ABSTRACT
Background: Many drugs were added bupivacaine to extend the total time of analgesia in the postoperative period. Dexmedetomidine was a successful additive in neuraxial block. It provides stable hemodynamics and better and prolonged analgesia after surgery. By this present work, we aim to assess the role of added dexmedetomidine to bupivacaine during combined sciatic and posterior lumbar plexus blocks during femur surgery regarding period of sensory and motor blocks, efficacy of its analgesic effect and incidence of adverse effects.
Methods: Our trial was done on 80 patients undergoing femur surgeries. They were all randomly chosen into two groups which have undergone combined sciatic nerve and posterior lumbar plexus blocks as solo anesthetic technique by peripheral nerve blocks. Group I: patients received bupivacaine 0.5% with no additives. Group II: patients received dexmedetomidine 100 µg with bupivacaine 0.5%.
Results: Adding dexmedetomidine prolongs total duration of motor and sensory block significantly by more than twofold (p = 0.001) as well as it took shorter time to achieve loss of pinprick sensation and also a shorter time was required to reach modified Bromage scale grade IV. Patients in Group 1 requested analgesia earlier after surgery, as well as they had significantly higher morphine consumption postoperatively compared to those in Group II (p = 0.001).
Conclusion: Adding of dexmedetomidine to the local anesthetic lessen the time till the beginning of the block and lengthen its total duration. It also prolongs analgesia postoperatively and has few side effects.
1. Introduction
Trauma is associated with many complications and morbidities. The traumatized patient may suffer physically, mentally and economically. Moreover, trauma has a negative impact on the whole society due to increased use of medical services and increased overall cost to the institution. Postoperative pain management following surgery planned for trauma patients with fractures has been achieved before using regional anesthetic techniques. Many techniques and drugs were used to enhance the total extent and quality of regional blocks with relieve of postsurgical pain [Citation1]. Opioids, epinephrine, neostigmine, magnesium sulfate, ketamine and clonidine were used in adjunction to local anesthetics (LA) for potentiation of neuraxial and regional blocks [Citation2–Citation7]. Dexmedetomidine is a profoundly selective α2 agonist. It has been tried with bupivacaine in neuraxial blockade and showed many advantages [Citation8,Citation9].
This trial was performed to assess the result of adding dexmedetomidine to bupivacaine in combined lumber plexus and sciatic nerve blocks. Our primary outcome was the comparison of the total time of motor and sensory block and our secondary outcomes were the assessment of the onset of motor and sensory block, consumption of morphine in 24 h postoperatively, the time of first request of analgesia and the noted adverse effects during the study period.
2. Patients and methods
After approval of local ethical committee and a written informed patient consent, 80 patients with physical status American Society of Anesthesiologist (ASA) I or ASA II who have undergone elective open reduction and internal fixation of femur fracture were included. Patients were excluded from the study if they had skin infection at the site of the block or they had coagulopathy or hypersensitivity to one of the study drugs and patients who refused participation.
2.1. Sample size and rationalization
The sample size is estimated from this equation: n = 2 δ2 (Zα + Z)2/∆2, where: n is the number of the subject of each group, Zα is the value of standard normal distribution for p-value 5% for two-sided test and it equals 1.96, Z is the value of a standard normal for the desired statistical power 90% and it equals 1.28, ∆ is the detectable difference between the means duration of sensory and motor block after adding dexmedetomidine and it equals 13.7 min, δ is the highest within group standard deviation and it equals 18 min. By calculation, n equals 36 with expected drop out of 10% had been added, so the required sample will be 40 patients/each group.
Study members were randomly allocated by using a computer-generated random number list to randomize the patients into one of two groups (Group I: patients who received only bupivacaine 0.5% with no additives and Group II: patients who received dexmedetomidine 100 µg with bupivacaine 0.5%). So the anesthesiologist who performed the block, the patients who received the block and the investigator who collected the data were totally blinded.
2.2. Preoperative assessment and premedication
Patients were asked about drug allergy, surgeries and drug history. Airway evaluation was done after brief general and systemic examinations. Proper preoperative preparation was done including 6 h fasting and all patients were premedicated by 2 mg midazolam before surgery. The whole procedure was explained using 10 cm visual analog scale (VAS; 0 – pain free and 10 – worst pain imaginable); modified Bromage score and sedation score were also discussed preoperatively.
2.3. Intraoperative measures
All patients were monitored by electrocardiogram (ECG), non invasive blood pressure (NIBP) and pulse oximeter. A statistical series were done by computer and it also determines which group the patient entered; the data of what will be done were undiscovered by the anesthesiologists either who record data or who administer anesthesia. The study drugs were prepared by an anesthesiologist who did not participate in the study; in 50 ml syringe. In Group I, patients received 49 ml of bupivacaine 0.5% and 1 ml normal saline, while in Group II, patients received 49 ml of bupivacaine 0.5% mixed with 100 μg dexmedetomidine in only 1 ml. All patients had the same anesthetic technique. Posterior lumbar plexus and sciatic nerve block was done in lateral decubitus position using nerve stimulator.
For the psoas block, the patient was placed in the lateral position while the site of operation was upright. Thigh is flexed with flexed knee (Sim’s position). Intercristal line was determined through the two iliac crests (line 1). Lumbar spinous processes were connected by another line. The posterosuperior iliac spine was determined. Another line parallel to the lumbar spines through the posterosuperior iliac spine was drawn (line 2). We inserted our needle where the two lines (1 and 2) intersect. We prepared skin with povidone iodine and infiltrated 2 ml of lidocaine 2%, then a 21-G 15-cm insulated needle was put at the site where lidocaine was infiltrated perpendicularly, looking for a quadriceps muscle contraction. If a contraction was not found at the initial insertion, from the same skin point the needle was inserted to the same depth toward an imaginary point in 1-cm increments more medially. After contraction of the quadriceps was elicited at less than 0.5 mA, 25 ml of study drugs was given gradually. We confirmed the psoas block by sensory loss in anterolateral aspect of the thigh.
At the same position (sim’s position), a line connects the posterior superior iliac spine to the greater trochanter. Midway along this line, a perpendicular line was drawn between the greater trochanter and the sacral hiatus. The 15-cm insulated needle was advanced in 90° angle through the buttock with elicited twitches in gluteus muscles which disappeared with further advancement and the sciatic nerve is usually located at 5–8 cm from skin. Planter flexion twitches were observed to detect stimulation of the tibial component of sciatic nerve. When the desired response reached at a current less than 0.5 mA, the other 25 ml of study drugs was incrementally injected.
Patients turned into supine position immediately after completion of the injection. Sensory and motor block was assessed; any cases of failure were excluded and undergone general anesthesia.
Oxygen (2 L/min) was administered via nasal cannula. Hypotension was considered if there was a drop in systolic arterial blood pressure (SAP); DAP (diastolic arterial blood pressure) >20% of preoperative readings and managed by 6 mg i.v. ephedrine and reused after 3 min if there is no accepted increase in SBP. Tachycardia was considered if heart rate (HR) was more than 90 and bradycardia when HR was less than 55. If HR decreased below 50/min, 0.5 mg of atropine was given i.v. side effects, like itching, sedation, bradycardia, hypotension, nausea and vomiting were documented. Assessment of sensory block was done by loss of pain to pinprick 23G needle tested 15–20 min after block. Assessment of motor block was made by Modified Bromage score [0 = full motor power, 1 = cannot raise straight leg but can flex knee, 2 = cannot flex knee but move foot freely, 3 = cannot move foot].
After achieving adequate sensory and motor blockade level, surgery was allowed. The patients, the anesthesiologist and the surgeon were blinded to the study groups. Data regarding the time to reach block from of injection of the drugs, duration of block and incidence of side effects were observed and written.
2.4. Postoperative measures
Patient-controlled analgesia was started intravenously just immediately after the patient transferred to the Post-Anesthesia Care Unit (PACU). The setup was done by morphine 0.5 mg/ml and to give 1 mg of morphine on demand with lockout time of 15 min. Four milligrams per hour was the maximum dose per hour. First analgesic request and total morphine consumption (mg) were documented postoperative for 24 h. VAS (0–10 scale, 0 – no pain and 10 – worst possible pain), at rest and on motion (passive knee flexion), was recorded every hour for the first 6 h, then every 2 h 5 times, then every 4 h 2 times.
3. Results
We compared the two groups regarding the age, gender, body mass index, ASA class and type of femur surgeries and there is no statistically significant difference (p-value >0.05; and ).
Table 1. Baseline demographic characteristics among the studied patients in both groups.
Table 2. Type of femur surgeries performed among both groups.
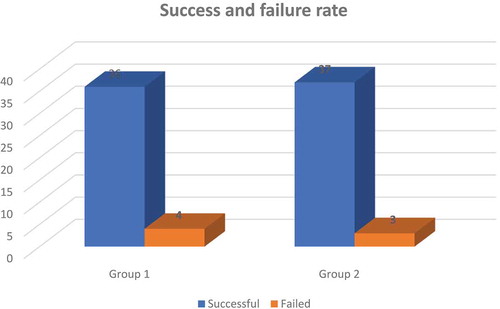
For assessment of success rate, demonstrated that there was no statistically significant difference among groups I and II regarding success and failure rate of the regional block used in the entire study. In Group II, 7.5% of patients had failed block compared to 10% of patients in Group I.
Figure 1. Comparison between success and failure rate between study groups.
showed that there was no statistically significant difference between both groups regarding success and failure rate of the block.
Failed block cases were excluded from the study and randomly replaced using computer-generated random number list to maintain the required sample size while preventing selection bias.
presents the difference between both groups regarding evaluation of block. It was found that by using dexmedetomidine as an adjuvant to bupivacaine in Group II, a significantly shorter time was required to achieve loss of pinprick sensation than in Group I (3.33 versus 5.45 min, in Groups II and I respectively, p = 0.001) and also a shorter time was required to reach motor block (modified Bromage scale grade IV) than in Group I (16.38 versus 17.93 min in Groups II and I respectively, p = 0.005). There was a statistically significant difference between both groups regarding the total duration of motor and sensory blocks. The mean duration of motor block in Group II was more than twofold than that of Group I, p = 0.001. In addition, the sensory block duration was also more than twofold than that of Group 1, p = 0.001.
Table 3. Outcome variables of the blockade among study groups.
The study showed the assessment of postoperative pain using VAS at rest and during passive motion (passive knee movement). It showed that there was no statistically significant difference regarding postoperative pain in the first 3 h postoperatively. Conversely, assessing of VAS from 6 to 24 h postoperatively was significantly lower in Group II compared to Group I, p = 0.001( and ).
Table 4. Postoperative scores of Visual Analog Scale (VAS) between the two study groups at rest (mean ± SD).
Table 5. Postoperative scores of Visual Analog Scale (VAS) between the two study groups on motion (passive knee flexion) (mean ± SD).
After evaluation of analgesic requirements postoperatively, we found that addition of dexmedetomidine to bupivacaine prolonged the sensory block and delayed the first postoperative rescue analgesics (8 h postoperatively in Group I versus 16.6 h postoperatively in Group II, p = 0.001). On the other hand, the patients in Group I had significantly higher morphine consumption in the first 24 postoperative hours compared to the patients in Group II (12.7 mg in Group I versus 7.15 mg in Group II) with statistically significant difference (p = 0.001) ().
Table 6. Comparison between the two study groups regarding the timing of the first morphine request and total morphine consumption in 24 h postoperatively (mean ± SD).
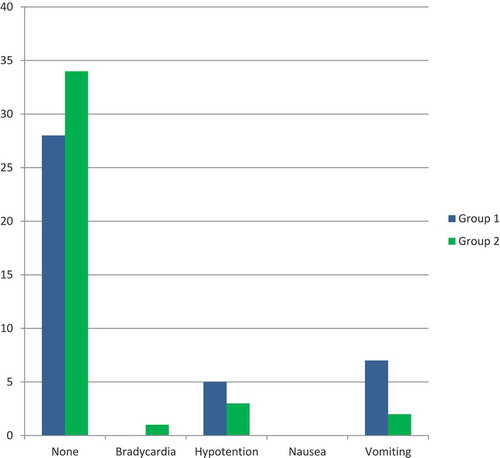
As shown in , the incidence of intraoperative side effects among the patients of the two groups, there was no statistically significant difference between both groups regarding the total intraoperative side effects; 72.5% of patients in Group II had no intraoperative complications compared to 62.5% of patients in Group I; 15% of patients in Group II developed bradycardia compared to 7.5% of patients in Group I; 7.5% of patients in Group II developed hypotension versus 10% of patients in Group I; 5% of patients in Group II suffered nausea compared to 2.5% of patients in Group I; no vomiting was reported in the patients of Group II compared to 17.5% of patients in Group I suffered vomiting.
Figure 2. Comparison of intraoperative complications between the two groups.
showed that there was no statistically significant difference between both groups regarding the total intraoperative complications
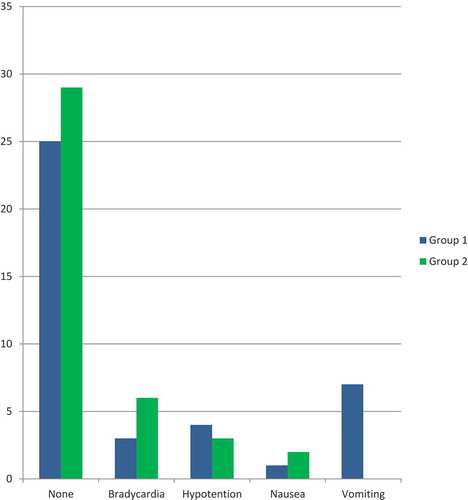
demonstrated that there was no statistically significant difference between the two groups regarding total postoperative complications. In Group II, 85% of the patients had no complications compared to 70% of the patients in Group I; 2.5% of the patients in Group II developed bradycardia while no patients suffered bradycardia in Group I; 7.5% of patients in Group II developed hypotension compared to 12.5% of the patients in Group I; no patients in both groups suffered nausea; 5% of the patients in Group II suffered vomiting compared to 17.5% of the patients in Group I.
4. Discussion
Peripheral nerve blocks have been under focus in the last two decades. Our study was created to evaluate the efficacy and safety of adding dexmedetomidine as an adjuvant to bupivacaine in combined posterior lumbar plexus and sciatic nerve blocks in patients with fracture femur. Peripheral nerve blocks are popular regarding using them as an anesthetic technique or as an adjuvant to general anesthesia to ensure intraoperative analgesia and postoperative analgesia as well.
Various methods and drugs had been tried to extend the duration of peripheral nerve blocks and attain postoperative pain alleviation [Citation1]. Dexmedetomidine added to LA improved sensory and motor blockade by increasing the duration of analgesia [Citation8,Citation10]. Additionally, it has a dose-dependent upsurge in analgesic duration and thermal antinociception [Citation11]. Many researches were done in peripheral nerve block procedures and proved the valuable effects of adding dexmedetomidine to LA [Citation5,Citation6].
There are different anticipated mechanisms of action of dexmedetomidine in peripheral nerve blocks. These predicted mechanisms are direct action on a peripheral nerve, diminution of the inflammatory response, analgesia centrally mediated and vasoconstrictive effects of α2B-adrenoceptor mediated even though they do not completely explain the effectiveness of dexmedetomidine in peripheral nerve blockades [Citation12].
On the other hand, trial was done by Fritsch et al. [Citation13] who prove that the outcome of adding dexmedetomidine to LA definitely prolongs the duration of regional blocks are not systemic in origin, but it was the direct effect on a peripheral nerve.
One of our encouraging findings that could influence possible upcoming routine use of addition of 100 μg dexmedetomidine to bupivacaine for lumbar plexus and sciatic nerve block significantly reduced the onset time of sensory and motor blocks in addition to the total duration of motor and sensory blocks they both were statistically significantly prolonged where the mean duration of both motor and sensory blocks were double the time by adding dexmedetomidine which mean improvement of potency and effectiveness of the block.
The results of our study are in agreement with Helal [Citation14] and his colleagues, who assessed the effects of perineural administration of dexmedetomidine in combination with bupivacaine in a femoral-sciatic nerve block, and they demonstrated that sensory and motor block onset times were shorter by 20% in dexmedetomidine group than in bupivacaine group. In addition to shorter onset time, sensory and motor blockade durations were longer in group BD (+45% and +40%, respectively) than in group B and duration of analgesia was also become longer in group BD by 75% than in group B.
Additionally, Abdulatif et al. [Citation15], in their randomized, controlled, double-blind study who evaluated the effects of three different doses of perineural dexmedetomidine (25, 50 and 75 μg) on the pharmacodynamic outline of femoral nerve block, found that the use of the 50 and 75 μg was associated with prolonged duration of block, decreased onset time, decreased postoperative morphine requirements and longed time to the first postoperative request for rescue analgesia.
Our results are also comparable to the results of Packiasabapathy et al. [Citation16], who assessed the effect of dexmedetomidine as an adjuvant to bupivacaine in femoral nerve block for perioperative analgesia in patients undergoing total knee replacement arthroplasty. They demonstrated that the addition of 100 μg dexmedetomidine to 0.5% bupivacaine was associated with a prolonged duration of analgesia, and lower pain score at rest. They recommended 100 μg of dexmedetomidine as an adjuvant for an equilibrium between effectiveness and sedation.
Supporting this study, Casati et al. informed that addition of α2-adrenoceptor agonist to 0.75% ropivacaine in sciatic nerve block combined with femoral nerve block prolonged nerve block duration, giving 3 h increase in postoperative pain alleviation and no hemodynamic side effects [Citation17].
In disagreement with this study, Helayel et al. reported that addition of α2-adrenoceptor agonist to 0.5% 40 ml ropivacaine in sciatic nerve block had no advantage in the duration and the quality of analgesia [Citation18], and there is another study conducted by Marhofer et al. [Citation19], who evaluated the effect of adding dexmedetomidine to ropivacaine for U∕S guided ulnar nerve block (UNB). They found that adding dexmedetomidine did not make any difference in sensory onset time of UNB, whereas motor onset time was significantly faster.
Another promising finding in our study was the lower VAS score at rest and during passive motion (passive knee flexion) in Group II comparing Group I at 6, 12 and 24 h postoperatively.
Regarding total analgesic requirements and time to first postoperative analgesic request, the current study demonstrated that the time for first-order analgesia in Group I was relatively longer than that in Group II and the total analgesic requirements in Group II were relatively lower than that of Group I.
In this study, the intraoperative complications between both groups were statistically insignificant. However, dexmedetomidine may cause a dose-related bradycardia, hypotension and excessive sedation when it was used by intravenous injection [Citation20], even perineural administration [Citation18].
In this study, it was found that adding dexmedetomidine (100 μg) induced bradycardia by 15% in Group II compared to 7.5% in Group I. In addition, the incidence of hypotension was 7.5% in Group II compared to 10% in Group I. In agreement with this outcome, Esmaoglu et al. [Citation21], who assessed the outcome of adding dexmedetomidine to levobupivacaine for brachial plexus blockade on 60 patients, planned for elective hand and forearm surgery. They showed the lower level of BP and HR in the dexmedetomidine group intraoperative with a high incidence of bradycardia.
A meta-analysis conducted by Xiao Liang et al. was done to assess the effectiveness of dexmedetomidine to prevent nausea and vomiting. The major finding was that the helpful outcome of dexmedetomidine on nausea and vomiting can be taken through IV rout only [Citation22], although the antiemetic effect of dexmedetomidine in regional anesthesia has not yet proven. Although our current study found that there is higher trend of occurrence of postoperative vomiting in Group I than in dexmedetomidine group which may suggest an anti-emetic effect potential of dexmedetomidine.
There are some limitations in this study. First, the dosage of dexmedetomidine for lumbar plexus and sciatic nerve block was selected on the basis of previous studies [Citation23,Citation24]. Up till now, a dose–response study was not done and is required to assess the optimum dose of dexmedetomidine for lumbar plexus and sciatic nerve block. Second, patients in this study were young and healthy (ASA I–II). It has not been confirmed whether these results were suitable for patients of other age populations or with comorbidities. Third, the systemic absorption of dexmedetomidine explaining the significant effects in this study could not be excluded; further studies are required to verify the local effects of dexmedetomidine by using an intravenous dexmedetomidine infusion in another third group and final limitation was not using ultrasound in block performance in addition to nerve stimulation.
5. Conclusion
Combined posterior lumbar plexus and sciatic nerve blocks is a suitable anesthetic approach for unilateral lower limb surgeries with low incidence of failure rate. Adding of dexmedetomidine to the LA decreases the onset and increases the duration of the block. It also prolongs the postoperative analgesia time and has few side effects as an additive in regional anesthesia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Smith OHW, Nielsen LA, Gaumann D, et al. Sensory changes and pain after abdominal hysterectomy: a comparison of anesthetic supplementation with fentanyl versus magnesium or ketamine. Anesth Analg. 1986;1:95–101.
- Popping DM, Elia N, Marret E, et al. Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: a meta–analysis of randomized trials. Pain. 2012;153:784–793.
- de Oliveira G, Balliu B, Nader A, et al. Dose-ranging effects of intrathecal epinephrine on anesthesia/analgesia: a meta–analysis and metregression of randomized controlled trials. Reg Anesth Pain Med. 2012;37:423–432.
- Ho KM, Ismail H, Lee KC, et al. Use of intrathecal neostigmine as an adjunct to other spinal medications in perioperative and peripartum analgesia: a meta-analysis. Anaesth Intensive Care. 2005;33:41–53.
- Pascual-Ramirez J, Gil-Trujillo S, Alcantarilla C. Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trials. Minerva Anestesiol. 2013;79:667–678.
- Khezri MB, Ghasemi J, Mohammadi N. Evaluation of the analgesic effect of ketamine as an additive to intrathecal bupivacaine in patients undergoing cesarean section. Acta Anaesthesiol Taiwan. 2013;51:155–160.
- Engelman E, Marsala C. Efficacy of adding clonidine to intrathecal morphine in acute postoperative pain: meta-analysis. BrJ Anaesth. 2013;110:21–27.
- Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–227.
- Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedure. Saudi Med J. 2009;30:365–370.
- Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71:447–449.
- Al-Ghanem SM, Massad IM, Al-Mustafa MM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–887.
- Leem JW, Choi Y, Han SM, et al. Conduction block by clonidine is not mediated by alpha2-adrenergic receptors in rat sciatic nerve fibers. Reg Anesth Pain Med. 2000;25:620–625.
- Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: A single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39:37–47.
- Gaarour I, Helal S, Eskandr A, et al. Effects of perineural administration of dexmedetomidine in combination with bupivacaine in a femoral-sciatic nerve block. Saudi J Anaesth. 2016 Jan-Mar;10(1):18–24.
- Abdulatif M, Fawzy M, Nassar H, et al. The effects of perineural dexmedetomidine on the pharmacodynamic profile of femoral nerve block: A dose-finding randomized, controlled, double-blind study. Anaesthesia. 2016;71:1177–1185.
- Packiasabapathy S, Kashyap L, Arora M, et al. Effect of dexmedetomidine as an adjuvant to bupivacaine in femoral nerve block for perioperative analgesia in patients undergoing total knee replacement arthroplasty: A dose–response study. Saudi J Anaesth. 2017 Jul-Sep;11(3):293–298.
- Casati A, Magistris L, Fanelli G, et al. Small-dose clonidine prolongs postoperative analgesia after sciatic-femoral nerve block with 0.75% ropivacaine for foot surgery. Anesth Analg. 2000;91:388–392.
- Helayel PE, Kroth L, Boos GL, et al. Effects of intramuscular and perineural clonidine on sciatic nerve block with 0.5% ropivacaine. Rev Bras Anestesiol. 2005;55:483–490.
- Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013;110:438–442.
- Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394.
- Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary Brachial Plexus Block. Anesth Analg. 2010;111(6):1548–1551.
- Liang X, Zhou M, Feng JJ, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8(6):8450–8471.
- Bharti N, Sardana DK, Bala I. The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: a randomized controlled trial. Anesth Analg. 2015;121:1655–1660.
- Rancourt MM, Albert NT, Cote M, et al. Posterior tibal nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–962.