ABSTRACT
Microbial aerosols in intensive broiler houses whose species and concentrations are closely related to human health are ubiquitous. Based on 16S rRNA gene sequencing, the aim of this study was to investigate the spatial distribution and diversity of bacterial aerosols in the air of broiler houses. Significant spatial variations in airborne bacterial concentrations were observed inside the poultry farmhouse. The results indicated that bacteria in the air samples could be grouped into a total of 1,674 OTUs. Alpha diversity analysis showed that the diversity of the microbial community at the entry of the broiler house was higher than that at the middle or the rear (p < 0.01). The Sankey diagram illustrated species dynamic changes in Proteobacteria, Firmicutes, and Actinobacteria among the different locations. From the aspect of LEfSe (LDA Effect Size) analysis, we discovered that the abundance of Planctomycetes was significantly higher in the entry than in the rear and middle. This study shows the spatial distribution of the entire bacterial community in intensive broiler houses, which offers a new perspective for studying airborne total bacteria in those environments.
Implications: The bacteria contained in air aerosols from poultry houses are closely connected to animal health and production. This study aimed to investigate the spatial distribution and diversity of bacterial aerosols in the air of broiler houses. The results observed that bacterial aerosol concentrations in the examined broilers house varied greatly at different positions, and a significantly higher exposure to bacterial aerosol was observed at the middle than at the other positions (p < 0.05). The alpha diversity analysis showed that the diversity of the microbial community at the entry of the broiler house was higher than that at the middle or the rear (P<0.01). Sankey diagram illustrated species dynamic changes of Proteobacteria, Firmicutes and Actinobacteria among the different locations. The microbial communities in genus level in the samples of entry and rear were closer, while the species diversity of middle and rear samples in chicken house was highly similar (P>0.05). Altogether, results revealed that the effects of spatial factors on the diversity and abundance of bacteria in the air of closed-cage broiler houses, which poses a potential threat to the health of animals and workers in those environments.
Introduction
In recent years, intensive, large-scale, and industrialized broiler farming has gradually replaced the traditional method of rural free-range farming in China. With the increasing density of animals used in intensive farming, large-scale farming faces many new challenges. The health and production status of poultry during the entire growth cycle are vulnerable to the external environment, and unfavorable environmental conditions are especially problematic under modern entire-house and high-density poultry farming conditions (Gladding et al. Citation2020; Xu et al. Citation2022). The in-house air environment is an important part of the broiler farming environment. Many microbes, primarily in the form of aerosols, exist in the air and can stay aloft for a long time. These aerosols contain a number of pathogenic and conditionally pathogenic bacteria, which can lead to the infection of both poultry keepers and surrounding residents (Dos Anjos Magri et al. Citation2021; Jegede et al. Citation2019).
The growing number of production and disease problems arising from the poor environment of livestock and poultry houses have attracted increasing attention (Bui et al. Citation2019; Shen et al. Citation2018; Zhou et al. Citation2021). While the importance of studies on improving air quality systems is recognized, environmental improvement has become the most important issue in modern livestock production. In recent years, the extensive use of antibiotics in the livestock and poultry farming industry has led to the occurrence of antibiotic-resistant bacteria and drug resistance genes in airborne aerosols, representing serious hazards in livestock and poultry houses (Bai et al. Citation2022; Song et al. Citation2022; Wang et al. Citation2021). Due to the high mobility and diffusivity of air, conditionally pathogenic bacteria present in the air can spread via air flows (Li et al. Citation2019). Microbial aerosols can be transmitted from poultry houses to the atmosphere by wind, with the aerosol distribution depending on the distance, degree of dispersion, and environmental parameters (Bai et al. Citation2022; You et al. Citation2019). With the development of large-scale poultry farming, an increasing number of broiler houses have adopted the enclosed farming model, in which high-density farming and inadequate ventilation conditions can lead to an increase in the concentration of bacterial aerosols (Zhao et al. Citation2015). Bacteria are small in size and more readily deeply inhaled into the respiratory tract than other microbes. Therefore, the monitoring of bacterial aerosols in poultry houses is of great importance.
Traditionally, microbes in broiler houses and in the surrounding air are studied using growth medium to culture microbes collected from air sampling (Sowiak et al. Citation2012). However, the selectivity of this approach renders it insufficient for an in-depth analysis of the microbes present in poultry houses and the surrounding air. In contrast, the use of a liquid impingement-based aerosol sampler to collect air samples can effectively avoid medium selectivity and provide bacterial enrichment and attenuation, thereby maximizing the collection of airborne microbes for genomic DNA extraction and sequence analysis (Nonnenmann et al. Citation2010). With the widespread use of second-generation high-throughput sequencing technologies in the field of microecology, an increasing number of biological fields have adopted this technology to investigate previously unsolved scientific problems. For example, it has been widely applied in animal gut, soil, and clinical microbiology applications (Gupta et al. Citation2021; Qiu et al. Citation2021; Yadav et al. Citation2021). However, few studies have used this technology to investigate the relationship between airborne bacterial communities and the environment in broiler houses. Because aerosols in poultry houses contain a number of pathogenic and conditionally pathogenic bacteria, an understanding of the abundance and type of bacteria present is of great importance for the environmental quality control of poultry houses (Beier et al. Citation2021; Bindari et al. Citation2021). High-throughput sequencing technology allows 16S rRNA gene analyses to be performed on poultry house samples by taking advantage of its ability to generate sequencing data without the need for culturing. This technology can provide a wealth of genetic information, and the ease with which data processing can be performed to identify microbial species allows for more convenient and efficient analyses than can be done using traditional analysis methods.
The poultry farming environment contains many microbial aerosols that can potentially impact animals, poultry keepers, and the natural environment. The dominant airborne bacterial species in different countries and regions are quite different, as are the dominant bacterial species in farming houses for various animals in the same region (Newton et al. Citation2021; Song et al. Citation2022). However, little work has been carried out on the levels of these organisms once they enter the intensive broiler house environment, which is key to understanding the impacts of mechanical ventilation and the dispersion of organisms. Bacteria that enter the aerosol environment can be transported during mechanical transfer via fans. The present study was carried out to address the issue of distribution and survival of key organisms in an intensive broiler house to assess bacterial community structure. In this study, air samples were collected from different locations of a broiler house using a BioSampler (a liquid microbial air sampler). The 16S rRNA content of bacterial aerosols at different locations of the broiler house was assessed by high-throughput sequencing. Understanding the distribution pattern of airborne microbes in broiler houses is of great importance to guide the improvement of poultry health management, control airborne infectious diseases, and improve the product quality of broilers.
Materials and methods
Ethics statement
The study design, protocol, and informed consent were approved by the Institutional Ethics Committee of Ludong University (No. LD0608–307).
Sampling sites
The experiment was designed and carried out in a standard intensive broiler breeding house randomly selected from a chicken farm located in Yantai City, Shandong Province, China. The chicken house was aligned north to south. The house was 100 m long, 14 m wide, 3.2 m high, and 1105 m2 in area, and it contained approximately 18,000 Arbor Acres (AA) broiler chickens. The house was equipped with automatic feed and water supply systems. A tunnel ventilation system was used. Air came in through the evaporative cooling pad on the front end wall. Ten exhaust fans (DJF-1380, Shandong, China) were installed at the other end wall, which kept the inside air flow at 3 m/s. The automatic control system used evaporative cooling pads and ventilation fans to keep the inside temperature and humidity suitable for the growth of broilers, generally ensuring that the temperature does not exceed 26°C and the humidity is 55%–65%.
Based on the distance from the vent and ventilator, three different positions were chosen inside the manure broiler houses, at the entry (near the evaporative cooling pad), middle, and rear (near the exhaust fan). The workers ventilated the house based on the temperature of the room, and negative pressure ventilation was adopted in the current chicken house.
Sample collection
For quantitative and qualitative analyses of microflora, airborne bacterial samples were collected by stationary sampling and liquid impingement air sampling. Cultivable bacteria were collected using the international standard Andersen 6-staged airborne microbe sample collector (SKC, Philadelphia, Pennsylvania, USA) with an airflow of 28.3 L/min for 2 min and replicated 3 times. The sampler was divided into 6 stages with 400 holes per stage. Based on the principle of the sampler, estimations can be made on the size of bacterial particles inhaled by the chickens and in which part of their body the sedimentation took place. Airborne particles of different sizes were collected in six stages using blood agar medium (Bahador et al. Citation2021). The diameter of the holes was gradually reduced from the top to the bottom, as follows: 7.0 μm (stage 1), 4.7–7.0 μm (stage 2), 3.3–4.7 μm (stage 3), 2.1–3.3 μm (stage 4), 1.1–2.1 μm (stage 5), and 0.65–1.1 μm (stage 6). The stationary samples were incubated at 37°C for 2 days and then stored at 4°C. The bioaerosol concentration (CFU/m3) was calculated according to the Equation C= (N × 1000)/(t×F), where C is the bacterial aerosol concentration, colony-forming unit (CFU)/m3; N is the number of colonies at each stage; t is the sampling time, min; and F is the gas flow rate at the time of sampling, L/min.
Simultaneously, aerosol samples were collected with a BioSampler (SKC, Philadelphia, Pennsylvania, USA) at a flow rate of 12.5 L/min for 60 min using 20 mL of phosphate buffered saline (PBS) as a sampling medium and replicated 3 times. The liquid samples were collected in sterile tubes, which were ultracentrifuged at 70,000 rpm for 2 h to harvest the bacteria. After ultracentrifugation, the supernatant was discarded, and the pelleted sample was frozen at −80°C. All samples were collected at a height of 0.5 m above ground, which is the breathing zone of broilers.
DNA extraction
Total bacterial RNA was extracted from the liquid samples using an EasyPure Viral DNA/RNA kit (Transgen Biotech, Beijing, China) according to the manufacturer’s instructions. In short, 20 μL of proteinase K and 200 μL of sample were added to a sterile 1.5 mL microcentrifuge tube. The released RNA is effectively purified after specifically binding to a silica-based spin column. RNA was reverse transcribed into cDNA using an EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (Transgen Biotech, Beijing, China) according to the manufacturer’s instructions. The cDNA was quantified using a Nanophotometer P300 (Implen, Munich, Germany), and the obtained samples were stored at −20°C prior to PCR amplifications.
HiSeq sequencing of 16S rRNA gene amplicons
To assess the diversity and structure of the bacteria present in each sample, cDNA was sent to Novogene Bioinformatics Technology (Beijing, China) to perform amplicon sequencing using HiSeq protocols. PCR amplifications were conducted with the primer set 515F/907 R (515F: GTGCCAGCMGCCGCGGTAA, 907 R: CCGTCAATTCCTTTGAGTTT), which targets the V4-V5 hypervariable region of the bacterial 16S rRNA gene (Guo et al. Citation2018). PCRs followed the general protocol described as follows: PCRs were performed in 30 μL volumes containing 15 μL of PCR Master Mix (2×), 0.2 μM primers, 10 ng of template DNA, and nuclease-free water to bring the final volume to 30 μL. The amplification conditions were as follows: 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, and elongation at 72°C for 60 s, with a final extension at 72°C for 5 min. The obtained PCR products were analyzed with a 2% agarose gel, and the DNA (~450 bp) was purified using the QIAquick PCR Purification kit (QIAGEN, Hilden, Germany). Next, the purified amplicons were used to generate sequencing libraries constructed using the NEB Next® Ultra™ DNA Library Prep Kit for Illumina (New England Biolabs, Inc., UK) according to the manufacturer’s recommendations. All prepared libraries were sequenced on the Illumina HiSeq platform. The data were deposited with links to BioProject accession number PRJNA860320 in the NCBI BioProject database.
Microbial community analysis
Raw sequencing data contain a certain amount of low-quality data that will interfere with downstream analyses. Therefore, when at least some of the reads overlapped the read generated from the opposite end of the same DNA fragment, the raw data were demerged and then used to merge paired-end reads of the original DNA fragments by using FLASH (Magoc and Salzberg Citation2011). Paired-end reads were assigned to each sample according to the unique barcodes.
After obtaining high-quality, clean tags; sequence analyses were performed using Uparse (Edgar Citation2013). Sequences were assigned to the same operational taxonomic unit (OTU) at a similar threshold of 97%. Representative sequences from each OTU were screened for further annotation and used to make taxonomic assignments through the SILVA database (Quast et al. Citation2013; Yilmaz et al. Citation2014).
The taxon abundance of each sample was assessed at the phylum, class, order, family, and genus levels. The bacterial diversity analyses were performed with QIIME and displayed with R (Caporaso et al. Citation2010).
Statistical analysis
One-way analysis of variance (ANOVA) was performed between groups. Tukey’s HSD test was applied for post hoc comparisons at α = 0.05 as needed. The figures were plotted using GraphPad Prism 7 (GraphPad Software, California, USA). All statistical analyses were carried out using SPSS (version 19.0; SPSS Inc., USA), and p < 0.05 was considered significant.
Results
Concentration of airborne bacteria
In the samples collected using a 6-stage Andersen impactor, the concentration of bacteria varied greatly among the different poultry house sampling positions. The range of culturable bacteria concentrations observed among the samples was 4.92~7.73 × 103 CFU/m3. It was observed that bacterial aerosol concentrations in the examined broiler house varied greatly at different positions, and a significantly higher exposure to bacterial aerosol was observed at the middle than at the other positions (p < 0.05) ().
Figure 1. The concentrations of bacteria in aerosols at different locations (a) and the size distribution of aerosols with culturable bacteria (b). The aerodynamic diameter ranges for the viable particle sizing sampler were>7.0 μm (stage 1), 4.7 to 7.0 μm (stage 2), 3.3 to 4.7 μm (stage 3), 2.1 to 3.3 μm (stage 4), 1.1 to 2.1 μm (stage 5), and 0.6 to 1.1 μm (stage 6).
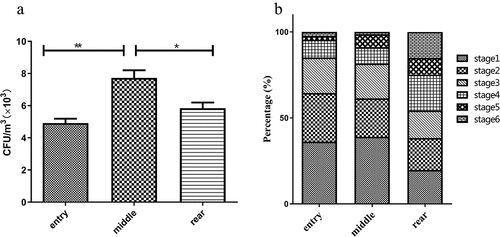
The size distributions of the aerosols with culturable bacteria at the three assayed locations were different. Particles larger than 7 μm (equivalent to stage 1 of the 6-staged sampler) are trapped in the nasal cavity. At the entry and middle, most of the bacteria were present in the stage 1 particles (7.0 μm). Bioaerosols with a diameter smaller than 4.7 μm (equivalent to stages 3–6 of the 6-staged sampler) might easily penetrate the respiratory system. At the rear, most were distributed in the stage 4 particles (2.1–3.3 μm). The results of this study showed that the aerosol concentration in the range of 0.65–2.1 μm (equivalent to stages 5–6) was higher at the rear than at other sites. This fraction of the bacterial aerosol has a small particle size and is prone to settle to the lungs. In addition, the greatest distribution rate was 36.2% for entry stage 1, and the lowest rate was 2.4% for entry stage 6, with other distributions shown in .
Bacterial 16S rRNA transcript amplicon profile
The total number of raw sequences in the samples was 787,552. After filtering the low-quality sequences, the effective tags obtained were arranged from 58,196 to 86,121. A rarefaction curve reflects the rationality of the amount of sequencing data. When the curve asymptotically approaches a plateau, it indicates that the amount of sequencing data can reasonably reflect the microbial community. As shown in , rarefaction curves of observed OTUs reached a plateau in all samples, demonstrating that sequencing data were adequate to cover the bacterial diversity in poultry houses.
Figure 2. Bacterial OTU (operational taxonomic unit) spatial distribution in the intensive broiler house. (a) Rarefaction curve of the observed number of OTUs from samples of 16S rRNA gene; (b) Venn diagram displays the number of shared and unique OTUs among the house’s entry, middle, and rear. Different colors represent different groups. The overlapping portions represent the common taxa between groups, and the nonoverlapping portions represent unique taxa in each group.
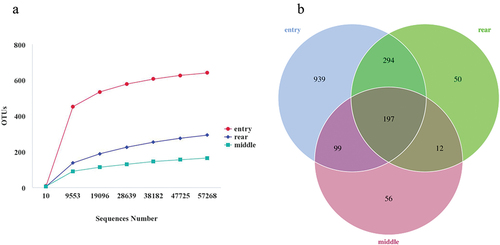
Total, shared, and unique OTUs in different locations are illustrated in a Venn diagram (). In the entry of the house, the aerosol had the most unique bacteria (939 OTUs), followed by middle (56 OTUs) and rear (50 OTUs). Shared bacterial components were observed: 296 OTUs between entry and middle, 491 OTUs between entry and rear, and 209 OTUs between middle and rear. A total of 197 common OTUs as a core microbiota were detected in all three sites of the intensive broiler house.
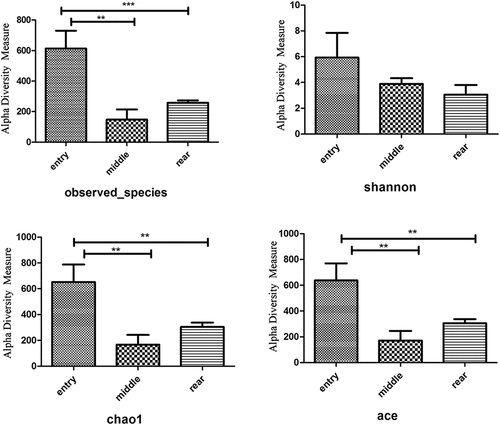
Alpha diversity analysis
The four alpha diversity indices (observed_species, Shannon, ACE, Chao1) of different samples are displayed in . Entry had higher values for observed_species, Shannon, ACE and Chao1. The Chao1 and ACE indices were used to assess the microbial diversity. Microbiota from the entry demonstrated that the Chao1 and ACE indices were significantly increased compared to the middle and rear groups (p < 0.01). The observed species, ACE and Chao1 indices indicated that the rear had higher diversity than the middle, but none of these differences were significant (p > 0.05).
Microbial diversities and compositions in different spatial locations
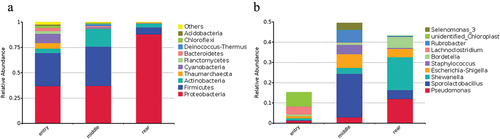
The differences in microbial composition characteristics at the phylum and genus levels, which closely mirrored the effects of different spatial locations on the richness of airborne bacteria, are presented in . At the phylum level, the top 10 abundant species were Proteobacteria, Firmicutes, Actinobacteria, Thaumarchaeota, Cyanobacteria, Planctomycetes, Bacteroidetes, Deinococcus-Thermus, Chloroflexi, and Acidobacteria (). The presence of the 10 dominant OTUs at the genus level was affected by the different positions assayed in the broiler house (). The genera Shewanella and Pseudomonas dominated the rear samples, accounting for 16.18% and 12.01% of the total OTUs, respectively. In contrast, in the entry samples, Shewanella and Pseudomonas only accounted for 0.90% and 1.24% of all OTUs, respectively. Interestingly, the proportions of Escherichia-Shigella reached 6.78% in the middle samples but only 1.17% and 4.12% in the entry and rear samples, respectively. The relative abundances of Lachnoclostridium were higher in the entry samples than in the rear samples, whereas Bordetella exhibited the opposite trend.
Figure 4. Microbial composition of each sample based on 16S rRNA genes. (a) Each bar represents the average relative abundance of each phylum in the broiler house’s entry, middle, and rear; (b) Each bar represents the average relative abundance of each genus of gut microbiota in the entry, middle, and rear of the broiler house.
Cluster analysis
To investigate the microbiota composition in different spatial locations, we calculated the relative abundance of 5 phyla shown in a Sankey diagram (). In the figure, the lines of different colors represent different bacterial phyla, and the height of the rectangles indicates the relative abundance in the groups (left) and phyla (right). At the phylum level, the five predominant phyla were Proteobacteria, Firmicutes, Actinobacteria, Thaumarchaeota, and Cyanobacteria. From the entry to rear, the air bacterial contamination of Thaumarchaeota and Cyanobacteria distinctly decreased, while the level of Proteobacteria notably increased.
Figure 5. The Sankey diagram (a) and Heatmap analysis (b). The heatmap colors represent the relative percentage of the microbial genus assignments within each sample. The samples with the highest and lowest bacterial levels are in red and blue, respectively.
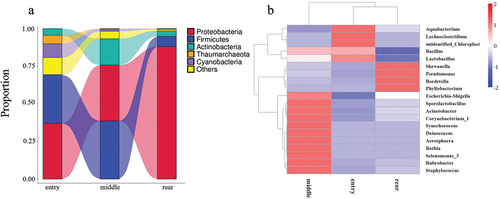
Heatmap representation of the clustered distant matrix data was used to visualize the microbial composition of different locations of samples by 16S rRNA sequencing. As shown in , the microbial communities of the middle group were different compared with those of the entry and rear groups at the genus level.
LEfSe analysis
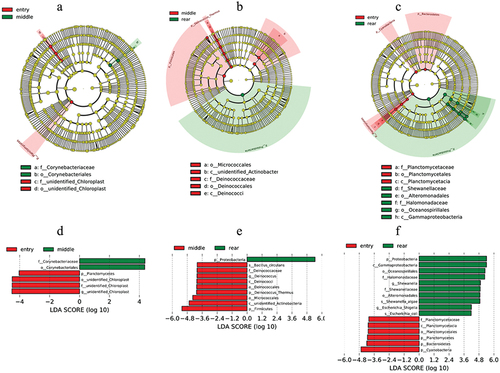
We used the LEfSe method to identify the specific bacterial phylotypes that were differentially altered between the entry, middle, and rear groups (). As seen in our analyses, the phylum Planctomycetes was significantly enriched in the entry group (). Following the middle and rear groups, the phylum Deinococcus-Thermus and the genera Deinococcus were significantly enriched in the middle group, and the phylum Proteobacteria was significantly enriched in the rear group (). The rear group was characterized by taxa in the phylum Proteobacteria (). Exceptions to this pattern were the rear group, having significantly more OTUs in the class Gammaproteobacteria, orders Alteromonadales and Oceanospirillales, and families Shewanellaceae and Halomonadaceae.
Figure 6. The potential biomarkers defined by LEfSe. (a)–(c), Cladogram analyses. Each circle represents the classified level from phylum to species. The diameter of the small circle is proportional to the relative abundance of air microbiota. (d)–(f), Histogram analyses. The threshold on the logarithmic LDA score for discriminative features was set to 4.0.
Discussion
In this study, bacterial aerosols were detected at different locations in an enclosed poultry house for cage-reared broilers to analyze the aerodynamic size distribution, bacterial community structure and diversity of airborne microbes. As a result of the normal activities of chickens and breeders, microorganisms and chemical particles in the environments of chicken farms can form aerosols (Cambra-Lopez et al. Citation2010; Radon et al. Citation2002). Chickens grown under intensive broiler production in mechanically ventilated sheds yielded total bacterial counts in the air that depended on the different stages of the animal (Lawniczek-Walczyk et al. Citation2013). Few studies have addressed the variation in microbes in aerosols in mechanically ventilated poultry houses. In this study, the results show that the total concentration of bacterial aerosols accumulated along with the airflow inside the house, with the total concentration of the sampling increasing from 4.92 × 103 CFU/m3 at the entry of the house to 7.73 × 103 CFU/m3 at the place close to the exhaust fans. An increase in the concentration of airborne bacteria can occur as a result of particulate matter transferring into the environment via feathers, feces, or litter material exiting the sheds during fan activity (Chinivasagam et al. Citation2010). The ventilation fans keep the air flowing from the water curtain to the back end wall of the building. As air flows, airborne bacteria also accumulate and form a high-concentration region inside houses.
In intensive breeding buildings, the high concentration of bacteria, which originates from the aerosolization of forage, feces, skin, and feather fragments during the processing of manure and movement of animals (Khan et al. Citation2022), can cause many health issues for both animals and farm employees (Gao et al. Citation2017). The particle size distribution of bacterial aerosols was different in the chicken house. Near the entrance of the poultry house, bacteria accounted for the highest proportion in stages 1–2, while at the rear of the house, bacteria were mostly distributed in stages 3–6. The six-stage Andersen impactor used for air sampling simulates the anatomical structure and aerodynamic characteristics of the human respiratory tract. When aerosol particles have a particle size of ≤4.7 μm (corresponding to stages 3–6 of the Andersen sampler), they will enter the trachea of the human body and pose serious harm to human health and are therefore named “inhalable particles” (Brook et al. Citation2004). The proportion of bacteria-carrying inhalable particles was the highest at the rear of the house, which was related to fan activity (Chinivasagam et al. Citation2010).
High-throughput sequencing provides rapid, sensitive, and accurate advantages in studying and evaluating the content and quality of microbial aerosols compared with traditional methods. The top five phyla in the air of the broiler house were Proteobacteria, Firmicutes, Actinobacteria, Thaumarchaeota, and Cyanobacteria at the entry, middle, and rear of the poultry house, respectively. Proteobacteria was the dominant phylum in the broiler house, and the farther away from the entry of the poultry house, the greater the proportion it accounted for in the air, with the highest concentration detected at the rear (88.04%). Studies have shown that the proportions of Proteobacteria and Firmicutes are 33.0% to 74.1% and 15.3% to 28.2%, respectively, in air (Bowers et al. Citation2013; Du et al. Citation2018; Gou et al. Citation2016), which is identical to the results of our research.
At the genus level, the bacterial community structure varied greatly among the samples. Shewanella had the highest bacterial abundance in the air in the house, and its concentration was the highest at the rear, followed by the middle location and the entry. Among these dominant bacterial genera, Pseudomonas, Staphylococcus, and Escherichia-Shigella were detected as conditionally pathogenic bacteria (Guo et al. Citation2020; Kang et al. Citation2018; Pang et al. Citation2019). The bacteria obtained in this study were also identified in poultry houses by other authors (Lawniczek-Walczyk et al. Citation2013; Roque et al. Citation2016), which indicates that these conditional pathogenic bacteria are ubiquitous in livestock and poultry houses. Escherichia-Shigella (6.78%) exhibited a higher content at the middle location than at the other locations of the house, whereas Pseudomonas (12.01%) had the highest distribution at the rear of the house. The same conclusion regarding Escherichia-Shigella content in poultry houses was drawn by Winkler et al. (Winkler et al. Citation2017). The high content of Escherichia-Shigella poses a potential threat to the health of broilers and people and might have factored into the high rate of E. coli infection in broiler houses (Yang et al. Citation2018). In our study, the E. coli concentration at the middle location of broiler houses was much higher than that at the rear of the house (Figure S1). This corroborates previous work demonstrating that poultry house sections contained more E. coli (Brooks et al. Citation2016), likely due to fan activity.
The difference in microbial diversity among the locations of the house was primarily related to environmental factors, including ventilation system, stocking density, and housing system (Shen et al. Citation2019; Wang et al. Citation2020; Wang-Li et al. Citation2013). In poultry farms, longitudinal ventilation systems are primarily used, with circulation heating from a water boiler used in winter (Chinivasagam et al. Citation2010). Low-speed high-flow ventilators are typically used for ventilation by mounting them to one end of the wall in the house and setting the air inlet on the opposite wall (Winkler et al. Citation2017). As winter has sunshine duration and is dry, windy, and cold, farms reduce ventilation to ensure heat preservation. Microbial growth depends on the temperature and humidity inside the house (Zhao et al. Citation2016), whereas the air flow of poultry houses can alter the temperature and the relative humidity; thus, the ventilation system can change the poultry house environment. This result was also identified in poultry houses by other authors (Wang et al. Citation2022). Based on the results in the investigation, it can be concluded that aerosol particles (≤4.7 μm) accumulate along with the airflow in the broiler house, with the smaller particle matter of stages 3–6 increasing from 38.6% at the center of the house to 61.7% at the place close to the exhaust fans. As the entry of the poultry house is located near the air inlet of the poultry house, air outside can enter the house, and the species of microbes increased at this location. The alpha diversity index analysis also showed that bacterial diversity at the entry was higher than that at the other locations. The middle location of the house, due to the high feeding density and the high concentration of animals, contained conditionally pathogenic bacteria. Therefore, to effectively control the microbial content in the house in winter, the relationship between ventilation and heat preservation must be well addressed, and the development of a reasonable disinfection control plan is needed to ensure an adequate growth environment for poultry.
Conclusion
The total concentration of bacterial aerosols in the broiler house gradually increased from the entry to the rear, with the highest concentration of inhalable microbes occurring at the middle of the boiler house.
The results of a high-throughput sequencing analysis showed that the entry of the broiler house had the most complex bacterial community structure, whereas the middle of the broiler house had the highest concentration of and the greatest species distribution of conditionally pathogenic bacteria.
In this study, the community structure of airborne microbes in different locations of a broiler house and the changing pattern of potentially conditionally pathogenic microbes were evaluated, and some suggestions and measures were put forward to control the concentration of bacteria present and to improve air quality in poultry houses.
Author contributions
Han Yan, Huan Chen and Linlin Jiang designed the experiments. Guozhong Chen, Nihong Jiang and Xin Yu carried out the experiments. Jianlong Zhang, Xiaoyu Zhao, and Wenli Tang analyzed the experimental results. Hongwei Zhu and Youzhi Li, wrote the manuscript. Xingxiao Zhang reviewed and edited the manuscript.
Supplemental Material
Download Zip (411.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10962247.2023.2193162.
Additional information
Funding
Notes on contributors
Han Yan
Han Yan is a postgraduate in the College of Life Science at Ludong University.
Huan Chen
Huan Chen is a postgraduate in the College of Life Science at Ludong University
Linlin Jiang
Linlin Jiang is associate professor of Environmental Engineering at Ludong University.
Jianlong Zhang
Jianlong Zhang is associate professor of Environmental Engineering at Ludong University.
Guozhong Chen
Guozhong Chen is an Environmental scientist.
Xin Yu
Xin Yu is associate professor of Environmental Engineering at Ludong University.
Hongwei Zhu
Hongwei Zhu is associate professor of Environmental Engineering at Ludong University.
Xiaoyu Zhao
Xiaoyu Zhao is an Environmental scientist.
Youzhi Li
Youzhi Li is an Environmental Engineer.
Wenli Tang
Wenli Tang is an Environmental Engineer.
Xingxiao Zhang
Xingxiao Zhang is Environmental Engineer.
Nihong Jiang
Nihong Jiang is an Environmental Engineer.
References
- Bahador, M., R.A. Alfirdous, T.A. Alquria, I.L. Griffin, P.A. Tordik, and F.C. Martinho. 2021. Aerosols generated during endodontic treatment: A special concern during the coronavirus disease 2019 pandemic. J. Endod. 47 (5):732–39. doi:10.1016/j.joen.2021.01.009.
- Bai, H., L. Y. He, D.L. Wu, F.Z. Gao, M. Zhang, H.Y. Zou, M.-S. Yao, and G.-G. Ying. 2022. Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk. Environ. Int. 158:106927. doi:10.1016/j.envint.2021.106927.
- Beier, R.C., J.A. Byrd, K. Andrews, D. Caldwell, T. L. Crippen, R. C. Anderson, and D. J. Nisbet. 2021. Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen Campylobacter jejuni isolated from the litter of broiler chicken houses. Poult. Sci. 100 (2):1024–33. doi:10.1016/j.psj.2020.10.045.
- Bindari, Y.R., S.K. Kheravii, C.L. Morton, S.B. Wu, S. W. Walkden-Brown, and P. F. Gerber. 2021. Molecular detection of Eimeria species and Clostridium perfringens in poultry dust and pooled excreta of commercial broiler chicken flocks differing in productive performance. Vet. Parasitol. 291:109361. doi:10.1016/j.vetpar.2021.109361.
- Bowers, R.M., N. Clements, J.B. Emerson, C. Wiedinmyer, M. P. Hannigan, and N. Fierer. 2013. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 47 (21):12097–106. doi:10.1021/es402970s.
- Brook, R.D., B. Franklin, W. Cascio, Y. Hong, G. Howard, M. Lipsett, R. Luepker, M. Mittleman, J. Samet, S.C. Smith, et al. 2004. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American heart association. Circulation. 109 (21):2655–71. doi:10.1161/01.CIR.0000128587.30041.C8.
- Brooks, J.P., M.R. Mclaughlin, A. Adeli, and D.M. Miles. 2016. Cultivation and qPCR detection of pathogenic and antibiotic-resistant bacterial establishment in naive broiler houses. J. Environ. Qual. 45 (3):958–66. doi:10.2134/jeq2015.09.0492.
- Bui, V.N., T.T. Nguyen, H. Nguyen-Viet, A.N. Bui, K.A. Mccallion, H.S. Lee, S.T. Than, K.K. Coleman, and G. C. Gray. 2019. Bioaerosol sampling to detect avian influenza virus in hanoi’s largest live poultry market. Clin. Infect. Dis. 68 (6):972–75. doi:10.1093/cid/ciy583.
- Cambra-Lopez, M., A. J. Aarnink, Y. Zhao, S. Calvet, and A. G. Torres. 2010. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 158 (1):1–17. doi:10.1016/j.envpol.2009.07.011.
- Caporaso, J.G., J. Kuczynski, J. Stombaugh, K. Bittinger, F.D. Bushman, E.K. Costello, N. Fierer, A.G. Peña, J.K. Goodrich, J.I. Gordon, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7 (5):335–36. doi:10.1038/nmeth.f.303.
- Chinivasagam, H.N., T. Tran, L. Maddock, A. Gale, and P.J. Blackall. 2010. The aerobiology of the environment around mechanically ventilated broiler sheds. J. Appl. Microbiol. 108 (5):1657–67. doi:10.1111/j.1365-2672.2009.04571.x.
- Dos Anjos Magri, C., R. Garofallo Garcia, E. Binotto, N. Duarte Da Silva Lima, I. De Alencar Naas, S. Sgavioli, and M. F. de Castro Burbarelli. 2021. Occupational risk factors in health of broiler-farm workers: A systematic review. Arch Environ. Occup. Health. 76 (8):482–93. doi:10.1080/19338244.2020.1832036.
- Du, P., R. Du, W. Ren, Z. Lu, and P. Fu. 2018. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 610-611:308–15. doi:10.1016/j.scitotenv.2017.07.097.
- Edgar, R.C. 2013. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 10 (10):996–98. doi:10.1038/nmeth.2604.
- Gao, M., R. Jia, T. Qiu, M. Han, and X. Wang. 2017. Size-related bacterial diversity and tetracycline resistance gene abundance in the air of concentrated poultry feeding operations. Environ. Pollut. 220:1342–48. doi:10.1016/j.envpol.2016.10.101.
- Gladding, T.L., C.A. Rolph, C.L. Gwyther, R. Kinnersley, K. Walsh, and S. Tyrrel. 2020. Concentration and composition of bioaerosol emissions from intensive farms: Pig and poultry livestock. J. Environ. Manage. 272:111052. doi:10.1016/j.jenvman.2020.111052.
- Gou, H., J. Lu, S. Li, Y. Tong, C. Xie, and X. Zheng. 2016. Assessment of microbial communities in PM1 and PM10 of Urumqi during winter. Environ. Pollut. 214:202–10. doi:10.1016/j.envpol.2016.03.073.
- Guo, Y., G. Song, M. Sun, J. Wang, and Y. Wang. 2020. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect. Microbiol. 10:107. doi:10.3389/fcimb.2020.00107.
- Guo, N., Y. Wang, T. Tong, and S. Wang. 2018. The fate of antibiotic resistance genes and their potential hosts during bio-electrochemical treatment of high-salinity pharmaceutical wastewater. Water Res. 133:79–86. doi:10.1016/j.watres.2018.01.020.
- Gupta, C.L., R. Avidov, K. Kattusamy, I. Saadi, V.S. Varma, S.E. Blum, Y. Zhu, X. Zhou, J. Su, Y. Laor, et al. 2021. Spatial and temporal dynamics of microbiomes and resistomes in broiler litter stockpiles. Comput. Struct. Biotechnol. J. 19:6201–11. doi:10.1016/j.csbj.2021.11.020.
- Jegede, A., Q. Fu, M. Lin, A. Kumar, and J. Guan. 2019. Aerosol exposure enhanced infection of low pathogenic avian influenza viruses in chickens. Transbound Emerg. Dis. 66 (1):435–44. doi:10.1111/tbed.13039.
- Kang, E., A. Crouse, L. Chevallier, S.M. Pontier, A. Alzahrani, N. Silue, F.-X. Campbell-Valois, X. Montagutelli, S. Gruenheid, and D. Malo. 2018. Enterobacteria and host resistance to infection. Mamm. Genome. 29 (7–8):558–76. doi:10.1007/s00335-018-9749-4.
- Khan, I., W. Wang, X. Ye, A. M. Isa, M.T. Khan, R. Sa, L. Liu, T. Ma, and H. Zhang. 2022. Comparison of bacterial community structure in PM2.5 within broiler houses under different rearing systems in China. Sustainability. 14 (3):1–15. doi:10.3390/su14031357.
- Lawniczek-Walczyk, A., R.L. Gorny, M. Golofit-Szymczak, A. Niesler, and A. Wlazlo. 2013. Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 20 (2):259–68.
- Li, Z., W. Zheng, Y. Wei, B. Li, Y. Wang, and H. Zheng. 2019. Prevention of particulate matter and airborne culturable bacteria transmission between double-tunnel ventilation layer hen houses. Poult. Sci. 98 (6):2392–98. doi:10.3382/ps/pez019.
- Magoc, T., and S.L. Salzberg. 2011. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27 (21):2957–63. doi:10.1093/bioinformatics/btr507.
- Newton, K., S.M. Withenshaw, S.A. Cawthraw, and R. Davies. 2021. In-depth farm investigations and an exploratory risk factor analysis for the presence of Salmonella on broiler farms in Great Britain. Prev. Vet. Med. 197:105498. doi:10.1016/j.prevetmed.2021.105498.
- Nonnenmann, M.W., B. Bextine, S.E. Dowd, K. Gilmore, and J.L. Levin. 2010. Culture-independent characterization of bacteria and fungi in a poultry bioaerosol using pyrosequencing: A new approach. J. Occup. Environ. Hyg. 7 (12):693–99. doi:10.1080/15459624.2010.526893.
- Pang, Z., R. Raudonis, B.R. Glick, T.J. Lin, and Z. Cheng. 2019. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37 (1):177–92. doi:10.1016/j.biotechadv.2018.11.013.
- Qiu, T., D. Wu, L. Zhang, D. Zou, Y. Sun, M. Gao, and X. Wang. 2021. A comparison of antibiotics, antibiotic resistance genes, and bacterial community in broiler and layer manure following composting. Environ. Sci. Pollut. Res. Int. 28 (12):14707–19. doi:10.1007/s11356-020-11469-6.
- Quast, C., E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies, and F. O. Glöckner. 2013. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41 (D1):D590–596. doi:10.1093/nar/gks1219.
- Radon, K., B. Danuser, M. Iversen, E. Monso, C. Weber, J. Hartung, K. Donham, U. Palmgren, and D. Nowak. 2002. Air contaminants in different European farming environments. Ann. Agric. Environ. Med. 9 (1):41–48.
- Roque, K., G.D. Lim, J.H. Jo, K.M. Shin, E.S. Song, R. Gautam, C.-Y. Kim, K. Lee, S. Shin, H.-S. Yoo, et al. 2016. Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings. J. Vet. Sci. 17 (4):531–38. doi:10.4142/jvs.2016.17.4.531.
- Shen, D., S. Wu, P.Y. Dai, Y.S. Li, and C.M. Li. 2018. Distribution of particulate matter and ammonia and physicochemical properties of fine particulate matter in a layer house. Poult. Sci. 97 (12):4137–49. doi:10.3382/ps/pey285.
- Shen, D., S. Wu, Z. Li, Q. Tang, P. Dai, Y. Li, and C. Li. 2019. Distribution and physicochemical properties of particulate matter in swine confinement barns. Environ. Pollut. 250:746–53. doi:10.1016/j.envpol.2019.04.086.
- Song, L., G. Jiang, C. Wang, J. Ma, and H. Chen. 2022. Effects of antibiotics consumption on the behavior of airborne antibiotic resistance genes in chicken farms. J. Hazard. Mater. 437:129288. doi:10.1016/j.jhazmat.2022.129288.
- Song, B., S. Yan, P. Li, G. Li, M. Gao, L. Yan, Z. Lv, and Y. Guo. 2022. Comparison and correlation analysis of immune function and gut microbiota of broiler chickens raised in double-layer cages and litter floor pens. Microbiol. Spectr. 10 (4):e0004522. doi:10.1128/spectrum.00045-22.
- Sowiak, M., K. Brodka, A. Kozajda, A. Buczynska, and I. Szadkowska-Stanczyk. 2012. Fungal aerosol in the process of poultry breeding--quantitative and qualitative analysis. Med. Pr. 63 (1):1–10.
- Wang, X., X. Dai, A. Wang, L. Wang-Li, M. Yang, H. Xiao, Y. He, and K. Wang. 2022. Size-segregated physicochemical properties of inhalable particulate matter in a tunnel-ventilated layer house in China. Environ. Res. 204:112064. doi:10.1016/j.envres.2021.112064.
- Wang-Li, L., Z. Cao, Q. Li, Z. Liu, and D.B. Beasley. 2013. Concentration and particle size distribution of particulate matter inside tunnel-ventilated high-rise layer operation houses. Atmos. Environ. 66:8–16. doi:10.1016/j.atmosenv.2012.03.064.
- Wang, Y., N. Lyu, F. Liu, W. J. Liu, Y. Bi, Z. Zhang, S. Ma, J. Cao, X. Song, A. Wang, et al. 2021. More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 153:106534. doi:10.1016/j.envint.2021.106534.
- Wang, Y., B. Niu, J.-Q. Ni, W. Xue, Z. Zhu, X. Li, and G. Zhou. 2020. New insights into concentrations, sources and transformations of NH3, NOx, SO2 and PM at a commercial manure-belt layer house. Environ. Pollut. 262:114355. doi:10.1016/j.envpol.2020.114355.
- Winkler, S., C. Coufal, D. Harmel, E. Martin, J. P. Brooks, S. Popham, and T. J. Gentry. 2017. Within-house spatial distribution of fecal indicator bacteria in poultry litter. J. Environ. Qual. 46 (5):1003–09. doi:10.2134/jeq2017.05.0188.
- Xu, X., W. Zhou, C. Xie, Y. Zhu, W. Tang, X. Zhou, and H. Xiao. 2022. Airborne bacterial communities in the poultry farm and their relevance with environmental factors and antibiotic resistance genes. Sci. Total Environ. 846:157420. doi:10.1016/j.scitotenv.2022.157420.
- Yadav, S., K.D. Caliboso, J.E. Nanquil, J. Zhang, H. Kae, K. Neupane, B. Mishra, and R. Jha. 2021. Cecal microbiome profile of Hawaiian feral chickens and pasture-raised broiler (commercial) chickens determined using 16S rRNA amplicon sequencing. Poult. Sci. 100 (7):101181. doi:10.1016/j.psj.2021.101181.
- Yang, W., M. Guo, G. Liu, G. Yu, P. Wang, H. Wang, and T. Chai. 2018. Detection and analysis of fine particulate matter and microbial aerosol in chicken houses in Shandong Province, China. Poult. Sci. 97 (3):995–1005. doi:10.3382/ps/pex388.
- Yilmaz, P., L.W. Parfrey, P. Yarza, J. Gerken, E. Pruesse, C. Quast, T. Schweer, J. Peplies, W. Ludwig, and F.O. Glöckner. 2014. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 42 (D1):D643–648. doi:10.1093/nar/gkt1209.
- You, J., Y. Wu, X. Zhang, X. Wang, J. Gong, Z. Zhao, J. Zhang, J. Zhang, Z. Sun, J. Li, et al. 2019. Efficient fecal-oral and possible vertical, but not respiratory, transmission of emerging Chlamydia gallinacea in broilers. Vet. Microbiol. 230:90–94. doi:10.1016/j.vetmic.2019.01.018.
- Zhao, Y., T.A. Shepherd, H. Li, and H. Xin. 2015. Environmental assessment of three egg production systems–part I: Monitoring system and indoor air quality. Poult. Sci. 94 (3):518–33. doi:10.3382/ps/peu076.
- Zhao, Y., D. Zhao, H. Ma, K. Liu, A. Atilgan, and H. Xin. 2016. Environmental assessment of three egg production systems – Part III: Airborne bacteria concentrations and emissions. Poult. Sci. 95 (7):1473–81. doi:10.3382/ps/pew053.
- Zhou, Y., M. Zhang, Q. Liu, and J. Feng. 2021. The alterations of tracheal microbiota and inflammation caused by different levels of ammonia exposure in broiler chickens. Poult. Sci. 100 (2):685–96. doi:10.1016/j.psj.2020.11.026.