Abstract
The “brain–gut axis” is a multicomponent conceptual model describing the bidirectional communication pathways connecting cognitive and emotional centres in the brain with neuroendocrine centres, the enteric nervous system and the immune system. This model enables novel explanations for the comorbidity of affective disorders, especially concerning mood and anxiety, in functional gastrointestinal disorders, the visceral hypersensitivity, as well as the effect of various acute and chronic stressors on gastrointestinal function. Furthermore, it offers the possibility to develop new biomarkers of integrated brain–gut function. This review discusses the possibilities to modify brain–gut interaction by nutritional means, offered by the brain–gut axis concept. Dietary interventions directed at serotonergic metabolism and its action in the brain, the hypothalamic–pituitary–adrenal axis, the metabolism of long-chain polyunsaturated fatty acids affecting both brain function and inflammatory cytokine profiles, and gut hormones are elaborated. The central role of the autonomic nervous system is also discussed. It is concluded that the concept of the brain–gut axis indeed enables the development of novel dietary intervention strategies in functional gastrointestinal disorders, but results directly transferable to daily practice are not immediately anticipated.
Introduction
It is well accepted that the most common disturbances of gut health, except for those caused directly by a gastrointestinal infection, are associated with aberrations of intestinal function rather than intestinal anatomy or substantial changes in intestinal morphology. Irritable bowel syndrome (IBS) is the most encountered functional gastrointestinal (GI) disorder, characterized by abdominal pain or discomfort associated with alterations in defecation, in the absence of structural or biochemical abnormalities that can be identified using currently available tests Citation1. As much as 10–20% of the Western population suffers from symptoms consistent with the diagnosis of IBS according to the Rome criteria Citation2.
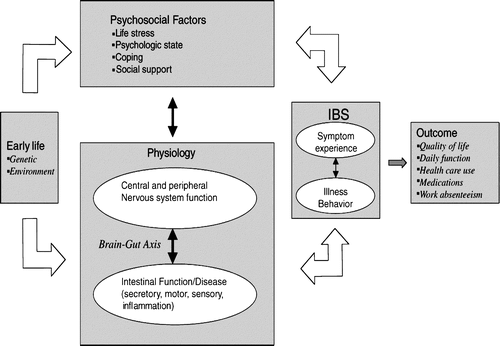
The pathophysiology of functional GI disorders is still not fully understood. Initially these disorders were attributed to a motor dysfunction. By the late 1980s it became apparent that a sensory dysfunction specific for visceral stimuli was a key feature in patients with functional GI disorders Citation3. It is now generally accepted that visceral hypersensitivity, characterized by the experience of pain in case of a normally non-painful visceral stimulus (allodynia) or a decreased threshold for the perception of a painful stimulus (hyperalgesia), is the hallmark of functional GI disorders, especially IBS Citation4Citation5. This change in paradigm enabled the invention of a new multicomponent conceptual model of functional GI disorders, involving physiological, affective, cognitive and behavioural factors Citation1 (). The brain–gut axis is a theoretical model describing the bidirectional neural pathways linking cognitive and emotional centres in the brain to neuroendocrine centres, the enteric nervous system and the immune system Citation7. The visceral hypersensitivity as well as the high occurrence of psychiatric symptoms, in particular those of affective dysregulation, fit well into this concept Citation4, Citation8, Citation9. In addition, psychosocial stressors play a prominent role in symptom generation Citation10 Citation11 Citation12 Citation13. The high rates of affective dysregulation in patients with functional GI disorders (up to 90% in IBS patients consulting a gastroenterologist) may be a specific and integral part of IBS, rather than a non-specific co-morbid syndrome related to a chronic GI disorder Citation14. It should be mentioned that such a holistic pathophysiological concept is rare in the traditionally dichotomic medical society (somatic versus psychiatric explanatory models).
Fig. 1 The “brain–gut axis” as an integrated part of a comprehensive conceptual model of irritable bowel syndrome (IBS). [Adapted from Ringel et al. Citation6.]
Brain–gut axis communication routes and nutritional intervention
The question arises as to whether nutrition may play a role in the (patho)physiology of the brain–gut axis and, consequently, whether diet can positively affect gut health by modifying physiological processes within the brain–gut concept. To expand our knowledge about this issue not only is more insight into the role of the brain–gut axis in dysregulation of GI function important, but the development of relevant biomarkers of brain–gut axis physiology and GI function is also of pivotal importance.
Based on the existing knowledge on the physiology of the brain–gut axis and the available hypotheses about how diet may interact with interorgan signalling routes, the following communication routes and modulators of the brain–gut axis were selected as the primary points of action for nutritional intervention, as well as for the development of relevant biomarkers of brain–gut function:
bioamines, especially serotonin (5-hydroxytryptamine, 5-HT)
the hypothalamic–pituitary–adrenal (HPA) axis
long-chain polyunsaturated fatty acids (LC-PUFAs) and associated inflammatory cytokines
gut hormones.
Autonomic nervous system (especially with regard to the development of biomarkers)
Serotonin
Serotonin (5-HT) is a biogenic amine that acts as a neurotransmitter and paracrine signalling molecule. It is peripherally involved, along with other factors, in the regulation of GI secretion, motility and perception, whereas it plays a role in the regulation of mood, appetite, sleep, cognition and sexual behaviour in the central nervous system (CNS). Most of the total body 5-HT is located in the GI tract (80%). The remainder is located in the CNS and blood platelets. Serotonin is synthesized from the essential amino acid tryptophan by two enzymic steps Citation15. Nearly all of the 5-HT in the blood is derived from the GI tract Citation16, and blood platelets avidly accumulate 5-HT from the plasma Citation17. The metabolism of 5-HT is rather complex and differs between the CNS and the periphery. Manipulation of serotonergic activity using 5-HT modulators, such as antidepressants, has been used in the treatment of both affective disorders and IBS Citation14 Citation18Citation19Citation20. Hence, 5-HT should be regarded as a key denominator of the brain–gut axis Citation14, Citation21. Disturbed serotonergic metabolism seems especially prevalent in the diarrhoea-associated alterations of intestinal function, as well as in visceral hypersensitivity, the key feature of IBS.
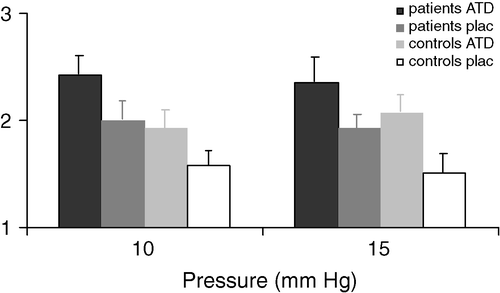
As tryptophan is the substrate for 5-HT, the question arises as to whether serotonergic metabolism and activity can be modulated by dietary means. It was recently demonstrated that acute lowering of 5-HT synthesis, using acute tryptophan depletion, was associated with altered brain–gut responses, i.e. enhanced visceral perception and impaired memory performance in diarrhoea-predominant IBS patients and healthy controls Citation22 (). This result could be regarded as a proof of principle that 5-HT activity can be modulated by nutritional means. However, increased central serotonergic activity, rather than tryptophan depletion, is regarded as advantageous with regard to brain–gut regulation, and could result in improved mood, cognition and visceral sensitivity. A simple increase in tryptophan intake will not result in the desired modulation of the brain–gut axis, because of the complex metabolic route of tryptophan and 5-HT and the regulation of its transport through the blood–brain barrier Citation23Citation24Citation25. In general, a systemically high tryptophan concentration in the presence of a low concentration of long-neutral amino acids (LNAAs) is regarded as beneficial to stimulate tryptophan transfer over the blood–brain barrier and consequently promote 5-HT synthesis. The blood concentration of LNAAs can be decreased by the anabolic action of insulin. In relation to 5-HT and stress resistance, it has been shown that dietary intervention can modify 5-HT metabolism and activity Citation26.
Fig. 2 Pressure-urge scores (mean±SEM) during intermittent pressure distension of the rectum in diarrhoea-predominant irritable bowel syndrome patients and control subjects, during acute tryptophan depletion (ATD) and placebo. ATD was significantly associated with increased urge scores compared with placebo (p<.0001) Citation22.
There is an important interindividual variation in the response to alterations of serotonergic metabolism in subjects with affective disorders. It has been assumed that these differences are predominantly of genetic origin and determined by gene polymorphisms. Such polymorphisms encoding for serotonin reuptake transporters (SERT) are selected as potential candidates Citation27, Citation28. Recently, a polymorphism encoding for the enzyme tryptophan hydroxylase-2 (Tph-2), which controls 5-HT production in the brain, has been identified. The mutated gene variant, changing the enzyme by a single amino acid, is associated with a very poor response to antidepressive treatment Citation29. Studies related to dietary intervention of serotonergic metabolism and action should take genetic heterogeneity into account.
It can be concluded that dietary intervention directed towards improvements in 5-HT regulation and abdominal comfort is feasible. However, initial efforts should investigate the question of how diet can induce a prolonged alteration in serotonergic activity at both CNS and peripheral level and how this affects GI function according to the brain–gut paradigm.
Dietary modulation of the metabolism and action of other biogenic amines, such as dopamine and norepinephrine, has not yet been systematically studied with regard to the brain–gut axis. Dopamine receptors are present in both the brain and the gut, and further studies with respect to dietary modulation and GI function are awaited.
Hypothalamic–pituitary–adrenal axis
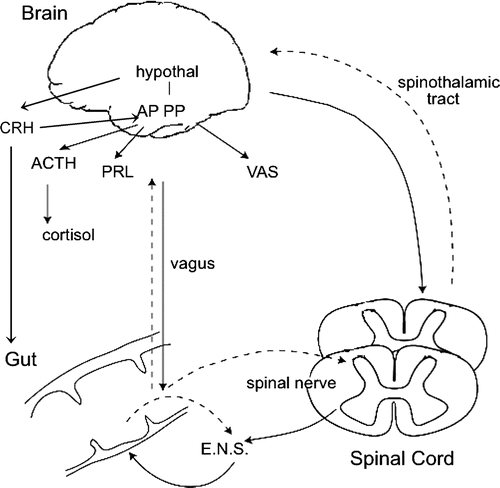
The HPA axis is the primary regulatory system of the body that copes with stress in a comprehensive sense, including physical, mental and metabolic (e.g. inflammation-related) stress Citation30. This (neuro)endocrine system regulates numerous metabolic routes and processes and is intimately connected with other regulatory mechanisms such as the autonomic nervous system (). Stimulation of serotonergic neurotransmission induces HPA axis-mediated neuroendocrine responses, which also offer the possibility for the development of biomarkers of serotonergic response. Furthermore, the HPA axis itself is important in brain–gut interaction. It has been shown that physiological and psychological stress-induced release of corticotropin-releasing hormone (CRH) from the hypothalamus is a main contributor to the stress-associated alterations in intestinal function, such as enhanced GI motility, decreased mucosal integrity resulting in impaired intestinal barrier function, and visceral hypersensitivity Citation32Citation33Citation34Citation35. The CRH release triggers, among other things, mast cell degranulation, with the release of histamine, tryptase, serotonin and substance P. This causes inflammatory reactions involving a complex cross-talk between mast cells and nerves with tryptase, substance P and nerve growth factor (NGF) as dominant players (neurogenic inflammation) Citation36Citation37Citation38. Recently, this CRH-associated mast cell release has been considered to be an important feature in the pathophysiology of IBS Citation39, Citation40 and offers another lead in the understanding of the co-morbidity of GI dysfunction and disorders of mood and anxiety regulation. Furthermore, this concept enables additional understanding of the pathophysiology of postinfectious IBS and the connection between IBS and inflammatory bowel disease (IBD).
Fig. 3 The hypothalamic–pituitary–adrenal axis integrated in the brain–gut axis concept. Neural control of visceral perception (gut sensation) and motility occurs at three primary levels: (a) the enteric nervous system (ENS) (local) of the gut, (b) the spinal cord and (c) the brain, integrated by the autonomic nervous system. Dysregulation at any level could explain the hypersensitivity and abnormal motor patterns seen in irritable bowel syndrome. [Adapted after Mertz Citation5.] Release of the hormones cortisol [mediated via the release of adrenocorticotropic hormone (ACTH)] and prolactin (PRL) is partly under the control of 5-hydroxytryptamine innervation from nerve terminals originating in the raphe nuclei that innervate the hypothalamus Citation31. Stress-induced secretion of CRH directly affects the gut by destabilizing mast cells. Hypothal: hypothalamus; AP: anterior pituitary; PP: posterior pituitary; VAS: vasopressin; CRH: corticotropin-releasing hormone.
Attenuation of stress-associated CRH release, increasing the stability of mast cells, or attenuating the effect of mast cell degranulation would be the preferable option with respect to functional bowel disorders. So far, it has not been shown that CRH release can be modified by dietary intervention. However, dietary modulation of HPA responses in general may be achieved by amino acids, peptides or proteins Citation41. Nutrition-induced stabilization of mast cells and attenuation of the effect of degranulation are under investigation by the present group and seem conceivable. However, implementation of this research from the bench into dietary practice is not expected within the next couple of years.
Long-chain polyunsaturated fatty acids
The involvement of LC-PUFAs, especially the n-3 and n-6 fatty acids, is based on the fact that these fatty acids and their metabolites are involved in local and systemic inflammatory responses, as well as in brain development and function.
The following propositions illustrate this involvement in the brain–gut axis and offer leads for the development of dietary intervention strategies:
There is a high prevalence of mood disorders in functional bowel disorders Citation14.
Mood disorders are bilaterally associated with a proinflammatory cytokine profile Citation42Citation43Citation44.
There is increasing evidence that several functional GI disorders are associated with chronic low-grade inflammation Citation45, Citation46.
Inflammatory cytokine profiles can be modified by LC-PUFA intake Citation47.
LC-PUFA intake is associated with the incidence and prevalence of mood disorders Citation48.
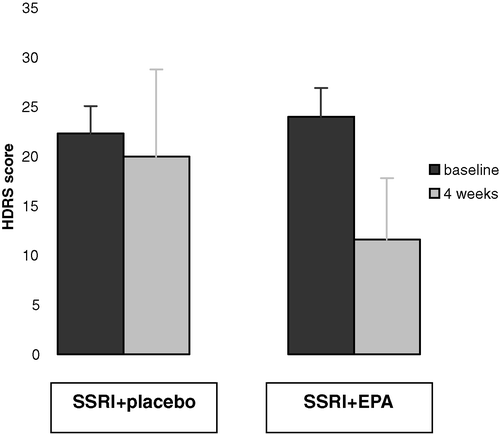
Oral administration of specific LC-PUFAs may have additional value in the treatment of depression Citation48, Citation49 ().
Chronic stress has a strong pathophysiological similarity with depression Citation50, Citation51 and is associated with a proinflammatory cytokine profile Citation52.
Chronic stress is associated with impaired mucosal integrity and intestinal barrier function in animal models Citation34.
Impaired intestinal barrier function may be causally related to postinfectious functional GI disorders and even Crohn's disease Citation45, Citation53, Citation54.
Fig. 4 Oral supplementation with eicosapentaenoic acid (EPA) (20:5 n-3) (right) enhances the effect of treatment with selective serotonergic reuptake inhibitors (SSRI) compared with placebo supplementation (left) in patients with recurrent unipolar depression. [Adapted after Nemets et al. Citation49.] HDRS: Hamilton Depression Rating Scale.
Hence, it can be postulated that a specific LC-PUFA intake, through its possible synergistic mood-enhancing effects and anti-inflammatory properties, may beneficially affect intestinal function. However, many issues remain to be solved. The systemic and brain bioavailability of fatty acids is of pivotal importance for their bioactivity. The specific fatty acid composition of dietary intervention should be determined against the background that eicosapentaenoic acid (EPA) (20:5 n-3) is generally considered a potent anti-inflammatory agent, while docosahexaenoic acid (DHA) (22:6 n-3) has specific effects on brain development and function (personal communication). Recently, it became apparent that the interaction between LC-PUFAs and cytokines is associated with a number of gene polymorphisms, which also may explain the variation in anti-inflammatory response in various intervention trials Citation47, Citation55. This knowledge offers the development of personalized dietary advice with regard to anti-inflammatory action. However, this comprises only one pathway of the possible beneficial bioactivity of LC-PUFAs on GI function.
The metabolism of LC-PUFAs is also related to immunological responses and to alterations in monoaminergic neurotransmission in the CNS, underlining the complexity of the brain–gut axis Citation56, Citation57.
Gut hormones
The physiological effect of a number of gut hormones is not restricted to the GI tract in a strict sense. Examples are ghrelin, cholecystokinin (CCK), neuropeptide Y (NPY) and peptide YY (PYY), which contribute to brain–gut signalling, but predominantly are considered to be associated with the regulation of food intake Citation58Citation59Citation60. It is well known that the intraluminal composition of the various segments of the gut and, hence, dietary composition heavily affect the secretion of these hormones. Whether this also affects brain–gut function with regard to functional GI disorders is largely unresolved. Interesting in this respect are observations that intraduodenal lipid infusion was able to modify visceral perception from colonic stimuli Citation61. These changes were more pronounced in IBS patients than in healthy controls, which may contribute to the explanation of the postprandial symptoms in IBS patients. One could speculate that separate influences on brain function may modify the central responses to the release of gut hormones. More research is needed to elucidate the role of gut hormones in brain–gut signalling and how this can be modified by diet. The example of ghrelin has shown that gut hormones may use an unexpected route (i.e. the somatotrophic axis) to achieve their biological effect.
Autonomic nervous system
The autonomic nervous system offers a major route in brain–gut communication and hence may yield important biomarkers of brain–gut signalling, especially in efferent pathways. Together with the CNS and the enteric nervous system it forms a highly integrated and complex regulatory system, as illustrated by the aberrations in GI function caused by the various neuropathies Citation62. The autonomic nervous system is intimately connected to other regulatory pathways within the brain–gut axis, such as the HPA axis and the serotonergic system.
The non-invasive and minimally invasive measurement of autonomic nervous system parameters has progressed substantially in recent years, and is applied most often in cardiovascular and metabolic research Citation63Citation64Citation65. The determination of autonomic nervous system parameters should be seen in relation to biomarkers of brain function and brain–gut interaction offering pathophysiological knowledge of the brain–gut axis. It is unlikely that the autonomic nervous response itself – separate from alterations in brain or intestinal stimuli – could be affected by dietary intervention in the near future.
Conclusion
The concept of the brain–gut axis offers a novel approach for the development of dietary intervention strategies with regard to functional GI disorders. Such a holistic approach necessitates a transdisciplinary approach involving, among others, nutritionists, gastroenterologists, neuroscientists and psychologists. Although results directly transferable to daily practice are not yet expected, this review substantiates the view that the novel approach may yield important breakthroughs in the dietary treatment of functional bowel disorders contributing to an improved quality of life in this large group of people. Progress may be hampered by the lack of availability of validated and applicable biomarkers of brain and gut function, as well as interindividual variation in brain–gut responses to dietary intervention.
References
- TalleyNJ, SpillerR. Irritable bowel syndrome: a little understood organic bowel disease? Lancet 2002 360 555–64.
- ThompsonWG, LongstrethGF, DrossmanDA, HeatonKW, IrvineEJ, Muller-LissnerSA. Functional bowel disorders and functional abdominal pain Gut 1999 45 Suppl 2 II43–7
- AzpirozF. Hypersensitivity in functional gastrointestinal disorders Gut 2002 51 Suppl 1 i25–8
- DelvauxM. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome Gut 2002 51 Suppl 1 i67–71
- MertzH. Review article: visceral hypersensitivity Aliment Pharmacol Ther 2003 17 623–33
- RingelY, SperberAD, DrossmanDA. Irritable bowel syndrome Annu Rev Med 2001 52 319–38
- MayerEA, GebhartGF. Basic and clinical aspects of visceral hyperalgesia Gastroenterology 1994 107 271–93
- WalkerEA, Roy-ByrnePP, KatonWJ, LiL, AmosD, JiranekG. Psychiatric illness and irritable bowel syndrome: a comparison with inflammatory bowel disease Am J Psychiatry 1990 147 1656–61
- DunlopSP, JenkinsD, NealKR, SpillerRC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS Gastroenterology 2003 125 1651–9
- NoyesR Jr, CookB, GarveyM, SummersR. Reduction of gastrointestinal symptoms following treatment for panic disorder Psychosomatics 1990 31 75–9
- WalkerEA, KatonWJ, JemelkaRP, Roy-BrynePP. Comorbidity of gastrointestinal complaints, depression, and anxiety in the Epidemiologic Catchment Area (ECA) Study Am J Med 1992 92 1A 26–30S
- ZaublerTS, KatonW. Panic disorder and medical comorbidity: a review of the medical and psychiatric literature Bull Menninger Clin 1996 60 2 Suppl A A12–38
- LydiardRB. Anxiety and the irritable bowel syndrome: psychiatric, medical, or both? J Clin Psychiatry 1997;58 Suppl 3:51–8; Discussion 59–61
- KilkensTO, HonigA, RozendaalN, Van NieuwenhovenMA, BrummerRJ. Systematic review: serotonergic modulators in the treatment of irritable bowel syndrome – influence on psychiatric and gastrointestinal symptoms Aliment Pharmacol Ther 2003 17 43–51
- TyceGM. Origin and metabolism of serotonin J Cardiovasc Pharmacol 1990 16 Suppl 3 S1–7
- LambertGW, KayeDM, CoxHS, VazM, TurnerAG, JenningsGL Regional 5-hydroxyindoleacetic acid production in humans Life Sci 1995 57 255–67
- Da PradaM, TranzerJP, PletscherA. Storage of 5-hydroxytryptamine in human blood platelets Experientia 1972 28 1328–9
- CreedF, FernandesL, GuthrieE, PalmerS, RatcliffeJ, ReadN The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome Gastroenterology 2003 124 303–17
- JacksonJL, O'MalleyPG, TomkinsG, BaldenE, SantoroJ, KroenkeK. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis Am J Med 2000 108 65–72
- ClouseRE. Antidepressants for irritable bowel syndrome Gut 2003 52 598–9
- KimDY, CamilleriM. Serotonin: a mediator of the brain–gut connection Am J Gastroenterol 2000 95 2698–709
- KilkensTO, HonigA, van NieuwenhovenMA, RiedelWJ, BrummerRJ. Acute tryptophan depletion affects brain–gut responses in irritable bowel syndrome patients and controls Gut 2004 53 1794–800
- PardridgeWM. Blood–brain barrier carrier-mediated transport and brain metabolism of amino acids Neurochem Res 1998 23 635–44
- O'KaneRL, HawkinsRA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier Am J Physiol Endocrinol Metab 2003 285 E1167–73
- RiedelWJ, SobczakS, SchmittJA. Tryptophan modulation and cognition Adv Exp Med Biol 2003 527 207–13
- MarkusCR, PanhuysenG, TuitenA, KoppeschaarH, FekkesD, PetersML. Does carbohydrate-rich, protein-poor food prevent a deterioration of mood and cognitive performance of stress-prone subjects when subjected to a stressful task? Appetite 1998 31 49–65
- SmitsKM, SmitsLJ, SchoutenJS, StelmaFF, NelemansP, PrinsMH. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: a systematic review Mol Psychiatry 2004 9 433–41
- CamilleriM. Is there a SERT-ain association with IBS? Gut 2004 53 1396–9
- ZhangX, GainetdinovRR, BeaulieuJM, SotnikovaTD, BurchLH, WilliamsRB Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression Neuron 2005 45 11–6
- CarrascoGA, Van de KarLD. Neuroendocrine pharmacology of stress Eur J Pharmacol 2003 463 235–72
- RaapDK, Van de KarLD. Selective serotonin reuptake inhibitors and neuroendocrine function Life Sci 1999 65 1217–35
- SantosJ, SaperasE, NogueirasC, MourelleM, AntolinM, CadahiaA Release of mast cell mediators into the jejunum by cold pain stress in humans Gastroenterology 1998 114 640–8
- TacheY, MartinezV, MillionM, WangL. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors Am J Physiol Gastrointest Liver Physiol 2001 280 G173–7
- SöderholmJD, PerdueMH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function Am J Physiol Gastrointest Liver Physiol 2001 280 G7–13
- MonnikesH, TebbeJJ, HildebrandtM, ArckP, OsmanoglouE, RoseM Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity Dig Dis 2001 19 201–11
- RaithelM, WinterkampS, PacurarA, UlrichP, HochbergerJ, HahnEG. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease Scand J Gastroenterol 2001 36 174–9
- VergnolleN, BunnettNW, SharkeyKA, BrusseeV, ComptonSJ, GradyEF Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway Nat Med 2001 7 821–6
- BielefeldtK, OzakiN, GebhartGF. Role of nerve growth factor in modulation of gastric afferent neurons in the rat Am J Physiol Gastrointest Liver Physiol 2003 284 G499–507
- MayerEA, NaliboffBD, ChangL, CoutinhoSV. V. Stress and irritable bowel syndrome Am J Physiol Gastrointest Liver Physiol 2001 280 G519–24
- BarbaraG, StanghelliniV, De GiorgioR, CremonC, CottrellGS, SantiniD Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome Gastroenterology 2004 126 693–702
- MarkusCR, OlivierB, PanhuysenGE, Van Der GugtenJ, AllesMS, TuitenA The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress Am J Clin Nutr 2000 71 1536–44
- SubletteME, RussMJ, SmithGS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review Bipolar Disord 2004 6 95–105
- AnismanH, MeraliZ, PoulterMO, HayleyS. Cytokines as a precipitant of depressive illness: animal and human studies Curr Pharm Des 2005 11 963–72
- SchiepersOJ, WichersMC, MaesM. Cytokines and major depression Prog Neuropsychopharmacol Biol Psychiatry 2005 29 201–17
- CollinsSM, VallanceB, BarbaraG, BorgaonkarM. Putative inflammatory and immunological mechanisms in functional bowel disorders Baillieres Best Pract Res Clin Gastroenterol 1999 13 429–36
- SpillerRC. Inflammation as a basis for functional GI disorders Best Pract Res Clin Gastroenterol 2004 18 641–61
- GrimbleRF, HowellWM, O'ReillyG, TurnerSJ, MarkovicO, HirrellS The ability of fish oil to suppress tumor necrosis factor alpha production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor alpha production Am J Clin Nutr 2002 76 454–9
- YoungG, ConquerJ. Omega-3 fatty acids and neuropsychiatric disorders Reprod Nutr Dev 2005 45 1–28
- NemetsB, StahlZ, BelmakerRH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder Am J Psychiatry 2002 159 477–9
- McEwenBS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders Ann N Y Acad Sci 2004 1032 1–7
- SherL. Type D personality: the heart, stress, and cortisol QJM 2005 98 323–9
- SongC, KenisG, van GastelA, BosmansE, LinA, de JongR Influence of psychological stress on immune-inflammatory variables in normal humans. Part II. Altered serum concentrations of natural anti-inflammatory agents and soluble membrane antigens of monocytes and T lymphocytes Psychiatry Res 1999 85 293–303
- MayerEA, CollinsSM. Evolving pathophysiologic models of functional gastrointestinal disorders Gastroenterology 2002 122 2032–48
- SöderholmJD, StreutkerC, YangPC, PatersonC, SinghPK, McKayDM Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha Gut 2004 53 1817–24
- MarkovicO, O'ReillyG, FussellHM, TurnerSJ, CalderPC, HowellWM Role of single nucleotide polymorphisms of pro-inflammatory cytokine genes in the relationship between serum lipids and inflammatory parameters, and the lipid-lowering effect of fish oil in healthy males Clin Nutr 2004 23 1084–95
- HibbelnJR, LinnoilaM, UmhauJC, RawlingsR, GeorgeDT, SalemN Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics Biol Psychiatry 1998 44 235–42
- ChalonS, VancasselS, ZimmerL, GuilloteauD, DurandG. Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission Lipids 2001 36 937–44
- WilliamsDL, CummingsDE. Regulation of ghrelin in physiologic and pathophysiologic states J Nutr 2005 135 1320–5
- BloomS, WynneK, ChaudhriO. Gut feeling – the secret of satiety? Clin Med 2005 5 147–52
- RehfeldJF. Clinical endocrinology and metabolism. Cholecystokinin Best Pract Res Clin Endocrinol Metab 2004 18 569–86
- SimrenM, AbrahamssonH, BjörnssonES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome Gut 2001 48 20–7
- De GiorgioR, CamilleriM. Human enteric neuropathies: morphology and molecular pathology Neurogastroenterol Motil 2004 16 515–31
- KobayashiH, IshibashiK, NoguchiH. Heart rate variability; an index for monitoring and analyzing human autonomic activities Appl Human Sci 1999 18 53–9
- FagiusJ. Sympathetic nerve activity in metabolic control – some basic concepts Acta Physiol Scand 2003 177 337–43
- KleigerRE, SteinPK, BiggerJT Jr. Heart rate variability: measurement and clinical utility Ann Noninvasive Electrocardiol 2005 10 88–101