ABSTRACT
Particulate matter (PM) concentrations have decreased dramatically over the past 20 years, thus lower method detection limits (MDL) are required for these measurements. Energy-dispersive X-ray fluorescence (XRF) spectroscopy is used to quantify multiple elements simultaneously in the U.S. Environmental Protection Agency (EPA) Chemical Speciation Network (CSN). Inductively-coupled plasma mass spectrometry (ICP-MS) is an alternative analysis with lower MDL for elements. Here, we present a side-by-side comparison of XRF and ICP-MS for elements in PM2.5 samples collected via the EPA’s CSN. For ICP-MS, a simple extraction and ICP-MS analysis technique was applied to a wide variety of samples to minimize effort and cost and serve as a feasibility test for a large monitoring network. Filter samples (N = 549) from various urban locations across the US were analyzed first analyzed via XRF at UC Davis and then ICP-MS at RTI International. Both methods measured 29 of the same elements out of the 33 usually reported to CSN. Of these 29, 14 elements (Na, Mg, Al, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, Pb) were found to be frequently detected (i.e. had more than 10% of values above both XRF and ICP-MS MDL). ICP-MS was found to have lower MDL for 26 out of 29 elements, namely Na, Mg, Al, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, As, Se, Rb, Sr, Zr, Ag, Cd, In, Sn, Sb, Cs, Ba, Ce, Pb; conversely, XRF had lower MDL for 3 elements, namely, P, K, Zn. Intra-method quality checks using (1) inter-elemental inspection of scatter plots using a priori knowledge of element sources and (2) scatter plots of routine versus collocated measurements reveal that ICP-MS exhibits better measurement precision. Lower detection limits for element measurements in nationwide PM monitoring networks would benefit human-health and source apportionment research.
Implications: We demonstrate that ICP-MS with adilute-acid digestion method would significantly improve the element detection rates and thus be avaluable addition to the current analysis techniques for airborne PM samples in anationwide monitoring network. In this paper, we show that a hybrid method of elemental analysis for airborne particulate matter (PM) would significantly improve the detection rates for elements in PM. This would be a valuable addition to the current analysis techniques for airborne PM samples in nationwide and other large-scale monitoring networks, such as the EPA’s Chemical Speciation Network (CSN). The techniques explored in this study (i.e., X-ray Fluorescence Spectroscopy or XRF and Inductively Coupled Plasma-Mass Spectrometry or ICP-MS) are relevant to the PM monitoring and regulatory community audience of JAWMA, especially agencies and states that are already involved in CSN. In addition, our results outline considerations that give insight on factors to consider for other large-scale and long-term ambient air monitoring efforts.
Introduction
Particulate matter (PM) is made up of many chemical components, including elements in the U.S. Environmental Protection Agency (EPA) list of Hazardous Air Pollutants (HAPs): Sb, As, Be, Cd, Cr, Co, Pb, Mn, Hg, Ni, and Se (U. S. Environmental Protection Agency). Furthermore, elements such as Zn, Fe, and Cu which are not listed as HAPs, are known to be toxic to humans at high concentrations or when present as a particular species or particle size (Brewer Citation2010; Hussain et al. Citation2022). To understand the health effects of PM, accurate analytical techniques are paramount.
The two nationwide monitoring networks operating in the United States — the Chemical Speciation Network (CSN) and Interagency Monitoring of Protected Visual Environments (IMPROVE) — collect PM2.5 samples on filter membranes for laboratory analysis of PM composition. Two techniques that have been widely used for elemental analysis are energy-dispersive X-ray fluorescence spectrometry (XRF) and inductively coupled plasma mass spectrometry (ICP-MS). XRF is a nondestructive nuclear analytical technique that requires no preparation for filter-based samples. XRF instruments are very stable, typically calibrated once per year or after major repairs. They are also easy to operate, thus well suited for automation. While XRF offers a relatively quick analysis that requires no sample preparation, method detection limits (MDL) may not be adequate for elements with lower concentrations.
Alternatively, ICP-MS has better MDL but requires extensive and destructive sample preparation. ICP-MS requires sample introduction in liquid form; PM must be extracted from filters using acids, which may require hazardous waste disposal, aided by heating, shaking, and/or microwave digestion. ICP-MS results are highly dependent on the extraction efficiency and susceptible to uncertainty in the sample preparation steps. ICP-MS instruments require frequent calibrations, maintenance, and quality control (QC) checks to ensure stability of the measurements. Tailoring the extraction protocol and ICP-MS analysis settings to the samples provides better results (e.g., heavily loaded samples are best analyzed after dilution; soil-rich samples need stronger acids to fully digest). The levels of expertise and effort required to perform ICP-MS analysis often make the analysis cost-prohibitive for monitoring efforts. Both techniques may suffer from interferences; however, careful choice of detectors, implementation of additional setups (such as a collision reaction cell for ICP-MS), or mathematical corrections during data processing can address many of these interferences.
Inter-instrumental comparisons of XRF and ICP-MS for elemental analysis are sparse for PM and similar media (Brown et al. Citation2010; Niu et al. Citation2010; Okuda et al. Citation2013; Sarkar et al. Citation2023; Yatkin, Gerboles, and Borowiak Citation2012). In general, the comparisons are good for elements measured above detection limits by both techniques although biases exist, likely because these used different calibration reference materials. As mentioned above, the comparisons are highly dependent on the extraction technique used for ICP-MS analysis; most use complex extraction protocols to ensure complete digestion of the samples (Brown et al. Citation2010; Kulkarni et al. Citation2003; Niu et al. Citation2010; Sarkar et al. Citation2023; Spada, Bozlaker, and Chellam Citation2012; Yatkin et al. Citation2016; Yatkin, Gerboles, and Borowiak Citation2012).
In this study, a simplified extraction and ICP-MS analysis technique was used to minimize effort and cost. We applied this approach to a wide variety of samples from different locations as a feasibility test for a large monitoring network. We present a side-by-side comparison of XRF and ICP-MS for elements in PM2.5 samples collected via the EPA’s CSN.
Materials and methods
Sampling
Archived PM2.5 filters (N = 549) sampled from various CSN locations in the US were analyzed here (Gorham et al. Citation2021). In CSN, PM2.5 is sampled for 24 hours via 47-mm polytetrafluoroethylene filters (PTFE, MTL Corporation, pore size 2 µm) using Met One SASS/Super SASS samplers at 6.7 L min−1 (mass flow controlled) according to the site-specific sampling schedule (e.g., 1 day in 3 or 1 day in 6). All routine CSN PTFE samples are analyzed by XRF at the University of California in Davis (UCD) for 33 elements (see Supplementary for complete list). Samples representing the 10th, 50th, and 90th percentiles in the network for each element were selected to span the range of mass concentrations. Some routine-collocated sample pairs were selected to provide sample precision estimates. The collocated samples were from Rutgers, NJ; Dudley Square, Boston, MA; Rubidoux, Riverside, CA; Bakersfield, CA; G.T. Craig, OH; and Deer Park, Houston, TX. These sites operate duplicate samplers for quality control purposes. Samples were originally collected from January 2019 to June 2022, and were pulled from the archive then sent to RTI International (RTI) for subsequent ICP-MS analysis.
XRF measurements
XRF analysis was performed at the Air Quality Research Center of UCD using the PANalytical Epsilon 5 (hereafter referred to as UCD XRF). The UCD XRF analyzer uses a three-dimensional (3D) geometry, defined by three orthogonal axes: (1) the primary beam, from a 100-kV side window X-ray tube with a dual Scandium/Tungsten (Sc/W) anode as an X-ray source, which then hits the secondary target; (2) the beam then travels from the secondary target to the sample in a second orthogonal axis, and (3) lastly, the characteristic X-rays from the sample travel to a solid-state Germanium (Ge) X-ray detector, which then outputs the spectra of characteristic photons emitted by excited elements in the sample. Spectrum evaluation is performed using non-linear least squares fitting based on AXIL (Vekemans Citation1994). Currently, 33 elements reported to CSN require seven different optimized analytical conditions, which are listed in the Supplementary Information.
ICP-MS measurements
Metals (M+) in PM2.5 collected on the PTFE filters were extracted using a method following Section 11.0, Hot Block Filter Strip Extraction, in 40 CFR Part 50, Appendix G (Appendix G to Part 50). Filters were soaked in 25 mL 5.0% v/v nitric acid (HNO3) in plastic tubes, which were previously soaked in 1% HNO3 for at least 24 hours then rinsed with reagent water prior to use. The extraction tubes were then placed in a heated graphite block for two hours at 95 ± 5°C to facilitate the extraction. Following heating, the samples were capped and vortex-mixed or shaken vigorously for a few seconds prior to ICP-MS analysis. The MDL for each metal was determined according to 40 CFR Part 136, Appendix B (Appendix B to Part 136). The method range for PM2.5 filters was demonstrated from 0.0025 µg/filter to 50 µg/filter, based on the low and high calibration curve standards. Calibration and check standards were prepared from a mixture of multi-element and single-element National Institute of Standards and Technology (NIST) traceable commercial standards to matrix-match the acid composition of the samples. Recovery from the leaching method was evaluated using NIST Standard Reference Material (SRM) 1648a (Urban Particulate Matter). ICP-MS analysis was then performed using a single quadrupole ICP-MS (Thermo Scientific™ iCAP™ RQ ICP-MS). The samples were aspirated, and the generated aerosol was transported by a flow of argon gas into the plasma torch. The ions produced (e.g., Pb+1) in the plasma were extracted via a differentially-pumped vacuum interface and were separated based on their mass-to-charge (m/z) ratio. The ions were quantified by a channel electron multiplier; the collected signal was processed by the instrument’s software. The final volume of 25 mL was built into the autosampler table so that the sample results were shown as ng M+/filter. For the collision reaction cell (CCT), which ensures selective removal of polyatomic interferences, Grade 6 helium was used.
Results and discussions
Out of the 33 elements measured by CSN, only 29 were measurable by ICP-MS in this analysis: Na, Mg, Al, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Rb, Sr, Zr, Ag, Cd, In, Sn, Sb, Cs, Ba, Pb, and Ce. Elements S, Si, Br, and Cl cannot be measured reliably by the ICP-MS method used herein but can be measured well using XRF (Figure S1).
Inter-instrumental comparison
To compare the performance of the two methods, two inclusion criteria were applied: detection frequency and ICP-MS extraction recovery for SRM 1648a (i.e., 100 ± 20%). shows the comparison of the detection frequency (above MDL) for both instruments. Of the 29 elements, only 14 (Na, Mg, Al, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, Pb) have more than 10% of measurements above the XRF MDL, while 28 out of 29 elements have more than 10% of the measurements above MDL for ICP-MS — only In did not meet this criterion. Extraction recovery values are shown in Table S4.
Figure 1. Comparison of the percentage of samples measured above the MDL for UCD XRF (left) and ICP-MS (right) for 33 elements reported to CSN. Elements that cannot be measured with the method described in-text are marked with two red asterisks.
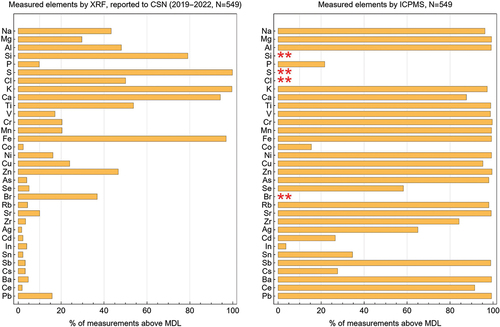
When measurements are above the MDL for both instruments (e.g., points are in the top right quadrant, Figure S2), the (x,y) pair for the measurements lies on the 1:1 line, indicating agreement—e.g., Fe, K, Cu, Zn. However, measurements in the other three quadrants, (i.e., below MDL for either or both methods) tend to deviate from the 1:1 line and show less agreement, often with the XRF measurements reported as zero or negative. Ca, Cr and Ni comparisons are poor even above the MDL estimates, suggesting the measurements are not quantifiable by one or both methods, or that the extraction (leaching) is incomplete. Unfortunately, these comparisons do not provide any information for assessing the quality of either measurement. Internal checks of the elements may provide a better measure of the quality in these cases.
Internal checks
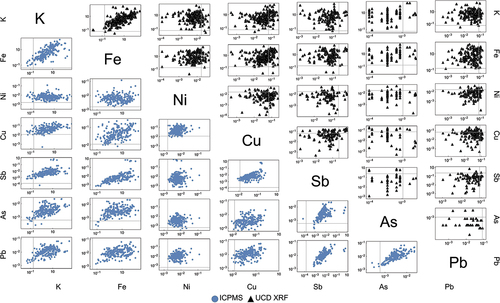
Internal checks were performed to evaluate the quality of the data. shows a matrix of intra-method, inter-elemental scatter plots of element pairs which were selected from a priori knowledge of the elements’ association. Elements from a common source are often correlated, such as soil elements (K, Fe) or elements that may come from anthropogenic urban sources such as industry, roadside or traffic-associated dust (Sb, As, Pb, Cu, Ni). The plots to the left of the diagonal show ICP-MS results only. The plots to the right of the diagonal show XRF results only. shows that ICP-MS inter-element correlations are much tighter than UCD XRF correlations, showing that ICP-MS exhibits better intra-method measurement precision. Ni, while expected to correlate with Cu, shows a poor relationship due to the incomplete leaching from the digestion method used. This is further explored in the next section.
Figure 2. Inter-elemental plots for select element pairs. On the bottom right (blue circles, below the diagonal) are scatter plots for ICP-MS element pairs; on the top right (black triangles, above the diagonal) are XRF element pairs. MDL are shown in light gray vertical and horizontal lines. Only measurements from collocated sites (values > 0) are plotted.
MDL and other sources of uncertainty
As another check on measurement quality, the inter-comparison was performed using collocated sample pairs. Analysis results from the routine and collocated samples from three sites were plotted against each other (Figure S4). The uncertainty between routine and collocated samples analyzed by the same method and instrument provided benchmarks for the inter-comparison (i.e., differences between two analytical techniques could be compared to differences between the same instrument analyzing two collocated samples). The collocated samples introduce a new source of uncertainty of about 4% arising from sampling itself (e.g., variations in flow rate, sampling media, handling) (Hyslop and White Citation2008). Collocated measurements above MDL tend to agree, but the lower ICP-MS MDL are obvious in these plots with collocated measurements agreeing down to much lower concentrations for ICP-MS than XRF (Sb, Pb, As). The lighter elements (K, Fe) show good collocated-routine agreement for both ICP-MS and XRF (Figure S4) and by two different XRF instruments (Figure S1).
In ICP-MS, spectroscopic interferences caused by atomic or molecular polyatomic ions with the same m/z as detected by the mass spectrometer are a concern. This can be minimized using reaction or collision cells for which a collision gas flow rate is optimized based on the elemental recoveries of QC materials. However, for some elements, the prevalence of polyatomic species formed by ubiquitous elements like O, N, C, H, e.g., 16O2 on 32S, makes compensation by techniques using collision cell challenging on a single-quadrupole ICP-MS, and thus these elements cannot be reliably analyzed with the current ICP-MS configuration (May and Wiedmeyer Citation1998). Also, analysis of anions like Br or Cl, is not practical using the current ICP-MS configuration. For these elements, XRF is reliable. In addition, cross-method validation procedures for CSN utilize S, Cl, and Si; thus, relying solely on the current ICP-MS configuration for the elemental measurements may hinder CSN data validation; this then is an opportunity to explore other inter-method cross-validation techniques for the network using elements detected by both methods. The dilute acid extraction (leaching) method used in this work is likely to result in incomplete extraction of silicates (see recoveries, Table S4) — which can be a major source of PM in dusty areas of the country — as well as Cr and Ni. Chemical mass balance-type calculations may also be affected if the measurements of elements abundant in soil (e.g., Si) are biased low. XRF measures silicates and the soil-related elements reliably, so the methods are complementary.
Cheatham, et al., mentions instrument drift as the most important cause of loss of analytical precision for ICP-MS (Cheatham, Sangrey, and White Citation1993). The condition and cleanliness of the sample and skimmer cones, or issues in sample transport systems (e.g., nebulizer and peristaltic pump) can cause values to meander. It can be corrected using internal or external standards. Measuring overlapping elements by ICP-MS and XRF provides an independent check for instrument drift, another advantage of using two analytical approaches together.
While XRF is stable, does not require frequent calibrations, and is nondestructive, XRF MDL are high compared to most elements measured by ICP-MS (Figure S5). In addition, instrument run time for a multi-elemental analysis is long compared to that of ICP-MS (e.g. ~1 hour vs. 4–9 minutes for ICP-MS). Conversely, sample preparation time for ICP-MS is long (typically 2 to 2.5 hours), while no sample preparation is necessary for XRF. Table S5 summarizes the operational differences between XRF and ICP-MS. Lastly, while reference materials that mimic atmospheric aerosols have been successfully generated on PTFE filters (Yatkin et al. Citation2018), no independently certified reference materials (CRM) are currently commercially available for XRF. While there are NIST-certified reference materials available, in the form of powders, to evaluate the performance of ICP-MS analyses (e.g., NIST SRM 1633c, 1648a, 2709a), they have not been used for XRF because they are not available deposited on membrane filters. Consequently, a shared, certified reference material cannot be used to compare instrument performance. Shared XRF and ICP-MS reference materials that challenge all aspects of the measurement processes are key to properly evaluating the inter-comparison and are under development.
Conclusion
Overall, XRF measurements compared well for elements routinely measured above MDL. Further, it was demonstrated that ICP-MS MDL are much lower for the trace elements. A combination of these two analytical methods for a major monitoring network could provide a more quantitative data set. Performing ICP-MS in addition to XRF will decrease the number of non-detects for the elements where ICP-MS has lower MDL, however the cost per sample will increase 2–3 times and the analysis turnaround time may increase. Thus, it is important to keep the sample preparation costs down. An inexpensive leaching method using dilute acid (5% HNO3) is adequate to extract many trace elements above ICP-MS detection limits, however, it is not enough to show good recovery with the current SRM used. Thus, it is recommended to find or develop an appropriate SRM that will be (1) representative of the trace element levels in PM and (2) inter-usable for both XRF and ICP-MS. It is also recommended to explore digestion with other acid mixtures that are inexpensive and require low effort but can dissolve more elements of concern.
Of the 14 elements evaluated here, it is recommended to measure Na, Mg, Al, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, and Pb with XRF; Mg, V, Mn, Cu, Zn, and Pb with ICP-MS with a dilute acid digestion and ICP-MS configuration as described herein. Of the remaining 19 elements not evaluated herein but reported to CSN — S, Si, Cl, and Br are recommended to be measured using XRF; Co, As, Se, and Cd with ICP-MS based on both acceptable recovery and frequency of detection above MDL; and P, Rb, Sr, Zr, Ag, Sn, Sb, Cs, Ba, and Ce using ICP-MS based only on frequency of detection above MDL.
Supplementary-rev1-July 31 2023-NoMarkup.docx
Download MS Word (383.4 KB)Acknowledgments
The authors would like to thank Joann Rice and Melinda Beaver of the EPA’s Office of Air Quality Planning and Standards Ambient Air Monitoring Group for their review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Measurements using UCD XRF reported to CSN are available via the EPA Air Quality System (AQS) at https://www.epa.gov/aqs. ICP-MS measurements will be made available and can be accessed in the Data Dryad repository (https://datadryad.org/) upon request.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10962247.2023.2247376.
Additional information
Funding
Notes on contributors
Colleen Marciel F. Rosales
Colleen Marciel F. Rosales is a postdoctoral scholar at the Air Quality Research Center at the University of California in Davis. She is also the Strategic Partnerships Director at the nonprofit organization OpenAQ. She obtained her Ph.D. in Environmental Science from Indiana University and her B.S. in Chemistry from the University of the Philippines in Diliman.
Frank Weber
Frank Weber is a research inorganic chemist in the Analytical Sciences center at RTI International.
Tracy L. Dombek
Tracy L. Dombek is a research chemist in the Analytical Sciences center at RTI International.
Keith Levine
Keith Levine is a research analytical chemist and is the Senior Director of the Analytical Sciences center at RTI International.
Andrea McWilliams
Andrea McWilliams is a research chemist in the Analytical Sciences center at RTI International.
Nicholas J. Spada
Nicholas J. Spada is a scientist in the Air Quality Research Center at the University of California at Davis. Dr. Spada conducts research on elemental profilings in mixed urban/industrial and remote environments.
Nicole P. Hyslop
Nicole P. Hyslop is the Associate Director for Quality Research in the Air Quality Research Center at the University of California at Davis. She earned her Ph.D. in Environmental Chemistry from UC Davis in 2010. Prior to joining UC Davis, Nicole worked at Sonoma Technologies, Inc. and earned her B.S. and M.S. degrees in Chemical Engineering at the University of Wisconsin at Madison and The University of Texas at Austin.
References
- Appendix B to Part 136, Title 40 Agency: Environmental Protection Agency Part 136 Authority: Secs. 301, 304(h), 307 and 501(a), Pub. L. 95–217, 91 Stat. 1566, et seq. https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-136/appendix-Appendix%20B%20to%20Part%20136.
- Appendix G to Part 50, Title 40 Agency: Environmental Protection Agency Part 50 Authority: 42 U.S.C. 7401, et seq. Accessed March 31, 2023. https://www.ecfr.gov/current/title-40/appendix-Appendix%20G%20to%20Part%2050.
- Brewer, G.J. 2010. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 23 (2):319–26. doi:10.1021/tx900338d.
- Brown, R.J.C., K.E. Jarvis, B.A. Disch, S.L. Goddard, E. Adriaenssens, and N. Claeys. 2010. Comparison of ED-XRF and LA-ICP-MS with the European reference method of acid digestion-ICP-MS for the measurement of metals in ambient particulate matter. Accred. Qual. Assur. 15 (9):493–502. doi:10.1007/s00769-010-0668-7.
- Cheatham, M.M.; W.F. Sangrey; W.M. White. 1993. Sources of error in external calibration ICP-MS analysis of geological samples and an improved non-linear drift correction procedure. Spectrochim. Acta Part B At. Spectrosc. 48 (3):487–506. doi:10.1016/0584-8547(93)80054-X.
- Gorham, K.A.; S.M. Raffuse; N.P. Hyslop; W.H. White. 2021. Comparison of recent speciated PM2.5 data from collocated CSN and IMPROVE measurements. Atmos. Environ. 244:117977. doi:10.1016/j.atmosenv.2020.117977.
- Hussain, S., M. Khan, T.M.M. Sheikh, M.Z. Mumtaz, T.A. Chohan, S. Shamim, and Y. Liu. 2022. Zinc essentiality, toxicity, and its bacterial bioremediation: A comprehensive insight. Front. Microbiol. 13:900740. doi:10.3389/fmicb.2022.900740.
- Hyslop, N.; W. White. 2008. An Evaluation of Interagency monitoring of Protected Visual Environments (IMPROVE) collocated precision and uncertainty estimates. Atmos. Environ. 42 (11):2691–705. doi:10.1016/j.atmosenv.2007.06.053.
- Kulkarni, P.; S. Chellam; G. Ghurye; M.P. Fraser. 2003. In Situ generation of hydrofluoric acid during microwave digestion of atmospheric particulate matter prior to trace element analysis using inductively coupled plasma mass spectrometry. Environ. Eng. Sci. 20 (6):517–31. doi:10.1089/109287503770736041.
- May, T., and R. Wiedmeyer. 1998. A table of polyatomic interferences in ICP-MS. At. Spectrosc. 19 (5):150–155.
- Niu, J.; P.E. Rasmussen; A. Wheeler; R. Williams; M. Chénier. 2010. Evaluation of airborne particulate matter and metals data in personal, indoor and outdoor environments using ED-XRF and ICP-MS and co-located duplicate samples. Atmos. Environ. 44 (2):235–45. doi:10.1016/j.atmosenv.2009.10.009.
- Okuda, T., E. Fujimori, K. Hatoya, H. Takada, H. Kumata, F. Nakajima, S. Hatakeyama, M. Uchida, S. Tanaka, K. He, et al. 2013. Rapid and simple determination of multi-elements in aerosol samples collected on Quartz fiber filters by using EDXRF coupled with fundamental parameter quantification technique. Aerosol Air Qual. Res. 13 (6):1864–76. doi:10.4209/aaqr.2012.11.0308.
- Sarkar, C.; N. Spada; S. Xu; M.M. Shafer; N.P. Hyslop. 2023. An inter-laboratory comparison of elemental loadings of PM2.5 samples using Energy-dispersive XRF and magnetic-sector ICP-MS. Atmos. Environ. 293:119463. doi:10.1016/j.atmosenv.2022.119463.
- Spada, N.; A. Bozlaker; S. Chellam. 2012. Multi-elemental characterization of tunnel and road dusts in Houston, Texas using dynamic reaction cell-quadrupole-Inductively coupled plasma–Mass spectrometry: Evidence for the release of platinum group and anthropogenic metals from motor vehicles. Anal. Chim. Acta 735:1–8. doi:10.1016/j.aca.2012.05.026.
- U.S. Environmental Protection Agency. 2022. Initial list of hazardous Air pollutants with modifications. Accessed March 31, 2023. https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications.
- Vekemans, B. 1994. Analysis of X-ray Spectra by Iterative Least Squares (AXIL): New developments. X-Ray Spectrom 23:278–85.
- Yatkin, S.; C.A. Belis; M. Gerboles; G. Calzolai; F. Lucarelli; F. Cavalli; K. Trzepla. 2016. An interlaboratory comparison study on the measurement of elements in PM10. Atmos. Environ. 125:61–68. doi:10.1016/j.atmosenv.2015.10.084.
- Yatkin, S.; M. Gerboles; A. Borowiak. 2012. Evaluation of standardless EDXRF analysis for the determination of elements on PM10 loaded filters. Atmos. Environ. 54:568–82. doi:10.1016/j.atmosenv.2012.02.062.
- Yatkin, S.; K. Trzepla; W.H. White; N.P. Hyslop. 2018. Generation of multi-element reference materials on PTFE filters mimicking ambient aerosol characteristics. Atmos. Environ. 189:41–49. doi:10.1016/j.atmosenv.2018.06.034.
