Abstract
Background
People with cognitive impairment often need support to perform everyday-life activities. Interventions are available, but evidence-based interventions are lacking.
Aim
This pilot RCT aimed to investigate use of an intervention with an interactive digital calendar with mobile phone reminders (RemindMe) in relation to change in outcomes and impact on occupational performance, independence, health-related quality of life, and psychosocial impact of the support for people with cognitive impairment.
Method
The study design was a multi-centre parallel-group pilot RCT [ClinicalTrails.gov, identifier: NCT04470219]. Fifteen participants from primary rehabilitation centres in Sweden were recruited and randomly assigned to intervention group (n = 8) receiving the intervention with RemindMe, or control group (n = 7) receiving usual treatment by an occupational therapist. Data were collected at baseline, after two- and four months, and analysed using descriptive and non-parametric statistics.
Result
The Canadian Occupational Performance Measure (COPM), and the Functional Independence Measure (FIM item n-r) showed significant differences. There were no significant differences in health-related quality of life nor in the psychosocial impact of the used support.
Conclusion
Plausible changes in outcome measures were found in COPM and FIM (items n-r). These instruments indicate change in outcome measures and impact on occupational performance and independence.
Introduction
People who have suffered a neurological injury or disease, such as stroke or traumatic brain injury, may develop cognitive impairments [Citation1,Citation2] that affect prospective memory or their ability to plan and structure everyday life i.e. knowing what to do and when to do it [Citation1,Citation3]. These functions are categorized in the ICF as ‘organization and planning’ and ‘time management’ [Citation4]. Activities that may become difficult to remember to perform are taking medication or keeping an appointment. Leisure activities may also become affected due to a reduced ability to initiate or plan activities. These problems in everyday life can indicate a need for support from others such as, family members [Citation5]; this reduces a person’s independence as well as having an impact on their perceived health-related quality of life [Citation6].
Recovery from a neurological impairment is complex. To some extent, the brain repairs itself, but full recovery is also dependent on rehabilitation interventions aimed at compensating for lost function. Interventions that appear to be most effective at improving cognitive ability are those based on activities in people’s natural environment together with coping strategies, and compensatory interventions such as, training in, and the use of assistive devices [Citation1,Citation7–9].
Interventions that compensate for lost cognitive ability often include the use of assistive technology for cognition [Citation10], which nowadays can be digital. Digital technology has the potential to support patients’ self-management, for example with personalized reminders [Citation11,Citation12]. Digital technology for people with cognitive impairment can be a specific device with a specific function, such as, a handheld computer [Citation13]. However, special devices can be experienced as stigmatizing [Citation12,Citation14]. Therefore, the development of mobile and smartphone digital technology is promising, as appropriate functions can be incorporated in a smartphone [Citation10,Citation11,Citation15]. This enables people with cognitive impairment to continue to use the technology they used prior to their injury, but with additional applications [Citation12]. These mainstream products improve the execution of everyday tasks and support people with cognitive impairments in everyday life activities. However, there is a lack of studies concerning their effectiveness for older people [Citation15].
To meet the need for a person-centred intervention with digital technology, for older people with cognitive impairment, an interactive digital calendar with mobile phone reminders (RemindMe) has been developed [Citation16]. RemindMe sends reminders using Short text Message Service (SMS). It has a unique feedback system that registers the user’s interaction with RemindMe and stores not only scheduled activities but also the user’s response to reminders [Citation16]. RemindMe consists of three core components: 1. The user schedules activities in a web-based calendar and the calendar sends a reminder SMS to the user’s mobile or smartphone; 2. The user actively confirms the reminder by answering the reminder SMS; 3. The feedback system registers the user’s interaction with the calendar by saving scheduled activities and confirmation answers [Citation16]. An intervention, using RemindMe and a predetermined procedure to provide training and weekly support in the use of RemindMe, has been tested for feasibility in patients’ real-life settings in three rehabilitation clinics. This has been perceived to be feasible by participating occupational therapists and by patients [Citation17].
Before moving to a large effectiveness trial, the Medical Research Council (MRC) recommends that interventions that include variables integrated in everyday practice are tested using a pilot study to explore strengths and weaknesses [Citation18]. Furthermore, the CONSORT group recommends conducting a pilot trial to investigate not only the effectiveness of the intervention, but also to evaluate the plausibility of the outcomes and measurement instruments [Citation19]. Studies have reported a lack of consensus of appropriate outcome measures to assess effects in everyday life of interventions for people with cognitive impairments [Citation7,Citation15,Citation20]. Therefore, the next step in the development of RemindMe and future digital intervention studies, is to explore outcomes and the plausibility of outcome measurements.
Aim
This pilot RCT aimed to investigate the use of an intervention with an interactive digital calendar with mobile phone reminders (RemindMe) in relation to change in outcomes and impact on occupational performance, independence, health-related quality of life, and psychosocial impact of the support for people with cognitive impairment.
Material and methods
Study design
The study was designed as a multi-centre parallel-group pilot randomized controlled trial [Citation19,Citation21] with an intervention group and a control group.
Participants and recruitment
Four primary care rehabilitation clinics in southeast Sweden (specializing in rehabilitation for people with neurological impairments) were informed from January to February 2016 of the study aim and procedure. The rehabilitation clinics accepted participation, and each clinic was estimated to have about 20 out-patients who could be eligible for participation. When the recruitment period started, one rehabilitation clinic could not participate due to their workload and one clinic participated one year later. For the pilot randomized controlled trial, the aim was to recruit 20 patients. The participants were consecutively recruited, by occupational therapists at the rehabilitation clinics from October 2016 until February 2018. Recruitment was based on the occupational therapists’ knowledge of the patients from their rehabilitation plan and the study’s inclusion and exclusion criteria. The inclusion criteria were patients who: (1) Had neurological disease/injury and who were experiencing a need for support in planning, organizing, and managing time in the activities of everyday life; (2) Had access to a computer and mobile phone/smartphone; (3) Had the linguistic ability to participate in the data collection. The exclusion criteria were patients who: (1) Had palliative care needs; (2) Had reduced vision and/or hearing loss that affects the ability to use a mobile phone/smartphone; (3) Had depression or psychiatric illness.
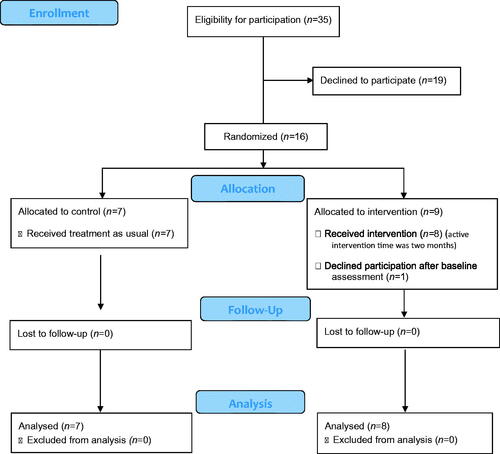
The occupational therapists informed patients about the study verbally and in writing, after which the patients gave their informed consent. Randomization took place after receiving informed consent. A total of 35 people were considered eligible by the recruiting occupational therapists ().
Procedure
Participants were randomly assigned using block-wise randomization to the intervention group or the control group. Randomization was performed at the rehabilitation centres. At each centre, a block of four sealed envelopes was stored, with two envelopes for the intervention group and two for the control group. When a participant was enrolled, they were randomized by allowing the participant to choose an envelope. Research personnel prepared the blocks so that the content of the envelopes was unknown to patients and personnel, and they updated the blocks as needed. The result of the randomization was not blinded for patients, occupational therapists, or research personnel.
Intervention group
Patients received usual treatment at the rehabilitation clinic together with an interactive digital calendar with mobile phone reminders (RemindMe).
RemindMe serves as a support for prospective memory as well as for planning and structuring everyday life. Work-related activities were not included in this study. In the intervention’s first session, the patient was given access to RemindMe and a member of the research team (occupational therapist) gave training in how to use RemindMe to the participant and, if required, to significant others. A user-friendly, written manual was given to the participants. Together with the occupational therapist, the participant chose activities for which he/she wished to receive reminders, and they established when to receive them. The patient could choose a support person with access to the calendar, which could be the treating occupational therapist or a significant other. The first session lasted about 90 min. Individual follow-up conversations were conducted at the rehabilitation clinic or by telephone once a week for two months by the occupational therapist or by a research assistant. At the weekly conversations, the participants were provided with support on further use of RemindMe and strategies for the coming week. After two months the participants decided whether they wanted to continue using RemindMe, and the weekly conversations stopped [Citation17]. The first two months of the intervention were the active intervention period. After that patient could choose to use or not use RemindMe according to the intention-to-treat approach [Citation22].
Control group
Patients received usual treatment by an occupational therapist at the rehabilitation clinic. Usual treatment included interventions that provided support in prospective memory as well as planning and structuring everyday life, such as weekly schedules and use of a paper calendar, digital calendars in smartphones, or other strategies. During the first two months, the participants in the control group were asked to use one specific calendar of their own choosing that they were accustomed to and used regularly. Four (4/7) participants chose to use a wall calendar and three (3/7) participants chose to use a calendar on their smartphone. The calendar was intended to provide a support in planning, organizing, and managing time in the activities of everyday life, and the calendar would serve as a comparison for the use of RemindMe.
In the period from the two-month follow-up session to the four-month follow-up session, the participants both in the intervention- and the control group decided which calendar they preferred to use.
Data collection and measurement
Data were collected by a member of the research team (occupational therapist) at baseline and after two and four months (). All data collection followed a predetermined protocol, with assessments performed by an occupational therapist in the same order and using the same instructions. Assessment sessions lasted 45–60 min, with a short break (5 min) after about 25 min, so that the assessment session would not be too exhausting to conduct. Selection of measurements on occupational performance, independence, health-related quality of life and Remind Me’s psychosocial impact were based on instruments used in clinical practice with good psychometric characteristics for people with cognitive impairment. The chosen instruments are expected to measure the impact of the intervention with RemindMe in various aspects on everyday life [Citation23–26].
Table 1. Overview of data collection.
Participant demographics
Background characteristics
Demographic data from the participants’ medical records were collected by the occupational therapist at the rehabilitation clinic during the baseline assessment. At baseline, participants filled in a questionnaire about their computer and mobile or smartphone skills and calendar use.
Cognitive ability
To measure various aspects of participants’ cognitive ability, two tests were conducted:
Subject-performed task (SPT) is a test of episodic long-term memory. In SPT the participant is shown 16 subjects and verbs and is asked to remember as many subjects and verbs as possible. The participant recalls as many verbs and subjects as possible for two minutes [Citation27].
Trail making test (TMT) is a test of executive function and consists of two parts: A and B. The TMT -index shows the time-difference to complete TMT-A compared with TMT-B. A higher index indicates more difficulty with cognitive ability [Citation28].
Outcome measures
Occupational performance
The Canadian Occupational Performance Measure (COPM) was used to measure occupational performance, i.e. COPM was used to assess participants’ perceptions of performance and satisfaction with performance. Patients identified activities in everyday life, related to cognitive impairment (memory and executive functioning – e.g. plan, structure, initiative) that were perceived as important, and their performance and satisfaction with their performance was measured. The assessment was made on a ten-point scale, from one ‘= not able to do/not satisfied at all’ to ten ‘= able to do it extremely well/extremely satisfied’. A two-point difference is considered a clinically significant difference [Citation23]. In this study, the focus was on activities that the participants experienced as difficult to perform due to cognitive impairment; for example, activities that the participant forgot to do or expressed a wish to perform. Examples of identified activities were remembering to take medication, attending planned appointments, planning and structuring instrumental- ADL (cooking, laundry, shopping), and initiating leisure activities. Activities related to physical abilities such as, opening a bottle, buttoning a shirt, walking without a cane, were excluded. COPM is usable in rehabilitation and has good sensitivity and good reliability [Citation23].
Independence
Independence was measured using the Functional Independence Measure (FIM) [Citation24]. FIM identifies the degree of independence in activities of daily living (ADL), transportation/mobility, communication, social interaction, and cognition on a seven-point scale. A higher number indicates more independence. FIM is a common outcome measure and has good psychometric characteristics [Citation24].
Health-related quality of life
Health-related quality of life was measured using the EQ-5D-VAS [Citation26] where participants rate their perception of health from 0 ‘=the worst health you can imagine’ to 100 ‘= the best health you can imagine’. EQ-5D has good validity and reliability for people with stroke [Citation29].
Psychosocial impact of the support used
At the assessments after two and four months, the participants assessed the perceived psychosocial impact of the used support by the Psychosocial Impact of Assistive Devices Scale (PIADS) which relates to 26 different aspects [Citation25]. The aspects are divided into three subscales: Competence, Adaptability, and Self-esteem. The score ranges from −3, indicating a negative effect, to 3, indicating a positive effect. 0 indicates that the participant does not perceive any psychosocial effect of the support. PIADS has shown good psychometric qualities [Citation25] and clinical relevance [Citation30].
Statistical analysis
IBM Statistical Package for the Social Sciences version 25 (SPSS, Chicago, Illinois) was used in all statistical analyses. We analysed data according to the intention-to-treat approach [Citation22]. Data analysis of baseline data for participant demographics (background characteristics, use of digital technology and calendars at baseline, and cognitive ability) was performed using descriptive statistical analysis. Continuous variables are presented with median, range, and standard deviation (SD), and categorical variables are described in numbers. Ordinal scale variables were analysed with non-parametric statistics: The Mann-Whitney U test was used to analyse differences between the intervention and control group (on COPM) and the Wilcoxon signed-rank test were used to analyse differences between the baseline and four-months assessments within the intervention group and within the control group (on FIM, EQ-5D-VAS and PIADS). The results will be interpreted in relation to if they are significant (alpha level of 0.05 used) and the magnitude of the effect size. Cohen’s rules of thumb for small (0.1 < r < 0.03), medium (0.3 < r < 0.05), and large (0.5 < r) will be used [Citation31].
Ethics
The project received ethical approval from the Regional Ethical Review Board in Linköping study code 2016/145-31, and 2018/263-32. Participation in the study was voluntary and participants could withdraw at any time without providing a reason. The patients were informed that the support person/research personnel had access to the calendar for as long as the participant agreed.
Trial registration
The study was registered through ClinicalTrails.gov, identifier: NCT04470219
Result
Baseline data for participant demographics
Background characteristics
Sixteen people were interested in participation, they received written and oral information and gave informed consent. The sixteen participants were randomized to either a control group (n = 7) or an intervention group (n = 9). One participant in the intervention group declined further participation after baseline assessment and is not included in the analyses. Therefore, the intervention group consists of six men and two women, with a median age of 58 (range 26–68, SD 13). The control group consisted of six men and one woman with a median age of 65 (range 54–79, SD 10). Most participants’ highest educational level was secondary school (10/15). The most common diagnosis were stroke (6/15) and traumatic brain injury (TBI) (4/15). There were no significant differences between the groups. Demographic data are presented in .
Table 2. Participant characteristics at baseline N = 15.
Use of digital technology and calendars at baseline
Thirteen participants (13/15) knew how to use a computer before the study and ten participants (10/15) used the internet daily. Thirteen participants (13/15) owned a smartphone and used it daily. Two participants (2/15) owned a mobile phone and used it a few times a week. A wall calendar (8/15) or a smartphone calendar (8/15) was most used for time management support. gives a detailed description of the participants’ use of digital technology and calendars.
Table 3. Description of participants’ use of digital technology and calendar use before and during the study N = 15.
Cognitive ability
In terms of cognitive ability, there were slight differences between patients in the intervention group compared with patients in the control group. Patients in the intervention group had fewer impairments in executive function (Trail making test, TMT) as well as in episodic long-term memory (Subject Performed Task, SPT) than patients in the control group. However, the differences were not significant.
Outcome measures
Occupational performance (COPM)
In the COPM subscale for assessing occupational performance, the Mann-Whitney test showed that there was no significant difference between the intervention and control groups at baseline or after two months. However, after four months the intervention group rated (Mdn = 8.04) their occupational performance significantly higher than the control group (Mdn = 5.00) with a large effect size (U = 5.00, z = −2.67, p = 0.006, r = −0.69). In both groups, there was an increase in occupational performance from baseline (intervention group Mdn = 5.13, control group Mdn = 2.67) to two months (intervention group Mdn = 6.75, control group Mdn = 7.00), which persisted in the intervention group while the perception of occupational performance of the control group decreased and was no longer significantly higher than baseline, see for more details.
Table 4. Self-assessed perception of occupational performance and satisfaction performance with The Canadian Occupational Performance Measure (COPM).
In the COPM subscale for assessing satisfaction with occupational performance, there was no significant difference between the intervention and control groups at baseline or after two months. However, after four months the intervention group assessed (Mdn = 7.75) their satisfaction with occupational performance as being significantly higher than the control group (Mdn = 3.33) with a large effect size (U = 5.50, z = −2.61, p = 0.006, r = −0.67). In both groups, there was an increase from baseline (intervention group Mdn = 4.00, control group Mdn = 2.67) to two months from baseline (intervention group Mdn = 7.50, control group Mdn = 6.17), which persisted in the intervention group while the satisfaction with occupational performance of the control group decreased and was no longer significantly higher than baseline, see for more details.
Independence (FIM)
The first part of FIM assesses a patient’s independence with performing personal care (getting dressed, hygiene, eating) and mobility. Both intervention and control groups had high scores, which indicates that participants in both groups were independent in these activities. No significant differences were found between the groups at any time point. A Wilcoxon signed-rank test indicated that there was no significant difference (small effect size) between baseline assessment (Mdn = 89.5) and assessment after four months (Mdn = 90) in the intervention group, (T = 3.00, z = −0.730, p = 0.456, r = −0.258) nor in the control group between baseline assessment (Mdn = 88) and assessment after four months (Mdn = 89) even if the effect size was large, (T = 6.00, z = 1.604, p = 0.109, r = 0.563), see for more details.
Table 5. Self-assessed independence with the Functional Independence Measure (FIM), subscale, a-m and subscale n-r and self-assessed health-related quality of life with the EQ-5D-VAS at each time point.
In the second part of FIM, independence in communication (understanding and expression) and social- and intellectual abilities (social interaction, problem-solving, memory) were measured, and several participants in both groups needed support from another person. No significant differences were found between the groups at any time point. A Wilcoxon signed-rank test indicated that there was a significant difference between baseline assessment (Mdn = 29) and assessment after four months (Mdn = 32.5) with a large effect size in the intervention group, (T = 28.00, z = 2.388, p = 0.017, r = 0.844). There was no significant difference (small effect size) in the control group between baseline assessment (Mdn = 30) and assessment after four months (Mdn = 32), (T = 9.00, z = 0.406, p = 0.684, r = 0.153), see for more details.
Health-related quality of life (EQ-5D-VAS)
There were no significant differences between the groups at any time point. A Wilcoxon signed-rank test showed that for the intervention group health-related quality of life was not significantly higher after four months (Mdn = 70) compared with baseline (Mdn = 67.5) and the effect size was minimal, (T = 12.50, p = 0.796, r = −0.067). However, for the control group the perception of health-related quality of life was significantly higher after four months (Mdn = 58) compared with baseline (Mdn = 50) with a large effect size (T = 15.00, p = 0.042, r = 0.525), see for more details.
Psychosocial impact of the support used (PIADS)
The PIADS assessment includes three subscales: competence, adaptability, and self-esteem. A Wilcoxon signed-rank test was performed for each subscale. The perception of competence is similar in both the intervention and control groups. Indicating that both groups self-assessed that the support used gave a perception of competence. No significant differences were found between the groups at any timepoint. The Wilcoxon signed-rank test indicated that there were no significant differences (small effect sizes) in the subscale of competence for the intervention group between assessment at two months (Mdn = 0.96) and four months (Mdn = 0.96), (T = 10.50, z = 0.813, p = 0.416, r = 0.287), nor for the control group between assessment at two months (Mdn = 0.66) and four months (Mdn = 0.83), (T = 12.00, z = −0.338, p = 0.735, r = −0.128). The intervention group increased their perceptions of their adaptability and self-esteem while the control group’s scores decreased. In the subscale of adaptability, the Wilcoxon signed-rank test indicated that there were no significant differences (minimal effect size) for the intervention group between assessment at two months (Mdn = 0.22) and four months (Mdn = 0.46), (T = 10.00, z = −0.106, p = 0.916, r = −0.037), nor for the control group between assessment at two months (Mdn = 0.83) and four months (Mdn = 0.50), even if the effect size was large (T = 0.00, z = −1.826, p = 0.068, r = −0.690). For the subscale of self-esteem, the Wilcoxon signed-rank test indicated that there were no significant differences (small and medium effect sizes) for the intervention group between assessment at two months (Mdn = 0.82) and four months (Mdn = 0.75), (T = 5.50, z = −0.544, p = 0.586, r = −0.192), nor for the control group between assessment at two months (Mdn = 0.50) and four months (Mdn = 0.38), (T = 4.00, z = −0.948, p = 0.343, r = −0.358) see for more details.
Table 6. Self-assessed perceived psychosocial impact of the support used by the Psychosocial Impact of Assistive Devices Scale (PIADS), subscales: Competence, Adaptability, Self-esteem at 2 months and, 4 months assessment.
Discussion
There is a lack of research evidence that describes the effect of interventions using digital technology [Citation7,Citation15,Citation20]. However, there are clinical reports that digital interventions work well for people with cognitive impairment with increased prospective memory as well as executive functioning [Citation7–9]. Well-designed RCT studies are needed to fill this knowledge gap [Citation32] and the present pilot study aims to identify plausible assessment methods. Research conducted with people suffering from cognitive impairment and mental fatigue must use plausible assessments that assess important aspects while at the same time are not exhausting for the participants. In this pilot study, we chose several instruments that measure outcomes that are important in everyday life for patients with cognitive impairment and which can be expected to change after using an interactive digital calendar with mobile phone reminders (RemindMe). The outcome measures of the instruments focus on activity and participation, and not on body functioning [Citation4]. It is vital to choose outcome measures that allow the real effects of compensatory rehabilitation to be measured.
Firstly, the COPM [Citation23] clarifies patient-perceived need, and thus supports person-centred care so that treatment is based on the patient’s perceived need. This is likely to increase a patient’s motivation to adhere to the treatment protocol [Citation33]. In this pilot study, we found that participants in both intervention and control groups increased their perception of their ability to perform activities in everyday life as well as their satisfaction with their performance. The improvements could be a result of spontaneous recovery [Citation8] for some participants. Both groups received usual treatment in their rehabilitation programme which could thereby improve occupational performance. For the intervention group, the impact on satisfaction with the performance remained higher after four months compared to the control group. This might be because the intervention included repeated support for two months, with a possible role of habit-forming [Citation34]. It might also be due to the individualized reminders that alert the participant instead of passive reminders (for example paper calendars, notes), such as those used by some participants in the control group. This would be in line with previous research that has advocated for active support for people with prospective memory deficits [Citation15,Citation35].
Secondly, we used FIM [Citation24] to assess participants’ independence in everyday life. There were no significant differences in independence (FIM) between the two groups. Looking more closely at this instrument, the first part of FIM (items a-m) assesses independence in personal care and mobility. The participants in this study were already independent in these activities and change was thus not expected. Use of this part of the FIM is relevant for participants with reduced motor function [Citation36] and its use with the present population may be questioned. However, the second part of the FIM (items n-r) is important, and vital, as it measures independence in communication and social- and intellectual abilities [Citation24]. The intervention group increased their independence in these items. These items are relevant to study as the results in this pilot study show that participants are less independent in these areas. Due to the small sample size, it is not possible to draw any conclusions and should therefore be further investigated. This is supported by Branco et al. who propose the use of FIM when investigating stroke rehabilitation outcomes in real-world settings [Citation37] and Kettlewell et al. [Citation20] that point out independence as a relevant outcome measure for people with cognitive impairment.
Thirdly, the EQ-5D-VAS [Citation26] was used to measure the participants’ perception of health-related quality of life. EQ-5D-5L also consists of the five dimensions: mobility, personal care, common activities, pain/discomfort, and anxiety/depression. However, these items overlap with some of the items (a-m) in FIM [Citation24] and EQ-5D-5L assesses neither communication nor memory impairment [Citation29]. In the present study, the items in FIM give a more relevant description of the participants than EQ-5D-5L. It is necessary to consider which assessment is relevant to use regarding the study aim. Brandt et al. [Citation15] conclude that there is a lack of studies investigating the cost-effectiveness of digital technology for people with cognitive impairments. To conduct this kind of study EQ-5D-5L might be a plausible assessment to use [Citation29] preferably in combination with FIM (items n-r) which measures independence in communication, and social- and intellectual abilities [Citation24].
Fourthly, we used the PIADS [Citation25] to measure the psychosocial impact of the support used. Results show an interesting difference in the subscale of adaptability. The intervention group increased their perception of adaptability while the control group’s perception decreased. This might suggest that receiving digital support and support with its use, increases a person’s ability to adapt to current everyday life situations [Citation38]. Furthermore, for the intervention group, the support used affected their self-esteem to a greater extent compared to the control group. The subscale of self-esteem includes feelings of security, sense of power and control [Citation25]. Further investigations could examine whether digital technology with active reminders results in a higher sense of self-esteem than passive reminders. In future studies, the PIADS can serve as a complement to other assessments, as it displays how an assistive device affects an individual psychosocially and will thus give a broader perspective of effects on everyday life [Citation25].
Limitations
This pilot study has limitations in addition to the limitation of generalizability applicable to all pilot studies. The study intended to explore the impact in the participants’ real-life settings and therefore the participants in the control group could use a calendar of their own choice, and they chose different calendars. This is a limitation when comparing results from the different groups. Another limitation is the fact that two participants in the intervention group chose to use the smartphone calendar they used prior to the study during the last two months, instead of RemindMe, this can have had an impact on the result. Especially the results from PIADS [Citation25] might have been affected because this assessment is related to the perceived impact of the support used. In this pilot study, we analysed the data according to intention-to-treat as is planned in larger RCT [Citation22]. Two participants did not use the RemindMe during the period from two to four months, the reason for this is not known. In future RCT’s assessment of intervention use (fidelity) is of major importance and a ‘pre protocol’ analysis should be considered. The participants in the intervention group were more experienced in the use of smartphone calendars, which might have had a positive impact on their possibilities in using RemindMe. This is something taking into account in future trials. In addition to the plausibility of the measurement and the description of the impact in a small group of participants, this pilot study gave insight into challenges related to recruitment. Recruitment of participants was performed consecutively by occupational therapists at the rehabilitation clinics. This approach to recruitment is considered to be relevant for future implementation and places a low burden on patients (since the patient does not meet increased numbers of personnel). However, it was difficult to recruit patients, in part because of the increased workload for the occupational therapists. Future studies should consider more intense involvement of research personnel through regular meetings at the rehabilitation clinic so that they can be informed of patients who meet the inclusion criteria. A limitation of this pilot RCT was that it was not blinded. Blinding increases a study’s validity and reduces the risk of bias [Citation21]. Blinding was not possible for the patients nor for the occupational therapists or research assistant (who undertook the weekly individual follow-up conversations). However, research personnel who conducted the assessments could have been blinded.
Conclusion
This pilot RCT study indicates changes in outcome measures for assessing occupational performance and independence for people with cognitive impairment, in the COPM together with FIM (items n-r). These instruments provide measurements that assess the impact of the intervention on supporting prospective memory and the ability to plan and structure everyday life and are therefore perceived as plausible instruments. Although no significant changes were found in this study, EQ-5D-VAS and PIADS provide measurements that assess variables that the intervention can possibly impact. The instruments are perceived as suitable to assess health-related quality of life, and psychosocial impact of the support. However, more research is needed to identify the most optimal instruments.
Acknowledgments
The authors would like to acknowledge associate Professor Inga-Lill Boman (Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm Sweden) for her contribution to the conception of the study. The authors would also like to thank patients and occupational therapists at the participating rehabilitation clinics in southeast Sweden for their valuable contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stephens JA, Williamson KNC, Berryhill ME. Cognitive rehabilitation after traumatic brain injury: a reference for occupational therapists. OTJR. 2015;35(1):5–22.
- Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80.
- Gillen G. Stroke rehabilitation: a function-based approach. St. Louis (MO): Elsevier Health Sciences; 2015.
- Organisation mondiale de la santé, World Health Organization, & World Health Organization Staff. International classification of functioning, disability and health: ICF. Geneva: World Health Organization; 2001.
- Viscogliosi C, Desrosiers J, Belleville S. Optimizing participation of older adults with cognitive deficits post-stroke: types of help and Caregiver Burden. Can J Aging. 2019;38(2):222–235.
- Lo Buono V, Corallo F, Bramanti P, et al. Coping strategies and health-related quality of life after stroke. J Health Psychol. 2017;22(1):16–28.
- Cicerone KD, Goldin Y, Ganci K, et al. Evidenced-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. 2019;100:1515–1533.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702.
- The National Board of Health and Welfare. National guidelines for stroke care- support for management. Stockholm (Sweden) 2018. (in Swedish); [cited 2021 Apr 16]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2018-12-57.pdf.
- Gillespie A, Best C, O’Neill B. Cognitive function and assistive technology for cognition: a systematic review. J Int Neuropsychol Soc. 2012;18(1):1–19.
- Bradbury K, Watts S, Arden-Close E, et al. Developing digital interventions: a methodological guide. Evid Base Compl Alternative Med. 2014;2014:1–7.
- Ferguson S, Friedland D, Woodberry E. Smartphone technology: gentle reminders of everyday tasks for those with prospective memory difficulties post-brain injury. Brain Inj. 2015;29(5):583–591.
- Lannin N, Carr B, Allaous J, et al. A randomized controlled trial of the effectiveness of handheld computers for improving everyday memory functioning in patients with memory impairments after acquired brain injury. Clin Rehabil. 2014;28(5):470–481.
- Boman IL, Persson AC, Bartfai A. First steps in designing an all-in-one ICT-based device for persons with cognitive impairment: evaluation of the first mock-up. BMC Geriatr. 2016;16(1):61.
- Brandt Å, Jensen MP, Søberg MS, et al. Information and communication technology-based assistive technology to compensate for impaired cognition in everyday life: a systematic review. Disabil Rehabil Assist Technol. 2020;15(7):810–824.
- Baric V, Andreassen M, Öhman A, et al. Using an interactive digital calendar with mobile phone reminders by senior people-a focus group study. BMC Geriatr. 2019;19(1):1–11.
- Andreassen M, Hemmingsson H, Boman IL, et al. Feasibility of an intervention for patients with cognitive impairment using an interactive digital calendar with mobile phone reminders (RemindMe) to improve the performance of activities in everyday life. Int J Environ Res Public Health. 2020;17(7):2222.
- Medical Research Council [MRC]. Developing and evaluating complex interventions. 2019; [cited 2021 Apr 16]. Available from: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance.
- Eldridge SM, PAFS consensus group, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
- Kettlewell J, das Nair R, Radford K. A systematic review of personal smart technologies used to improve outcomes in adults with acquired brain injuries. Clin Rehabil. 2019;33(11):1705–1712.
- Polit DF, Beck CT. Nursing research: principles and methods. Philadelphia: Lippincott Williams & Wilkins; 2017.
- Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
- Carswell A, McColl MA, Baptiste S, et al. The Canadian occupational performance measure: a research and clinical literature review. Can J Occup Ther. 2004;71(4):210–222.
- Pretz CR, Kean J, Heinemann AW, et al. A multidimensional Rasch analysis of the Functional Independence Measure based on the national institute on disability, independent living, and rehabilitation research traumatic brain injury model systems national database. J Neurotrauma. 2016;33(14):1358–1362.
- Day H, Jutai J, Campbell KA. Development of a scale to measure the psychosocial impact of assistive devices: lessons learned and the road ahead. Disabil Rehabil. 2002;24(1–3):31–37.
- EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199.
- Rönnlund M, Nyberg L, Bäckman L, et al. Recall of subject-performed tasks, verbal tasks, and cognitive activities across the adult life span: parallel age-related deficits. Aging Neuropsychol Cogn. 2003;10(3):182–201.
- Tamez E, Myerson J, Morris L, et al. Assessing executive abilities following acute stroke with the trail making test and digit span. Behav Neurol. 2011;24(3):177–185.
- Hunger M, Sabariego C, Stollenwerk B, et al. Validity, reliability and responsiveness of the EQ-5D in German stroke patients undergoing rehabilitation. Qual Life Res. 2012;21(7):1205–1216.
- Devitt R, Chau B, Jutai JW. The effect of wheelchair use on the quality of life of persons with multiple sclerosis. Occup Ther Health Care. 2004;17(3–4):63–79.
- Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale (NJ): L. Erlbaum Associates; 1988.
- Bateman DR, Srinivas B, Emmett TW, et al. Categorizing health outcomes and efficacy of mHealth apps for persons with cognitive impairment: a systematic review. J Med Internet Res. 2017;19(8):e301.
- Taylor RR, Kielhofner G. Kielhofner’s model of human occupation: theory and application (5nd ed.). Philadelphia: Wolters Kluwer; 2017.
- Fritz H, Cutchin MP. The transactional perspective on occupation: a way to transcend the individual in health promotion interventions and research. J Occup Sci. 2017;24(4):446–457.
- Gentry T, Wallace J, Kvarfordt C, et al. Personal digital assistants as cognitive aids for individuals with severe traumatic brain injury: a community-based trial. Brain Inj. 2008;22(1):19–24.
- Grimby G, Gudjonsson G, Rodhe M, et al. The functional independence measure in Sweden: experience for outcome measurement in rehabilitation medicine. Scan J of Rehabil Med. 1996;28:51–62.
- Branco JP, Oliveira S, Sargento-Freitas J, et al. Assessing functional recovery in the first six months after acute ischemic stroke: a prospective, observational study. Eur J Phys Rehabil Med. 2018;55:1–7.
- Axelsson W, Melander Wikman A. Ready for eHealth. Older Swedes’ perceptions of eHealth Services: using the PIADS scale as a predictor for readiness. Technologies. 2016;4(3):29.