ABSTRACT
Objective: To investigate effect of ketamine–bupivacaine in thoracic paravertebral block (TPVB) on acute and chronic pain after breast cancer surgery.
Study Design: Prospective randomized controlled study.
Setting: Cancer Institute.
Methods: Ninety patients were assigned to receive TPVB with 20 ml bupivacaine 0.25% (Group I), combined with ketamine 0.5 mg/kg (Group II) or 1 mg/kg (Group III). Assessments included; analgesia time, post-operative morphine consumption, VAS scores, hemodynamics, sedation, adverse events and Douleur Neuropathic 4 questions (DN4) scores in 1st, 2nd and 3rd post-operative months.
Results: Time to first request of analgesia was (17.76 ± 10.85h) in group I vs. (23.00 ± 1.51h, P = 0.188) and (37.33 ± 2.06h, P < 0.000) in group II and III, with significant difference between group II and III (P < 0.000). Dose of morphine in group I was (10.80 ± 2.53 mg) vs. (7.25 ± 2.31 mg, P = 0.135) and (6.20 ± 1.20 mg, P < 0.02) in group II and III, with no difference between group II and III. VAS scores were lower in Group III compared with group I and II (P < 0.05). Mean DN4 score in 1st month postoperative in Group I was (3.50 ± 0.90) vs. (3.07 ± 1.17, P = 0.114) and (2.70 ± 0.65, P < 0.000) in Group II and III, with no difference between Group II and III. Twelve patients in group I had DN4 Score ≥4 vs. 10 (P = 0.372) and 3 (P < 0.000) patients in group II and III, with significant difference between Group II and III (P < 0.001). DN4 score showed no difference between groups in 2nd and 3rd postoperative months.
Conclusion: Ketamine-bupivacaine in TPVB controlled acute post-operative pain in a dose-dependent manner and decreased DN4 scores one month after breast cancer surgery.
Trial Registration: ClinicalTrials.gov Identifier: NCT02462681.
1. Introduction
Regional blocks are more conclusively effective than pharmacologic modalities in providing analgesia to patients following surgery for breast cancer [Citation1]. Pre-emptive thoracic paravertebral blocks (TPVBs) have been shown to reduce postoperative pain and decrease opioid consumption in patients undergoing breast cancer surgery [Citation2].
Poorly controlled acute postoperative pain is considered an independent risk factor for the development of chronic pain afterwards [Citation3]. Through triggering central sensitization [Citation4], it leads to hyperalgesia and chronic pain [Citation5]. Chronic pain hinders the function and quality of life causing distress and mood changes [Citation6], and in many cases it becomes permanent and difficult to treat. Prevention of chronic pain has become more important than its treatment.
Central sensitization is a comprehensive stepwise process and so, the integration of nociceptive impulses over time leads to persistent postoperative pain [Citation4,Citation5]. Blocking nociception during any part of the perioperative experience may prevent persistent pain after surgery, raising the importance of pre-emptive or even preventive regional analgesic techniques [Citation7]. Current literature is focused upon the value of TPVBs and how its analgesic efficacy in the early postoperative period aids in the prevention of chronic pain [Citation8], and moreover, the possible prevention of recurrence after breast cancer surgery, and current evidence is still evolving [Citation9].
Being a N methyl D aspartate (NMDA)- receptor antagonist, ketamine’s anti-hyperalgesic properties have gained much interest [Citation10,Citation11]. Ketamine reduced chronic post-surgical pain (CPSP) when administered in a pre-incisional IV loading dose of 0.15–1.0 mg/kg, with additional intraoperative infusions [Citation11]. However, the role of ketamine administered perineurally in preventing chronic pain has not been established, yet.
We tested the hypothesis that the performance of pre-emptive unilateral mutli-level single-shot TPVB to patients scheduled for cancer breast surgery would improve the quality of analgesia in the early post-operative period, thereby decreasing the probability of developing chronic pain afterwards. We aimed to investigate the effect of adding ketamine (in two doses, 0.5 and 1 mg/kg) to bupivacaine in TPVB on acute postoperative pain after modified radical mastectomy with axillary dissection for breast cancer and on the possible development of chronic neuropathic pain later on.
2. Patients and methods
2.1. Enrollment
This study was approved by the Research Ethics Committee of the South Egypt Cancer Institute, Assiut University, Assiut, Egypt. Our protocol was prospectively registered in Clinical Trials. gov trial registry (identifier: NCT02462681) and strictly followed the regulations and amendments of Helsinki Declaration. Enrolled in this prospective randomized double-blind comparative study, women aged 30–60 years, ASA I-III, scheduled for unilateral modified radical mastectomy with axillary dissection for breast cancer and planned to receive single-shot thoracic paravertebral block in conjunction with general anesthesia. All study participants provided their written informed consent. Patients were excluded from the study if patient refused to participate in the study or if they had contraindications to the nerve block (coagulation defects, platelet disorders, infection at puncture sites), significant cardiac, respiratory, renal, central nervous system or hepatic disease; pregnancy; body mass index (BMI) ≥30 kg/m2; allergy to study drugs and history of opioid use for pain management the time of enrollment or drug addiction, stroke or psychiatric disease that could affect the perception of pain.
2.2. Randomization and blinding
A total of 90 patients were randomly allocated in three groups of 30 patients each, based on a computer-generated randomization table. Patients received an ipsilateral thoracic paravertebral block with 20 ml bupivacaine 0.25% alone (Group I), or combined with ketamine 0.5 mg/kg (Group II), or ketamine 1 mg/kg (Group III).
Each study participant had to complete the two stages of the study; A). Stage I: Acute postoperative pain assessments in the first 48hours postoperatively, and B). Stage II: Chronic pain assessments every month for the first three consecutive post-operative months. The attending anesthesiologist, surgeon, data collection personnel, and the patient were blinded to the patient group assignment.
2.3. Study intervention
Oral diazepam (5 mg) was administered the night before surgery and before the blocks in the operating room, intravenous access was established and standard monitors were attached. All patients received IV midazolam 1 mg plus fentanyl 50 μg titrated to produce sedation while maintaining verbal contact. All patients received an ipsilateral ultrasound-assisted thoracic paravertebral block from the second to sixth thoracic vertebrae (SonoSite ®, Inc., U.S.A). Patients were positioned in the sitting position similar to that required for neuro-axial anesthesia. The upper thoracic portion of the back, ipsilateral to the surgical side was scanned using a linear array ultrasound transducer probe of high frequency (10–12 MHZ) to identify the transverse processes. The superior aspect of the spinous processes of T2–T6 was marked [Citation12]. After cleaning the skin with an iodine solution, 2% lidocaine 0.2 ml was infiltrated subcutaneously at each point of needle insertion. An 80 mm 21G needle (Pajunk ®, SonoPlex Stim cannula U.S.A) attached through extension tubing to a syringe containing the study drugs was used. The needle was inserted perpendicular to the skin for a distance of 2 to 4 cm until the transverse process was contacted. The needle was withdrawn and walked cephalad off the transverse process and advanced not more than 1.5 cm. A staff anesthesiologist, who was not involved in the management of the patient or the study, prepared the injectate according to the patient’s group assignment. After negative aspiration for air, cerebrospinal fluid or blood, patients received 20 ml bupivacaine 0.25% (Group I), or combined with 0.5 mg/kg ketamine (Group II) or 1 mg/kg ketamine (Group III). The injected drug was prepared as 10 ml of 0.5%bupivacaine (group I) + 0.5 mg/kg (group II) or 1 mg/kg ketamine (group III) and diluted by normal saline 0.9% up to 20 ml to reach final concentration of bupivacaine to 0.25%. The study drugs were injected paravertebral and divided into 4 ml in each level. The patients and all staff involved in patient management and data collection were unaware of the group assignment. After 10 min, the success of the block was tested by decreased pin prick sensation at the expected dermatome level. Patients with failed block or unsatisfactory sensory loss were excluded from the study.
2.4. Study protocol
After establishment of the TPVB, anesthesia and muscle relaxation were induced with fentanyl 2 μg/kg, propofol 2 to 3 mg/kg, lidocaine 1.5 mg/kg and cisatracurium 0.15 mg/kg. Isoflurane 1–1.5 MAC in a 50% oxygen/air mixture and cisatracurium 0.03 mg/kg were used for maintenance of anesthesia and muscle relaxation, respectively. Monitoring included electrocardiogram, pulse oximetry, end-tidal carbon dioxide capnography, and noninvasive arterial blood pressure. One gram IV paracetamol (Perfalgan; Bristol-Myers Squibb, New York, New York), and 6 to 8 mL/kg/h lactated Ringer’s infusion were administered to patients. At the end of surgery, muscle paralysis was reversed with standard doses of intravenous neostigmine and atropine. Patients were extubated awake and transferred to the surgical intensive care unit (SICU). Postoperatively, all patients received intravenous patient-controlled morphine analgesia (B.Braun Melsungen, Melsungen, Germlany). The IV-PCA solution contained 100-mg morphine in 100 mL 0.9% normal saline (1 mg/mL), and the pump was programmed to provide an initial morphine bolus of 0.1 mg/kg once pain was expressed by the patient or if the VAS score was ≥3 followed by 1-mg bolus with a 15-min lockout time and without continuous infusion.
2.5. Surgical technique
All patients underwent modified radical mastectomy by total mastectomy and axillary lymph node dissection which was including level I and II lymph nodes through transverse elliptical incision. Interpectoral lymph nodes were removed by retraction of pectoralis minor muscle. Drains were removed when the output was less than 30 mL in a two successful days. Patients were encouraged to ambulate early and begin arm movements.
2.6. Assessment parameters
2.6.1. Acute postoperative pain assessments included
Acute postoperative pain assessments included; the patients’ demographic and clinical characteristics, intraoperative and postoperative hemodynamics (non-invasive arterial blood pressure, heart rate and pulse oximetry), postoperative sedation score (using a modified Observer’s Assessment of Alertness/Sedation scale (where 6 = agitated to 0 = does not respond to deep stimulus), Visual Analogue Scale Scores, time to first request of IV-PCA (which is defined as the time between the end of operation and tracheal extubation to the first request for supplemental analgesics and its administration to the patient) and the cumulative consumption of morphine PCA in the 1st 48 h postoperatively. The assessment time points were; baseline (upon admission to SICU) and 2, 4, 6, 12, 24, 36 and 48h postoperatively. Perioperative adverse events were treated and recorded such as nausea, vomiting, hypotension, hypertension, bradycardia, tachycardia, nystagmus, dizziness, emergence phenomenon, and sedation. Also any complications of the TPVB (e.g., accidental pneumothorax or vascular puncture) were treated and recorded. Also any post-operative surgical complications (like hematoma, seroma, lymphedema, and wound breakdown) were treated and recorded.
2.6.2. Chronic pain assessment parameters
Chronic pain assessment parameters were conducted in the pain clinic during the patients’ visits every month for the first three consecutive months after operation. A physician who was blinded to the study group assignment examined patients. Chronic neuropathic pain was assessed using the Douleur Neuropathique 4 questions (DN4) questionnaire (Appendix A) [Citation13]. Additional information was gathered including adjuvant treatments for cancer such as hormonal therapy, radiotherapy or chemotherapy. Adjuvant chemotherapy was started within 2–6 weeks after surgery. Patients with lower-risk disease, chemotherapy regimen were six cycles of anthracycline-based chemotherapy (FEC). FEC (5- fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) every 3 weeks for total of 6 cycles. For patients with higher-risk, three cycles of FEC followed docetaxel 100 mg/m2 every 3 weeks for three cycles. Only patients with HER2-positive breast cancer offered adjuvant trastuzumab.
2.7. Statistical analysis
2.7.1. Power of the study
The primary outcome parameter was defined as the difference in the VAS scores during the first 48 post-operative hours between the three treatment groups. Based on our previous research [Citation14], 26 patients in each group would be sufficient to detect a difference between means of 1.5, assuming an SD of 2 for the primary end point with a power of study 80% and a 2-sided type I error of 5%. To compensate for patient dropout, 90 patients were recruited and were equally distributed between the three treatment groups.
2.7.2. Data analysis
Distribution of baseline variables was assessed by the Shapiro-Wilk tests. Continuous variables were reported as mean (±SD) and were analyzed using the one-way analysis-of-variance test with post hoc multiple comparisons. Categorical data were reported as numbers and percentages and were analyzed using the χ2 test or Fisher exact test with the Bonferroni correction to calculate adjusted P values. Nonparametric data were analyzed using the Mann-Whitney U test. P < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS statistics version 20 (SPSS Inc., Chicago, Illinois).
3. Results
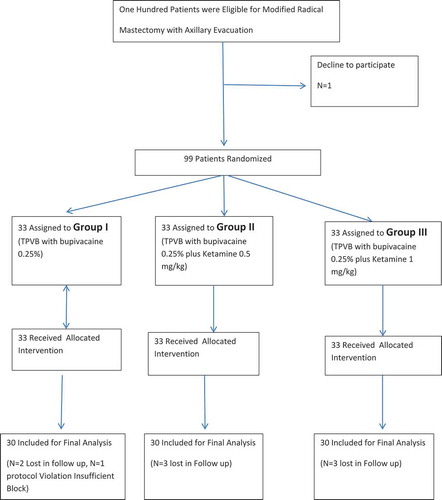
Among the 100 patients who were screened for eligibility, 90 patients were finally analyzed and were equally distributed in the three studied groups (n = 30), (). There were no significant differences between groups in the demographic or clinical data ().
Table 1. Patients’ demographic and clinical data.
3.1. Stage I assessments
There were no significant differences between groups in the hemodynamic parameters or sedation score at any time point in the study (data not represented).
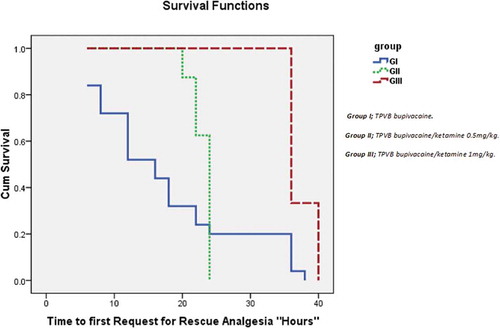
The mean time to first request for rescue analgesia was (17.76 ± 10.85 h) in group I vs (23.00 ± 1.51h, P = 0.188) and (37.33 ± 2.06h, P < 0.000) in group II and III, respectively, with a highly significant difference between group II and III (P < 0.000), (). The cumulative consumption of morphine IV-PCA in group I was (10.80 ± 2.53 mg) vs. (7.25 ± 2.31 mg, P = 0.135) and (6.20 ± 1.20 mg, P < 0.02) in group II and III, respectively, with no significant difference between group II and III (P = 0.493). A total of 25 (83%) patients in group I received morphine IV-PCA in the first 48 hours, while eight (26.6%) and six (20%) of patients in the group II and III, respectively ().
Table 2. Consumption of intravenous patient controlled morphine analgesia in the first 48h postoperatively and postoperative adverse effects.
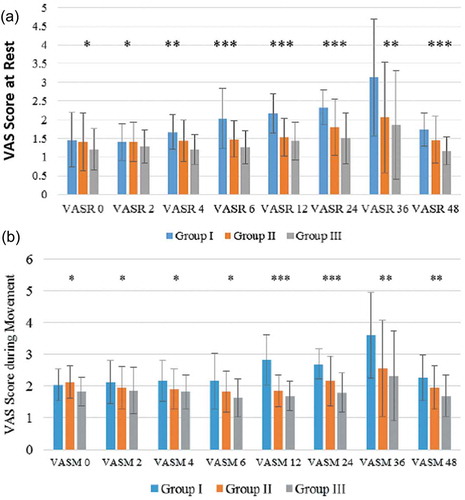
Since the admission to the SICU till end of the acute stage assessments, the Visual Analogue Scale Scores during rest and at ipsilateral arm movement were significantly lower in Group III compared with group I and II (P < 0.05), (().
Figure 3. (a) Visual analogue scale scores during rest (VAS.R). (b) Visual analogue scale scores during movement (VAS.M).
There were no significant differences between the studied groups in the incidence of postoperative adverse effects or surgical complications and their relative distribution is represented in .
3.2. Stage II assessments
The mean DN4 score in the 1st month postoperative in Group I was (3.50 ± 0.90) vs. (3.07 ± 1.17, P = 0.114) and (2.70 ± 0.65, P < 0.000) in Group II and III, respectively, with no significant difference between Group II and III (P = 0.140). A total 12 patients in group I had DN4 Score≥ 4 versus 10 (P = 0.372) and 3 (P < 0.000) patients in group II and III, respectively, with a significant difference between Group II and III (P < 0.001).
There were no significant differences between the three studied groups in the mean DN4 scores in the 2nd (P = 0.309) either in the 3rd (P = 0.132) postoperative months, respectively. Also, the number of patients who exhibited a DN4 score≥4 didn’t show a significant difference between groups in the 2nd (3 vs. 3 and 0, P = 0.736) either in the 3rd (3 vs. 1 and 0, P = 0.230) postoperative months ().
Table 3. The DN4 in the first, second and third months postoperatively.
4. Discussion
The main findings in this study were that adding ketamine (0.5 mg/kg or 1 mg/kg) to bupivacaine in pre-emptive single-shot multilevel unilateral TPVB effectively controlled early acute postoperative pain in a dose dependent manner with the longest analgesia time and lowest morphine PCA consumption were recorded in the higher ketamine dose. The mean DN4 score and the number of patients who developed neuropathic pain in the first post-operative month were reduced in patients received the higher ketamine dose with no significant difference between groups in the second and third postoperative months.
Chronic pain is a well-known risk after modified radical mastectomy with axillary evacuation for breast cancer that ranges from 20 to 47% [Citation15]. It has neuropathic characteristics and was commonly attributed to the injury to the intercostobrachial nerve (a cutaneous branch of T1-T2) during axillary evacuation [Citation16]. Persistent pain often results from nociceptive communication from injured peripheral tissue to the central nervous system via afferent nerves in the immediate postoperative period, and it is this communication that peripheral nerve blocks can dramatically attenuate [Citation8]. The perioperative period should be considered as a justifiable target for interventions aimed to reduce the incidence and severity of chronic pain after modified radical mastectomy. Thereby, decreasing the effort and cost spent on the management of chronic post-mastectomy pain syndromes (PMPS).
The effect of Pre-incisional TPVB on chronic pain after modified radical mastectomy operations has been investigated in many studies [Citation17–Citation20]. Kairaluoma et al. in their study through utilizing a 14-day symptom diary and telephone interviews 1, 6, and 12 months after surgery, demonstrated that, pre-emptive TPVB effectively controlled acute postoperative pain and reduced the prevalence of chronic pain for 1 year after surgery [Citation17]. Ibara et al. followed their mastectomy patients for 5 months through telephone questionnaire for chronic pain and concluded that fewer cases of chronic pain developed in patients received pre-incisional TPVB compared with patients received general anesthesia alone [Citation18]. Karmakar et al. found that the pre-emptive TPVB did not provide a significant difference in the incidence or relative risk of chronic pain at 3 and 6 months after modified radical mastectomy. However, the severity of chronic pain was reduced and the physical and mental health-related quality of life were better in patients received TPVB [Citation19]. Gacio et al. demonstrated that Single-injection paravertebral block allows proper control of acute pain with less intraoperative and postoperative consumption of opioids but apparently it cannot prevent pain chronicity. In their study on 80 females undergoing modified radical mastectomy with (n = 40) and without TPVB (N = 40), who were evaluated for 6month postoperatively, and found that fewer cases of neuropathic pain were recorded in the TPVB group (3 vs. 7 cases, P > 0.05). They concluded that further studies are needed to clarify the role of paravertebral block in pain chronicity in major breast surgery [Citation20]. In this study, we demonstrated that controlling early postoperative pain pre-emptively by the TPVB was translated afterwards into reduced mean DN4 scores.
The above studies investigated the role of pre-incisional TPVB using a local anesthetic only. However, adding an adjuvant to the local anesthetic can also add a benefit. Being a NMDA antagonist with anti-hyperalgesic effect, ketamine can be a suitable adjuvant to TPVB in patients undergoing modified radical mastectomy. In this study, the addition of ketamine significantly decreased the mean DN4 score and the number of patients with DN4 score≥4 in the 1st month after surgery with no difference between groups in the 2nd or 3rd months, postoperatively. The paucity of clinical studies on the role of perineural ketamine on chronic pain urges the need for future studies on such topic.
The role of systemic ketamine in chronic pain has been recently studied with conflicting results [Citation11,Citation21–Citation23]. Chaparro et al. in their systemic meta-analysis studied the efficacy of different systemic drugs for the prevention of chronic pain for 3 months after surgery in adults. They investigated 14 randomized controlled trials on intravenous ketamine. Their meta-analysis suggested a modest but statistically significant reduction in the incidence of chronic pain after surgery following treatment with ketamine but not gabapentin or pregabalin. To avoid over estimation of the treatment effect of ketamine, they suggested further studies of larger sample size (>100 in each treatment arm) [Citation11]. Hu et al, investigated the use of pre-incisional ketamine 1 mg/kg iv, followed by 72-h infusion of 2 μg/kg/minute after thoracotomy, compared to saline placebo, and found no differences in postoperative pain scores in 2nd either 6th month postoperatively. They concluded that, perioperative ketamine infusion does not prevent chronic pain after thoracotomy [Citation23]. Jendoubi et al., investigated the effects of ketamine: bolus of 0.15 mg/kg followed by infusion of 0.1 mg/kg/h intra-operatively and for 24 h postoperatively in comparison with lidocaine infusion and placebo control. They found that both ketamine and lidocaine infusion significantly reduced early postoperative morphine consumption. However, Lidocaine, but not ketamine, reduced significantly the development of neuropathic pain at 3 months after open nephrectomy (P < 0.05) [Citation23].
The current study has some limitations. First, the analgesic effect of ketamine might be in part due to systemic absorption; serum levels of ketamine if present could have confirmed or declared the analgesic efficacy of perineural ketamine. Also, it would have been more informative to include a group of patients receiving intravenous ketamine as a systemic control. Second, the sample size we selected is small. Indeed a larger sample size was needed to confirm our results. Third, we followed our patients for three months post-operatively. We think that a longer follow up interval is needed. Most of research studies on chronic pain use pain questionnaires completed by the patient through telephone or e-mail contacts permitting longer follow up intervals. In our research group we use pain scores such as LANSS and DN4 questionnaire which need patient examination [Citation13,Citation24]. We followed our patients for 3 months postoperatively, as this is the period in which patients frequently visit or become admitted in our Institute to complete their adjuvant cancer therapy courses.
In conclusion, the addition of ketamine to bupivacaine in pre-incisional TPVB effectively controlled the early acute postoperative pain in a dose-dependent manner and decreased the mean DN4 scores one month after modified radical mastectomy for breast cancer. Further studies are needed to support or declare these findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cheng GS, Ilfeld BM. A review of postoperative analgesia for breast cancer surgery. Pain Manag. 2016 Nov;6(6):603–618.
- Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multi-center clinical trial. Can J Anesth. 2015 Mar;62(3):241–251.
- Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634.
- Sandkuhler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12:18–27.
- Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quovadimus? Anesth Analg. 2011;113:1242–1253.
- Ferreira VTK, Dibai-Filho AV, de Oliveira AK, et al. Assessing the impact of pain on the life of breast cancer survivors using the brief pain inventory. J Phys Ther Sci. 2015;27:1361–1363.
- Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111(5):711–720.
- Heesen M, Klimek M, Rossaint R, et al. Paravertebral block and persistent postoperative pain after breast surgery: meta-analysis and trial sequential analysis. Anaesthesia. 2016 Dec;71(12):1471–1481.
- Finn DM, Ilfeld BM, Unkart JT, et al. Post-mastectomy cancer recurrence with and without a continuous paravertebral block in the immediate postoperative period: a prospective multi-year follow-up pilot study of a randomized, triple-masked, placebo-controlled investigation. J Anesth. 2017;31:374–379.
- Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anaesth. 2011;107:123–126.
- Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;7:CD008307.
- Tighe SQM, Greene MD, Rajadurai N. Paravertabral block. Anaesth Crit Care Pain. 2010;10(5):133–137.
- Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36.
- Mohamed SA, Fares KM, Mohamed AA, et al. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician. 2014;17:E589–E598.
- Stevens PE, Dibble SL, Miakowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: an investigation of women’s experiences. Pain. 1995;61:61–68.
- Gartner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992.
- Kairaluoma PM, Bachmann MS, Rosenberg PH, et al. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006 Sep;103(3):703–708.
- Ibarra MM, S-Carralero GC, Vicente GU, et al. Chronic postoperative pain after general anesthesia with or without a single-dose preincisional paravertebral nerve block in radical breast cancer surgery. Rev Esp Anestesiol Reanim. 2011 May;58(5):290–294.
- Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014 Jul-Aug;39(4):289–298.
- Gacio MF, Lousame AM, Pereira S, et al. Paravertebral block for management of acute postoperative pain and intercostobrachial neuralgia in major breast surgery. Braz J Anesthesiol. 2016 Sep-Oct;66(5):475–484.
- Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain. 2015;19:451–465.
- Hu J, Liao Q, Zhang F, et al. Chronic post thoracotomy pain and perioperative ketamine infusion. J Pain Palliat Care Pharmacother. 2014 Jun;28(2):117–121.
- Jendoubi A, Naceur IB, Bouzouita A, et al. A comparison between intravenous lidocaine and ketamine on acute and chronic pain after open nephrectomy: a prospective, double-blind, randomized, placebo-controlled study. Saudi J Anaesth. 2017 Apr-Jun;11(2):177–184.
- Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157.