bioaerosol-loaded sites. Based on strong correlations, we suggest the reconsideration of exposure limits in WWTPs. Workers should be encouraged to use personal respiratory protection to limit the risk of health problems, especially during long-term work.
Implications: The work presented herein showcases significant correlations between concentrations of endotoxins, cultivable bacteria, gram-negative bacteria, and total bacteria by qPCR from air collected in indoor wastewater treatment plants. These correlations lead us to propose new limit of exposure values, revisited to fit the endotoxin exposure limits recommendations. The results can serve as guidelines for future proposals for air quality indicators.
Introduction
Wastewater treatment plants (WWTP) are associated with an inherent risk of human exposure to biological hazards. Wastewater is laden with a multitude of microorganisms including some pathogenic microorganisms mainly coming from human flora, which workers often come into contact with (Dutkiewicz et al. Citation2011; Kay et al. Citation2008). For example, wastewater can contain coliform bacteria that range from 108 and 1012 cells/100 mL depending on the time of the day (Drapeau and Jankovic Citation1977). Bioaerosols, defined as airborne biological particles including bacterial and fungal cells, viruses and by-products of microbial metabolism, are generated during multiple steps in the wastewater treatment process and, depending on size distribution, can remain in the air for prolonged periods and eventually be inhaled by workers (Sánchez-Monedero et al. Citation2008). If not inhaled, these particles may settle and use indirect transmission to oral exposure route through contact with soiled surfaces. Some studies have demonstrated the presence of various bacterial markers such as Escherichia coli, Klebsiella spp. and Pseudomonas spp. in air (Fracchia et al. Citation2006; Korzeniewska and Harnisz Citation2012; Shannon et al. Citation2007). Most studies concluded that WWTP employees are at risk of being exposed to several pathogens, which could contribute to an increased frequency of gastrointestinal or respiratory illness amongst wastewater workers.
Rylander et al. described the multiple health problems among these populations (headaches, weakness, fever, nausea, coughing, rhinitis, gastroenteritis, etc.) and, grouped them under the term “sewage worker syndrome” (Rylander et al. Citation1976). Several other health problems have arisen in populations of WWTP workers such as infections, various respiratory and gastroenteric problems, systemic symptoms, and even certain cancers (Divizia et al. Citation2008; Gangamma et al. Citation2011; Kindzierski et al. Citation2015a; Smit, Spaan, and Heederik Citation2005; Thorn and Beijer Citation2004; Thorn and Kerekes Citation2001; Vidal et al. Citation2012). Different agents in WWTP aerosols could be responsible for the multitude of health problems among these employees, including harmful gases, endotoxins and a wide variety of pathogenic bacteria and viruses (Elia et al. Citation2005; Fannin, Vana, and Jakubowski Citation1985; Ivens et al. Citation1999; Rylander Citation1999; Rylander et al. Citation1977; Smit, Spaan, and Heederik Citation2005). One challenge of studying bioaerosols is that several of these agents can be responsible for the same symptoms previously described. For example, according to Smit et al. (Citation2005), the risk of diarrhea appears to increase in workers exposed to more than 50 endotoxin units per cubic meter of air (EU/m3; 10) while other authors associate diarrhea with the probable presence of viruses (Fannin, Vana, and Jakubowski Citation1985). In 2010, the Dutch Expert Committee on Occupational Safety (DECOS) increased the recommended limit threshold of endotoxin exposure for eight-hour shifts to 90 endotoxin units per cubic meter of air (EU/m3, 21).
The wastewater-bioaerosol literature focuses mainly on outdoor settings because of the potential impacts of pathogen dispersion on neighboring populations. Findings vary greatly from study to study depending on the type of treatment and environment. Based on a written survey, results show that close proximity to a WWTP could coincide with the deteriorated health of nearby residents (Jaremkow, Noga, and Pawlas Citation2018; Jaremkow et al. Citation2017; Vantarakis et al. Citation2016). Studies that included the examination of air samples, typically using culture-based methods, most often concluded that there is no risk to the surrounding population, but rarely focus on potential occupational risks (Grisoli et al. Citation2009). However, in Eastern Canada, due to harsh winter conditions, many of the steps in the wastewater treatment process occur indoors in modern WWTP. The consequence of the treatment process confinement is that workers in these indoor facilities are at higher risk of exposure, especially if there is inadequate ventilation, hygiene or personal protection (Kindzierski et al. Citation2015b). It is noteworthy that in a northern climate, the summer season is usually linked with better ventilation and consequently, lower bioaerosols exposure in indoor settings such as pig barns (Nehme et al. Citation2008). In the situation where temperature could influence microbial activity in the bioaerosols source (wastewater), the impact of the seasons could be different from what one could expect.
In addition to the differences in treatment process and environmental parameters, comparison of existing studies of bioaerosols from WWTPs are difficult because of the wide range in reported concentrations of microorganisms (Carducci et al. Citation2000; Han et al. Citation2018; Sánchez-Monedero et al. Citation2008). This variation can be caused by several factors such as differences in sampling and analysis techniques, ventilation impacting on measured concentrations and statistical analyses used. Evaluation of bacterial populations in indoor WWTPs showed significant regional disparities (Han et al. Citation2018). The unique climatic and geographic conditions in Eastern Canada certainly affect the ventilation regimes, effluent water volume and temperature and, consequently, the nature and concentration of the bioaerosols from WWTPs.
The lack of consistency throughout previous studies makes it difficult to assess recommendations given the limited information available in the scientific literature. It is necessary to identify factors promoting worker exposure and also to determine the most appropriate bioaerosol hazard indicator for monitoring these types of environments. The aim of this project was to compare and identify key biomarkers to easily monitor the biological air quality in WWTP by doing stationary sampling during multiple indoor wastewater treatment steps during both cold and warm seasons along with ventilation and ambient parameters analyses. The composition of bioaerosols was evaluated using culture methods and molecular analyses to target specific human pathogenic bacteria.
Material and methods
Sampling sites
Eight WWTPs, treating municipal sewage, located in Quebec, Canada, were each visited once during the cold season (from February to March) and once during the warm season (from July to September). All visits occurred between 2015 and 2016. Sampling was conducted under normal operating conditions for each plant. For the wastewater treated, the largest plant treats an annual average of 400,000 m3 of water/day, and the smallest 36,000 m3 of water/day – the average is 170,000 m3/day. Only indoor treatment steps, where workers perform daily tasks and representing higher risk of being exposed to bioaerosols, were selected. Based on these criteria and availability, four different indoor treatment steps, could be studied: screening, degreasing/grit removal, settling tanks and biofiltration. Pertinent details associated with each step are presented in . For each measured parameter (endotoxins, culturable bacteria, and molecular analyses of bacteria), at each site, samples were collected in triplicate. Samplers were installed on the tables at one meter above the floor and special attention is used not to select areas where ventilation supply and exhaust may disturb the sampling process. Sampling was run simultaneously at each investigated site. Calibration of all sampling equipment was performed prior to every sampling campaign.
Table 1. Description of treatment steps and number of sites sampled.
Ambient temperature and the relative humidity were measured with a psychrometer (Cole-Parmer®, Montréal, Canada). The number of air changes per hour (ACH) was measured with the tracer gas sulfur hexafluoride (SF6), a common tracer gas used for ventilation studies, using the concentration decay method proposed by ASTM (Citation1993). The number of ACH was measured several times at different points per sites (~4 times) during sampling under normal operations of treatment plants within the day.
Culturable bacteria
The culturable airborne bacteria were collected using a six-stage Andersen impactor (Thermo Fisher Scientific, Waltham, Masschusettes, USA) (Andersen, Citation1958) loaded with petri dishes containing either Difco™ tryptic soy agar (TSA) (BD Canada, Oakville, Canada) with amphotericin B (5 µg/mL) to collect total culturable aerobic bacteria or with gram-negative selective agar (GNSA) for culturable gram-negative bacteria (Moore et al. Citation2003). Sampling was performed at three time points and averaged to represent exposure across the work shift. Sampling was performed using a high volume pump (Gast Manufacturing Inc., Benton Harbor, USA) with an airflow rate of 28.3 L/min, 20 min for GNSA and 10 min for TSA. The flow rate was adjusted with a calibrated rotameter at each use. The TSA and GNSA plates were incubated at 37°C, for 48 hr. Colonies were enumerated according to the positive-hole correction method (ASTM Citation1993). The concentration of culturable microorganisms was expressed in colony-forming units per cubic meter of air (CFU/m3).
Endotoxins
To determine airborne endotoxin concentrations, 37 mm cassettes (SKC Inc., Eighty Four, PA, USA) loaded with binder-free glass fiber filters with a porosity of 1 µm were used in duplicate at each site for 240 to 360 min. The cassettes were connected to a Gilian GilAir-5 pump (Sensidyne Industrial Health and Safety Instrumentation, St Petersburg, FL, USA) at a flowrate of 2 L/min with inlet pointing downward. After sampling, cassettes were frozen at −20°C prior to analysis. Endotoxins were extracted from the filters using 20 mL of sterile pyrogen-free saline solution (0.9% NaCl) plus 0.025% Tween 20 and vortexed at maximum speed for 60 min (Multi-Pulse Vortexer; Glas-Col®, Terre Haute, IN, USA). The suspensions were then centrifuged at 1000 g for 10 min and supernatant was used for endotoxin quantification using the kinetic chromogenic limulus amoebocyte lysate assay (Lonza, Walkersville, MD, USA) without the β-G-blocker and according to the manufacturer’s instructions. Inhibition/enhancement tests were performed to determine the appropriate dilution of the samples (1:20). The endotoxin concentrations were expressed in EU/m3. Blank filters were used as a field negative control, brought to the sampling site and analyzed using the same procedure that was used for the other filters. Blank value was subtracted from sample results.
Molecular analyses of bacteria
Air sampling
Samples were collected with a SASS 3100 (Research International, Inc., Monroe, WA, USA), a high-efficiency sampler that collects bioaerosols using a charged electret filter. Thirty cubic meters of air were sampled at 300 L/min. Filters were kept at 4°C until the extraction using the SASS 3010 Particle Extractor (Research International, Inc.). Filters were eluted in 5 mL of SASS extraction buffer. Blank filters were brought to the sampling site and analyzed using the same procedures used for bacteria. Each air sample was divided into three aliquots (1.5 mL each) and centrifuged at 21,000 g for 10 min. The pellets were stored at −20°C until DNA extraction. DNA from all samples was extracted in 50 µL of elution buffer using the PowerLyzer UltraClean Microbial DNA Isolation Kit (Bio-Rad Laboratories, Mississauga, ON, Canada). In addition to total bacteria, specific qPCR were used to monitor bacteria from human flora: E. coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and fresh water environment: Aeromonas hydrophila. An external control sample (field blanks) was also collected upwind of each facility, during summer only and was treated the same way as samples. A negative extraction control was included in each batch of samples processed for DNA extraction. Total bacteria PCR amplification signal from blanks was subtracted from the values of corresponding samples. The extremely cold temperatures in winter prevented us from sampling outdoors.
Quantification using real-time quantitative PCR (qPCR)
A Bio-Rad CFX384 thermocycler (Bio-Rad Laboratories) was used for DNA amplification. The sequences of primers and probes used were purchased from Integrated DNA Technologies (Coralville, IA, USA) and are listed in . All standard curves were calculated based on 10-fold dilutions of genomic DNA to quantify corresponding bacteria – E. coli was used as a standard for total bacteria. For all qPCR runs, both no template controls, extraction blanks and field blanks were added to estimate potential background amplification. The results were analyzed using Bio-Rad CFX Manager software version 3.0.1224.1015 (Bio-Rad Laboratories).
Table 2. Primers and probes used to detect selected microorganisms by qPCR.
Statistical analysis
Data in tables were expressed using min–max and mean values. Graphical representation of ambient parameters (humidity, temperature and air flow) are expressed using means ±SEM. To compare the different treatment sites from eight WWTPs, a linear mixed model was performed. Three experimental factors were defined: one being associated to WWTPs (random factor), one associated to the different treatment sites (fixed factor) and another factor linked to the season effect (fixed factor) and analyzed as a repeated-measure factor. An interaction term between the two fixed factors was added to the statistical model. The normality assumption was verified with the Shapiro–Wilk tests on the error distribution from the statistical model after a Cholesky factorization. Data from concentrations of endotoxins, culturable gram-negative bacteria, total culturable bacteria, total bacteria and pathogenic bacteria were expressed using means ±SEM after a log10 transformation to stabilize the variances among treatment sites from the summer and winter period. The same statistical approach was used to perform statistical analyses. The results were considered significant with p-values ≤ 0.05. All analyses were conducted using the statistical packages SAS v9.4 (SAS Institute Inc., Cary, NC, USA) and R v3.5 Edgar Brunner, Sebastian Domhof, Frank Langer (R Core Team 2018; Domhof and Langer, Citation2002).
Results
Ambient parameters
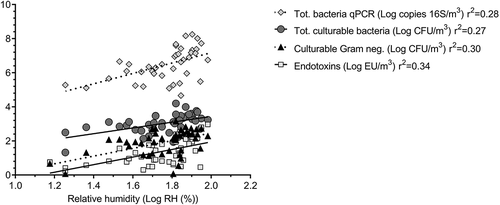
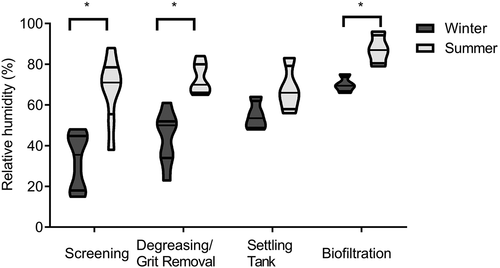
The average room temperature was significantly higher in summer (approximately 22°C) compared to winter (17°C; p < 0.0001). The temperature was stable from one site to another during both seasons. The average ACH was slightly higher in summer (4.25) than in winter (3.35; p = 0.015). The pretreatment sites (screening and grit/FOGs removal) exhibited the highest rates of air change for both seasons. No significant correlation was observed between these parameters and the concentration of airborne bacteria. The average relative humidity (RH) was lower (p < 0.0001) in winter (48%) than in summer (72%). There were also significant differences in the RH between treatment steps (p < 0.005) for both seasons. The screening step exhibited the lowest RH and biofiltration had the highest RH (). A significant correlation was observed between RH and most of the parameters used to examine the content of bioaerosols in the air (): increase in the RH corresponds with increase in the microbiological load in the air. The concentration of endotoxins showed the strongest correlation with RH (R2 = 0.34/p < 0.0001) of all of the parameters tested. Wastewater temperature collected from visited sites goes from 6°C to 12°C and 17°C to 25°C for both cold and warm seasons, respectively (data in supplementary figure).
Figure 1. Relative humidity measured during different treatment steps at all WWTPs visited in winter and summer. The average relative humidity was lower in winter than in summer (p < 0.0001). There were also significant differences (*) in the relative humidity between treatment steps for both seasons (screening p = 0.002; degreasing/grit removal p = 0.0003; Biofiltration p = 0.005).
Culturable gram-negative bacteria
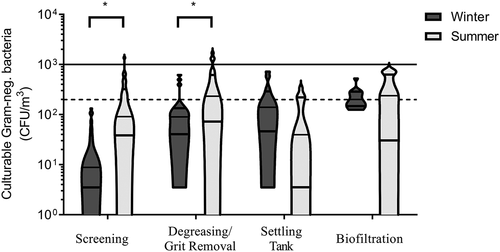
The average concentration was approximately 100 CFU/m3 for both seasons with values ranging from below the detection limit for both seasons () and 540 CFU/m3 (winter) or 1400 CFU/m3 (summer). There was no significant difference in culturable gram-negative bacteria concentrations between winter and summer at each site, with the exception of the screening site (p = 0.003) and the degreasing/grit removal site (p = 0.01). There was no significant difference between sites in summer. There was a slight difference observed between sites in winter (p = 0.023). Concentration tended to increase at each step throughout the wastewater treatment process and only two WWTPs showed concentrations higher than the Institut Robert Sauvé en Santé et en Sécurité du Travail (IRSST) recommended exposure limit (1,000 CFU/m3, 34). One breach of this limit was at the screening site and the other was at a grit/FOGs removal site. While those were the only two instances that exceeded the recommended limit, several values were close to the limit.
Figure 3. Concentrations of culturable gram-negative bacteria measured in air during different treatment steps at all WWTPs visited in winter and summer. There was a significant difference (*) in culturable gram-negative bacteria concentrations between winter and summer at the screening site (p = 0.003) and the degreasing/grit removal site (p = 0.01). The solid line represents the IRSST recommended limit and the dotted line represents the recommended limit based on endotoxins/culturable gram-negative correlations in this study.
Total culturable bacteria
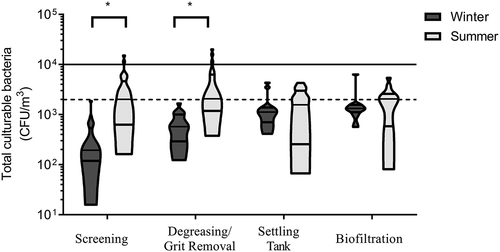
The average concentration of culturable bacteria was 600 CFU/m3 in winter and 2000 CFU/m3 in summer (). The minimum concentration measured was 20 CFU/m3 in winter and 86 CFU/m3 in summer, reaching maximum concentration of 3000 CFU/m3 (winter) and 16000 CFU/m3 (summer). At each site, average concentrations were significantly higher in summer only for the screening (p = 0.001) and the grit/FOGs removal (p = 0.003). There was no significant difference between sites in summer and a slight difference in winter (p = 0.036). The bacterial concentrations tended to increase at each step throughout the wastewater treatment process. Trends observed with gram-negative bacteria were also observed here. The same WWTPs and the same specific sites (screening and grit/FOGs removal site) had concentrations higher than the exposure limit recommended for culturable bacteria.
Figure 4. Concentrations of total culturable bacteria measured in air during different treatment steps at all WWTPs visited in winter and summer. Concentrations were significantly higher (*) in summer for the screening (p = 0.001) and the grit/FOG removal (p = 0.003). The solid line represents the IRSST recommended limit and the dotted line represents the recommended limit based on endotoxins/total culturable bacteria correlations in this study.
Endotoxins
The average concentration of endotoxins in winter was 7.31 EU/m3 with a minimum of 2 EU/m3 and a maximum of 331 EU/m3. In summer, the average concentration was 50 EU/m3 with a minimum and maximum of 3 and 924 EU/m3, respectively. The endotoxin concentrations were significantly higher in summer for most sites (p = 0.002) and the concentrations varied according to the treatment sites for both seasons (p = 0.0005). Average concentrations of endotoxins were lowest during screening and highest during biofiltration ().
Figure 5. Concentrations of endotoxins measured in air during different treatment steps at all WWTPs visited in winter and summer. Concentrations were significantly higher (*) in summer compared to winter in screening (p = 0.01), degreasing/grit removal (p = 0.0003), and biofiltration (p = 0.03). The dotted line represents the Dutch Expert Committee on Occupational Safety (DECOS) recommended limit of exposure (LOE) for endotoxin concentration.
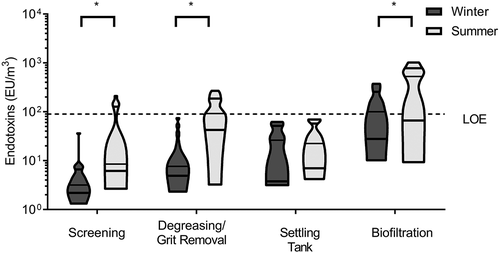
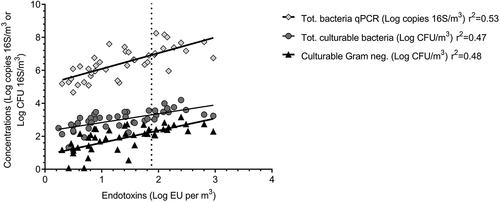
Several air samples contained concentrations above the limit recommended by DECOS (90 EU/m3), mainly in summer. As shown in (limit represented by the dotted line) 9% of samples collected during winter and 39% of samples collected during summer are above that recommended limit. Several WWTPs had higher concentrations of endotoxins in the air at the screening, grit/FOG removal and biofiltration sites. The biofiltration site appeared to be the most problematic as samples from this site from more than half of the WWTPs were above the exposure limit during both seasons. shows correlations between endotoxins and other parameters monitored in this study.
Total bacteria (qPCR)
The total bacteria concentration presented in is expressed in 16S copies per cubic meter of air (16S copies/m3). The average concentration of bacteria in winter and summer were 1 × 106 16S copies/m3 and 1 × 107 16S copies/m3, respectively. The minimum concentrations detected were 4 × 104 16S copies/m3 (winter) and 1 × 105 16S copies/m3 (summer). The maximum concentration detected in winter was 3 × 107 16S copies/m3 and 1 × 108 16S copies/m3 in summer. Bacterial concentrations were significantly higher in summer at every treatment site (p = 0.001). There was a strong correlation between bacterial concentration obtained by culture and by qPCR (R2 = 0.48; data not shown), but the qPCR method led to an estimate up to 3 orders of magnitude greater than that obtained by culture, as shown in other types of environments (Nehme et al. Citation2008)
Figure 7. Concentrations of total bacteria obtained by qPCR during different treatment steps at WWTPs visited in winter and summer. Concentrations were significantly higher in summer (*) for the screening (p = 0.002), the degreasing/grit removal removal (p = 0.0003), and the settling tank (p = 0.006). The dotted line represents the recommended limit based on endotoxins/total bacteria by qPCR correlations in this study. The detection limit of the method is 100 copies 16S/m3).
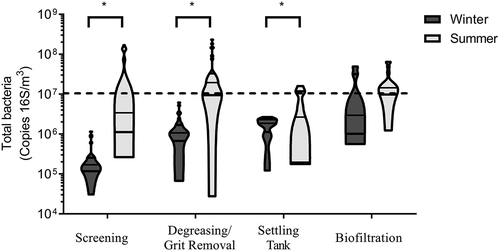
There were also differences between sites for both seasons (p = 0.048). There was no observable pattern in summer, but in winter, trends were similar to those observed for culturable bacteria. The bacterial concentrations tended to increase at each step throughout the wastewater treatment process.
Specific bacterial markers
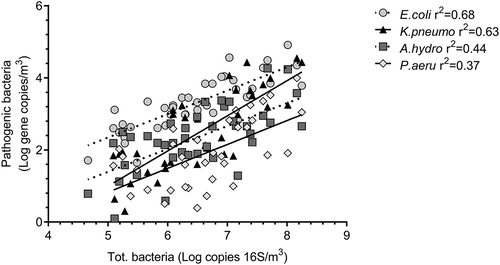
Bacteria of interest in a human health perspective were selected to evaluate them as potential indicators: E. coli, K. pneumonia, A. hydrophila and P. aeruginosa. shows the minimum and maximum as well as the average concentration (in brackets) of these bacteria which were all detected.
Table 3. Concentrations of pathogenic bacteria found in the air at WWTPs in winter and summer at each of the treatment sites (gene copies/m3 air).
E. coli was present in all air samples and at all treatment sites. Concentrations were greater in summer than in winter (p = 0.045). This species was present in higher concentrations than any of the others identified. The average concentration in winter was 1 × 103 gene copies/m3 and 4 × 103 gene copies/m3 in summer.
The average concentration of K. pneumoniae in winter and summer was 60 and 800 gene copies/m3, respectively. Only the screening (p = 0.002) and grit/FOGs sites (p = 0.05) had significantly higher concentration in summer than in winter. The average concentration of A. hydrophila species in aerosols was 100 and 400 gene copies/m3 in winter and summer, respectively. Concentrations were higher in summer (p = 0.011) than in winter for all sites except for samples collected during biofiltration where concentrations were not significantly different. For P. aeroginusa, the average concentration found were 6 gene copies/m3 in winter and 100 gene copies/m3 in summer. It was present in higher concentrations in summer (p = 0.011).
In general, bacterial concentrations were almost always higher in summer than in winter. In addition, their concentration correlated with total bacteria obtained by qPCR (), as the presence of specific bacterial markers increased with increasing total concentration of bacteria in the air.
Discussion
Endotoxins, cultivable and potentially pathogenic bacteria were detected in the air of all WWTPs visited in this study during summer and winter. Concentrations were slightly higher in biofiltration site in winter and at the screening and the grit/FOG sites in summer, while concentrations did not vary significantly among any of the other treatment steps. This is true for both seasons, indicating that the presence of specific human pathogenic species in the air may not depend on the stage or type of wastewater treatment. There is a risk associated with inhaling potentially pathogenic bacteria at all stages of wastewater treatment.
Endotoxins are one of the most monitored variables in bioaerosols occupational exposure studies. However, there is no determined universal limit established for human exposure but recommendations. The threshold recommended by the Dutch Expert Committee on Occupational Safety (DECOS) in 2010 is 90 endotoxin units per cubic meter of air (EU/m3) during eight hours of exposure (DECOS Citation2010). The current IRSST recommendations for assessing air quality also include limits of 1,000 CFU/m3 for culturable gram-negative bacteria and 10,000 CFU/m3 for total cultivable bacteria (Goyer et al. Citation2001). In this study, endotoxin concentration was the parameter that reached or exceeded the IRSST recommended limit most frequently. These results (presented in ) support the conclusion that a significant health risk could exist at many of these treatment plants and are consistent with the current relevant literature. The evaluation of endotoxins or correlating parameters should be prioritized to have proper estimation of potential workers’ exposure and to monitor impact of bioaerosols attenuation interventions.
Results from endotoxin measurements show strong correlation with culturable bacterial concentrations in this study () as gram-negative and total culturable bacteria concentrations increased proportionally with endotoxin concentration. Using correlation statistics with measured endotoxins levels to estimate the concentration of culturable gram-negative bacteria results in concentrations of approximately 200 CFU/m3 compared to the recommended 1,000 CFU/m3. Meaning that current interpretation of potential exposure could be greater at lower concentrations than that previously expected. The current recommendations were established many years ago and may not be applicable to present day conditions. In this study, based on endotoxins/culturable gram-negative correlations, the percentage of samples above the recommendation would rise from 0% to 22% in winter and from 4% to 52% in summer.
Using the correlation established between endotoxins and total culturable bacteria, the concentrations of bacteria would be closer to 2,000 CFU/m3 compared to the recommended 10,000 CFU/m3 when reaching the recommended threshold. Using this correlation, the number of samples above the recommended exposure limit for bacteria would rise from 0% to 9% in winter and from 9% to 52% in summer. Laitinen et al. (Citation1992) have already demonstrated that it is possible to estimate endotoxin concentration based on culturable gram-negative bacteria. The concentrations obtained in this study are comparable with the results found in the literature supporting the idea that it is appropriate to reevaluate the recommendations for these two variables in these specific environments (Sánchez-Monedero et al. Citation2008; Teixeira et al. Citation2013).
Actually, there is no official recommended exposure limit for total bacteria as measured by qPCR. In this study, all concentrations of endotoxins, culturable bacteria and bacteria obtained by qPCR correlate with each other. Using this relationship, it is possible to suggest the consideration of an exposure limit value (during eight hours of exposure) for total bacteria obtained by qPCR for WWTPS workers at 1 × 107 16S copies/m3.
Almost all plants had some samples that exceed recommended concentrations at the degreasing and biofiltration sites during summer. The biofiltration step appeared to be the most problematic site as the recommended concentration were exceeded at several plants, even in winter when the water temperature is lower and the microbial activity more limited. In biofiltration the wastewater is sprayed on the surface of the biofilter at a height around 50 cm above the surface, which could generate more aerosols, compared to other processes in the WWTP. These results suggest that workers in biofiltration and occasionally in several areas at WWTPs could be exposed to high bioaerosol concentrations that may lead to health problems.
Bioaerosols in WWTPs come mainly from the agitation and mechanical mixing of wastewater during treatment and are linked to the water biomass and presence microbial activity. The aerosolization of wastewater particles into the air results in an increase in relative humidity in treatment rooms. An article by Haas et al. (Citation2010) states that effluent flow rate and air relative humidity each play an important role in increasing the concentration of airborne particles.. The concept is confirmed in the current work by the correlation observed between RH and the concentrations of endotoxins, cultivable bacteria and total bacteria by qPCR as shown in . Based on the results of the current study, the concentration of bioaerosols would exceed the current endotoxin recommendations when relative humidity is approximately 80% or higher. This variable can be controlled in several ways such as increasing the ACH in the room or possibly by confining the basins that can be the main sources of bioaerosol and RH production.
There is a strong correlation between total culturable bacteria and total bacterial genomes quantified by qPCR. In this study we found that the qPCR method leads to 100 to 10,000 times higher genome concentrations compared to culturable bacteria quantified by culture. However, culture approaches allow comparison with existing literature as well as isolation of some collected microorganisms and does not include free DNA measurement. Using both approaches together helps to get a more representative picture of the environment being examined.
Specific bacterial markers were present at all sites with the highest concentrations measured in summer. Specifically, we quantified airborne bacterial species ubiquitous in different environments including, E. coli, A. hydrophila, Klebsiella spp, and P. aeruginosa. These bacteria are detected in feces, are associated with gastroenteritis, and have the potential to be ingested after airborne exposure (Bin Kingombe et al. Citation1999; Fracchia et al. Citation2006; Horneman, Ali, and Abbott Citation2007; Hsu et al. Citation2010; Janda and Abbott Citation2006; Rusin et al. Citation1997). However, the concentrations detected in this study will not likely lead to the ingestion of significant numbers of bacterial cells. In particular, P. aeruginosa is an opportunistic pathogen that can be transmitted through the ingestion of contaminated water, and also through the inhalation of aerosols since it is able to survive inside droplet nuclei and remain infectious for a long period of time (Mena and Gerba Citation2009; Rusin et al. Citation1997). P. aeruginosa was detected at all levels of treatment and in significant concentrations.
E. coli and K. pneumoniae are the bacterial agents that correlated the most with the overall airborne bacterial biomass in the WWTPs visited in this study. Although K. pneumoniae is a bacterium that normally lives without causing any disease inside human intestines, its presence in other organs, can cause pneumonia or bloodstream infections (Ashurst and Dawson Citation2021). K. pneumoniae represents a potentially good marker to determine bioaerosol hazard and for monitoring bioaerosols and potential exposure in WWTPs. This investigation also demonstrates the importance not to rely solely on temperature and the ACH to assess occupational exposure.
One of the limitations of our study is the relatively small number of samples. However, the main takeaway from this work is the need to reconsider the outdated recommendations, which were established using methods that do not represent the current advancements. The correlations we found and the new limit values we propose are an example of why it is essential to open this conversation amongst scientists in the field to start building the necessary capacities, including the appropriate study designs, to move toward new bioaerosol exposure guidelines.
Conclusion
The evaluation of bacteria in bioaerosol composition, including concentrations of bacteria and endotoxins, from eight WWTPs was achieved using several approaches as well as ambient and ventilation parameters. Comparisons were made between summer and winter, where summer showed higher more consistent concentration, establishing a significant difference for the first treatment steps between seasons. Increasing the rate of ventilation in summer, when water temperature favors microbial activity and biomass, would be beneficial to reduce the concentration of bioaerosols during this time of the year. In winter, the biofiltration site had the highest concentrations of bioaerosols. Therefore, increasing the ventilation rate at this site during the cold season or confining the basins would also help minimize the risk of exposure to harmful bioaerosols. Data support the need for accurate risk assessment and personal protective equipment promotion.
This study highlights the presence of many pathogen indicators and bacteria at all steps in the treatment process, with no correlations with actual recommended exposure limits, which can pose potential risks to the health of workers. The correlation between concentrations of pathogen indicators and total bacteria suggest the use of K. pneumonia as a biomarker is suitable for monitoring exposure to opportunistic pathogen in WWTPs. In addition, significant correlations between concentrations of endotoxins, cultivable bacteria, gram-negative bacteria, and total bacteria by qPCR support the recommendation to propose or reconsider the LOE in WWTPs. In this study, we propose new LOE values for the mentioned parameters – revisited to fit the endotoxin exposure limit recommendations. Based on these results, workers are encouraged to use personal respiratory protection to limit the risk of health problems especially during long-term work in wastewater treatment rooms. Qualitative microbial risk assessment studies could be a pertinent perspective to assess specifically infectious risks associated with wastewater bioaerosols.
Data availability
The data that support the findings of this study are available on request from the corresponding author, CD.
Supplementary_Material.xlsx
Download MS Excel (9.2 KB)Supplementary_Figure.jpg
Download JPEG Image (167 KB)Acknowledgment
This work was funded by Institut de Recherche Robert-Sauvé en Santé et Sécurité du Travail du Québec (IRSST Grant 2010-0050). Hamza Mbareche received a postdoctoral fellowship from Fond de Recherche du Québec – Nature et Technologie and is the recipient of the Lab Exchange Visitor Program for the Canadian Society of Virology. Vanessa Dion-Dupont received a CRIUCPQ scholarship. Caroline Duchaine is the holder of Tier-1 Canada Research Chair on Bioaerosols. The authors are thankful to Serge Simard for statistical analyses, Yves Beaudet (IRSST for technical assistance and to Amanda Toperoff and Michi Waygood for English revision of the manuscript. The authors are also indebted to the wastewater treatment plant managers and staff for their participation in the study and their collaborative efforts.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this paper can be accessed on the publisher’s website
Additional information
Funding
Notes on contributors
Hamza Mbareche
Hamza Mbareche is a consultant, researcher and trainer who works at the convergence of microbial occupational exposure, the microbiome and genomics, with implications for public health. He ranks in the top 0.25% of Air Microbiology experts worldwide according to Expertscape. He harnesses cutting-edge technologies to improve the environmental safety of workplaces.
Vanessa Dion-Dupont
Vanessa Dion-Dupont is a microbiologist and has research experience in bioaerosols field sampling and analyses.
Marc Veillette
Marc Veillette has a Master degree in bioaerosols sciences and is a microbiologist and molecular biologist. He is managing major research projects within the Canada Research Chair on Bioaerosols and has published numerous articles and abstracts.
Evelyne Brisebois
Evelyne Brisebois has a master degree in microbiology and has an expertise as an application scientist and regulatory affairs.
Jacques Lavoie
Jacques Lavoie is an industrial hygienist and had a fruitful career in occupational health and bioaerosols research.
Caroline Duchaine
Caroline Duchaine is holder of Tier-1 Canada Research Chair on Bioaerosols and her research is pioneer in molecular methods for bioaerosols assessment, impacts on human health and in vitro bioaerosols behaviour.
References
- Andersen, A. A. 1958. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 76 (5):471–84. doi:10.1128/jb.76.5.471-484.1958.
- Ashurst, J. V., and A. Dawson. 2021. Klebsiella Pneumonia. StatPearls [Internet]. Accessed February 5, 2021. Treasure Island (FL): StatPearls Publishing; January. https://www.ncbi.nlm.nih.gov/books/NBK519004/.
- ASTM. 1993. Standard test methods for determining air change in a single zone by means of a tracer gas dilution. In Designation E, 741–93. Philadelphia: ASTM.
- Bach, H.-J., J. Tomanova, M. Schloter, and J. C. Munch. 2002. Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. Journal of Microbiological Methods 49 (3):235–45. doi:10.1016/S0167-7012(01)00370-0.
- Bin Kingombe, C. I. B., G. Huys, M. Tonolla, M. J. Albert, J. Swings, R. Peduzzi, T. Jemmi. 1999. PCR detection, characterization, and distribution of virulence genes in aeromonas spp. Appl. Environ. Microbiol. 65(12):5293–302. doi:10.1128/AEM.65.12.5293-5302.1999.
- Carducci, A., E. Tozzi, E. Rubulotta, B. Casini, L. Cantiani, E. Rovini, M. Muscillo and R. Pacini. 2000. Assessing airborne biological hazard from urban wastewater treatment. Water Res. 34(4):1173–78. doi:10.1016/S0043-1354(99)00264-X.
- DECOS. 2010. Endotoxins: Health-based recommended occupational exposure limit.
- Divizia, M., B. Cencioni, L. Palombi, and A. Pana. 2008. Sewage workers: Risk of acquiring enteric virus infections including Hepatitis A. The new microbiologica 31 (3):337–41.
- Domhof, S., and F. Langer. 2002. Nonparametric analysis of longitudinal data in factorial experiments. In Book Nonparametric analysis of longitudinal data in factorial experiments, 261. Hoboken, New Jersey: Wiley-Interscience.
- Drapeau, A. J., and S. Jankovic. 1977. Manuel de microbiologie de l’environnement. In Book Manuel de microbiologie de l’environnement, Editor. 8–18. Geneva, Switzerland: Organisation mondiale de la santé.
- Dutkiewicz, J., E. Cisak, J. Sroka, A. Wojcik-Fatla, and V. Zajac. 2011. Biological agents as occupational hazards - selected issues. Ann. Agric. Environ. Med. 18 (2):286–93.
- Elia, V. J., C. S. Clark, V. A. Majeti, P. S. Gartside, T. MacDonald, N. Richdale, C. R. Meyer, G. L. Van Meer, K. Hunninen. 2005. Hazardous chemical exposure at a municipal wastewater treatment plant. Environ. Res. 32(2):360–71. doi:10.1016/0013-9351(83)90118-4.
- Fannin, K. F., S. C. Vana, and W. Jakubowski. 1985. Effect of an activated sludge wastewater treatment plant on ambient air densities of aerosols containing bacteria and viruses. Applied and Environmental Microbiology 49 (5):1191–96. doi:10.1128/aem.49.5.1191-1196.1985.
- Fracchia, L., S. Pietronave, M. Rinaldia, and M. G. Martinotti. 2006. Site-related airborne biological hazard and seasonal variations in two wastewater treatment plants. Water Res. 40 (10):1985–94. doi:10.1016/j.watres.2006.03.016.
- Gangamma, S., R. S. Patil, S. Mukherji, D. Kay, J. Crowther, C. M. Stapleton, M. D. Wyer, L. Fewtrell, A. Edwards, and C. A. Francis. 2011. Characterization and proinflammatory response of airborne biological particles from wastewater treatment plants. Environ. Sci. Technol. 45 (8):3282–87. doi:10.1021/es103652z.
- Goyer, N., J. Lavoie, L. Lazure, and G. Marchand. 2001. Bioaerosols in the workplace: Evaluation, control, and prevention Guide. Technical Guide. IRSST Études et Recherches T–24.
- Grisoli, P., M. Rodolfi, S. Villani, E. Grignani, D. Cottica, A. Berri, A. Maria Picco, C. Dacarro. 2009. Assessment of airborne microorganism contamination in an industrial area characterized by an open composting facility and a wastewater treatment plant. Environ. Res. 109(2):135–42. doi:10.1016/j.envres.2008.11.001.
- Haas, D., M. Unteregger, J. Habib, H. Galler, E. Marth, F. F. Reinthaler. 2010. Exposure to bioaerosol from sewage systems. Water, Air, and Soil Pollution. 207(1–4):49–56. doi:10.1007/s11270-009-0118-5.
- Han, Y., Y. Wang, L. Li, G. Xu, J. Liu, and K. Yang. 2018. Bacterial population and chemicals in bioaerosols from indoor environment: Sludge dewatering houses in nine municipal wastewater treatment plants. Sci. Total Environ. 618:469–78. doi:10.1016/j.scitotenv.2017.11.071.
- Horneman, A. J., A. Ali, and S. L. Abbott. 2007. Aeromonas. In Manual of clinical microbiology, ed. P. R. Murray, E. J. Baron, M. L. Landry, J. H. Jorgensen, and M. A. Pfaller, 9th ed., 715–22. Washington, DC: American Society for Microbiology.
- Hsu, B.-M., S.-F. Wu, S.-W. Huang, Y.-J. Tseng, -D.-D. Ji, J.-S. Chen, F.-C. Shih. 2010. Differentiation and identification of Shigella spp. and enteroinvasive Escherichia coli in environmental waters by a molecular method and biochemical test. Water Res. 44(3):949–55. doi:10.1016/j.watres.2009.10.004.
- Ivens, U. I., N. O. Breum, N. Ebbehøj, B. H. Nielsen, O. M. Poulsen, H. Würtz. 1999. Exposure-response relationship between gastrointestinal problems among waste collectors and bioaerosol exposure. Scand. J. Work Environ. Health. 25(3):238–45. doi:10.5271/sjweh.430.
- Janda, J. M., and S. L. Abbott. 2006. The Genera Klebsiella and Raoultella. In The Enterobacteria, 2nd ed., 115–29. Washington, USA: ASM Press.
- Jaremkow, A., L. Noga, and K. Pawlas. 2018. Respiratory system symptoms in the neighborhood of a wastewater treatment plant. Pol. J. Environ. Stud. 27 (1):117–27. doi:10.15244/pjoes/74131.
- Jaremkow, A., Ł. Szałata, B. Kołwzan, I. Sówka, J. Zwoździak, K. Pawlas. 2017. Impact of a sewage treatment plant on health of local residents: gastrointestinal system symptoms. Pol. J. Environ. Stud. 26(1):127–36. doi:10.15244/pjoes/64793.
- Kay, D., J. Crowther, C. M. Stapleton, M. D. Wyer, L. Fewtrell, A. Edwards, C. A. Francis, A. T. McDonald, J. Watkins, J. Wilkinson, et al. 2008. Faecal indicator organism concentrations in sewage and treated effluents. Water Research. 42(1–2):442–54. doi:10.1016/j.watres.2007.07.036.
- Kindzierski, W. B., Bari, A., Wang, X., Wetmore, T., Maal-Bared, R., Michaels, C. 2015b. Evidence of wastewater treatment plant worker biohazard exposure and health symptom responses. Conference: Canadian Society for Bioengineering, Edmonton, Alberta, Canada. July.
- Kindzierski, W., M.A. Bari, X. Wang, T. Wetmore, R. Maal-Bared, and C. Michaels. 2015a. Evidence of wastewater treatment plant worker biohazard exposure and health symptom responses. Can. Soc. Bioeng.:CSBE15–090.
- Korzeniewska, E., and M. Harnisz. 2012. Culture-dependent and culture-independent methods in evaluation of emission of enterobacteriaceae from sewage to the air and surface water. Water Air Soil Pollut. 223 (7):4039–46. doi:10.1007/s11270-012-1171-z.
- Laitinen, S., A. Nevalainen, M. Kotimaa, J. Liesivuori, and P. J. Martikainen. 1992. Relationship between bacterial counts and endotoxin concentrations in the air of waste-water treatment plants. Appl. Environ. Microbiol. 58 (11):3774–76. doi:10.1128/aem.58.11.3774-3776.1992.
- Liu, J., J. Gratz, A. Maro, H. Kumburu, G. Kibiki, M. Taniuchi, A.M. Howlader, S.U. Sobuz, R. Haque, K.A. Talukder, et al. 2012. Simultaneous detection of six diarrhea-causing bacterial pathogens with an in-house PCR-luminex assay. J. Clin. Microbiol. 50(1):98–103. doi:10.1128/JCM.05416-11.
- Malinen, E., A. Kassinen, T. Rinttilä, and A. Palva. 2003. Comparison of real-time PCR with SYBR Green I or 5’-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 149 (Pt 1):269–77. doi:10.1099/mic.0.25975-0.
- Mena, K. D., and C. P. Gerba. 2009. Risk Assessment of Pseudomonas aeruginosa in Water. In Reviews of environmental contamination and toxicology, ed. D. M. Whitacre, Vol. 201, 71–115. New York: Springer.
- Moore, J. E., J. Xu, B. C. Millar, J. Courtney, and J. S. Elborn. 2003. Development of a Gram-negative selective agar (GNSA) for the detection of Gram-negative microflora in sputa in patients with cystic fibrosis. J. Appl. Microbiol. 95 (1):160–66. doi:10.1046/j.1365-2672.2003.01956.x.
- Nehme, B., V. Létourneau, R. J. Forster, M. Veillette, and C. Duchaine. 2008. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ. Microbiol. 10 (3):665–75. doi:10.1111/j.1462-2920.2007.01489.x.
- Rusin, P. A., J. B. Rose, C. N. Haas, and C. P. Gerba. 1997. Risk assessment of opportunistic bacterial pathogens in drinking water. Rev. Environ. Contam. Toxicol. 152:57–83. doi:10.1007/978-1-4612-1964-4_2.
- Rylander, R. 1999. Health effects among workers in sewage treatment plants. Occup. Environ. Med. 56 (5):354–57. doi:10.1136/oem.56.5.354.
- Rylander, R., K. Andersson, L. Belin, G. Berglund, R. Bergström, L.-Å. Hanson, M. Lundholm, I. Mattsby. 1976. Sewage workers syndrome. Lancet. 2(7983):478–79. doi:10.1016/S0140-6736(76)92583-6.
- Rylander, R., K. Andersson, L. Belin, G. Berglund, R. Bergström, L. Hanson, M. Lundholm, I. Mattsby. 1977. Studies on humans exposed to airborne sewage sludge. Schweiz. Med. Wochenschr. 107 (6):182–84.
- Sánchez-Monedero, M. A., M. I. Aguilar, R. Fenoll, and A. Roig. 2008. Effect of the aeration system on the levels of airborne microorganisms generated at wastewater treatment plants. Water Res. 42 (14):3739–44. doi:10.1016/j.watres.2008.06.028.
- Shannon, K. E., D. Y. Lee, J. T. Trevors, and L. A. Beaudette. 2007. Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Sci. Total Environ. 382 (1):121–29. doi:10.1016/j.scitotenv.2007.02.039.
- Smit, L. A., S. Spaan, and D. Heederik. 2005. Endotoxin exposure and symptoms in wastewater treatment workers. American Journal of Industrial Medicine 48 (1):30–39. doi:10.1002/ajim.20176.
- Teixeira, J. V., S. Miranda, R.A. Monteiro, F.V. Lopes, J. Madureira, G.V. Silva, N. Pestana, E. Pinto, V.J. Vilar and R.A. Boaventura. 2013. Assessment of indoor airborne contamination in a wastewater treatment plant. Environ. Monit. Assess. 185(1):59–72. doi:10.1007/s10661-012-2533-0.
- Thorn, J., and L. Beijer. 2004. Work-related symptoms and inflammation among sewage plant operatives. Int. J. Occup. Environ. Health 10 (1):84–89. doi:10.1179/oeh.2004.10.1.84.
- Thorn, J., and E. Kerekes. 2001. Health effects among employees in sewage treatment plants: A literature survey. American Journal of Industrial Medicine 40 (2):170–79. doi:10.1002/ajim.1085.
- Vantarakis, A., S. Paparrodopoulos, P. Kokkinos, G. Vantarakis, K. Fragou, and I. Detorakis. 2016. Impact on the quality of life when living close to a municipal wastewater treatment plant. J. Environ. Public Health 2016: 8.
- Vidal, A., J. F. Blanchemain, C. Verdun-Esquer, M. Rinaldo, and P. Brochard. 2012. Respiratory effects of chronic and subacute hydrogen sulfide exposure: Case reports of workers in wastewater purification plants. Arch. des Mal. Prof. et de l’Environnement 73 (5):799–805. doi:10.1016/j.admp.2012.07.002.