ABSTRACT
Background: The aim of this study was to evaluate the effect of deep and moderate neuromuscular block on surgical exposure quality, intraoperative lung mechanics, and postoperative respiratory functions, during laparoscopic bariatric surgery.
Methods: Sixty adult morbid obese patients were enrolled in the study. They were randomly distributed in two equal groups: the deep block group, where rocuronium infusion was given to maintain the post-tetanic counts above 1, and the moderate block group, where increments of rocuronium were delivered to maintain the train of four 1–2. The surgeon was asked to assess intraoperative relaxation. The increase in intra-abdominal pressure and reported abdominal or diaphragmatic movements were recorded. Intraoperative lung mechanics were studied, and preoperative and postoperative pulmonary function tests were done.
Results: The scores reported by the surgeons for intraoperative surgical exposure were indifferent among the two groups (5 (4–5) in the deep block group and 4 (3–5) in the moderate block group, P = 0.243). The difference in the increase in intra-abdominal pressure and reported abdominal or diaphragmatic movements was insignificant between both (P = 0.299 and 0.424). Intraoperative pulmonary mechanics and postoperative pulmonary functions were comparable (P > 0.05), and the postoperative pain score was indifferent between both groups (P > 0.05).
Conclusion: During laparoscopic bariatric surgery of morbidly obese patients, the quality of abdominal relaxation and surgical exposure, intraoperative lung mechanics, and postoperative pulmonary functions were indifferent with the use of moderate or deep neuromuscular block.
1. Introduction
The introduction of neuromuscular blocking agents in anesthesia has been associated with numerous benefits. It specifically improves the intubation condition, increases the depth of anesthesia, decreases airway edema, and improves surgical exposure [Citation1–Citation3]. Despite that, it has also been associated with several drawbacks, such as postoperative dysfunction of respiratory muscles that predispose to postoperative pulmonary complications [Citation4,Citation5]. The risk of postoperative respiratory complications increases significantly in morbidly obese patients, sometimes approaching 100% incidence, especially with increasing body mass index (BMI) and presence of obesity hypoventilation syndrome [Citation6]. Respiratory complications must be considered and properly managed in the perioperative management of bariatric surgeries, as they account for about 12% of mortality rates [Citation7].
Laparoscopic surgeries for morbidly obese patients require deep muscle relaxation from the surgeons’ point of view, as they require good visualization. However, anesthesiologists give special consideration to postoperative pulmonary dysfunction, which may be associated with abuse of muscle relaxants [Citation8]. Maintaining deep neuromuscular blockade was thought for long to be hazardous due to the potential risk of awareness and residual curarization postoperatively [Citation9,Citation10]. A deep neuromuscular block is considered when the train of four (TOF) count is zero, with post-tetanic counts (PTC) of 1 or more, while a moderate block is considered when the TOF count is 1–3.
Kopman suggested that deep neuromuscular block provides satisfactory surgical exposure during laparoscopic surgery [Citation11]. In addition, studies support this in laparoscopic cholecystectomy [Citation12] and gynecological [Citation13] and urological surgeries [Citation14]. However, the evidence is still under debate. The use of deep neuromuscular block in laparoscopy may shorten the recovery period of non-depolarizing muscle relaxants, with an increased risk of postoperative residual blockade and respiratory complications [Citation15].
Sugammadex, a new muscle relaxant reversal agent, allows rapid and complete reversal of rocuronium, compared to the traditionally used muscle relaxant reversal agent neostigmine [Citation16]. Thus, it may allow the use of deep neuromuscular block during the intraoperative period for satisfactory surgical exposure and rapid reversal of deep relaxation, to decrease the incidence of postoperative pulmonary complications [Citation17].
When anesthetizing obese patients, the question that comes to the minds of all anesthesiologists is, which is better, deep or moderate neuromuscular block? The purpose of this study is to compare the effects of each on the intraoperative surgical space conditions, the intraoperative pulmonary mechanics, and the postoperative pulmonary functions, in morbidly obese patients undergoing laparoscopic bariatric surgeries.
2. Material and methods
This clinical randomized double-blind study was approved by the local research ethics committee (Tanta Faculty of Medicine Research Ethics Committee, Tanta Faculty of Medicine, Tanta, Egypt) on the 23rd of August 2017 (registration number: 31,724/08/2017). It was then registered in the Pan-African Clinical Trial Registry (unique identification number: PACTR201710002663258). The study was conducted at Tanta University Hospitals for 6 months (October 2017-April 2018). Patients who accepted to participate in the study signed informed written consent. Patients 25 to 45 years old, American Society of Anesthesiologists (ASA) class III, BMI above 40 kg/m2 and less than 50 kg/m2 and scheduled for laparoscopic bariatric surgeries were enrolled. They were reassured and given an adequate explanation of the benefits, technique, and potential hazards of this research work. All obtained data of the patients were kept in secret files to maintain their privacy.
The patients were properly assessed preoperatively. Patients’ history was taken, general and local examination was done, and an analysis of complete blood count, liver function test, renal function test, and coagulation studies were requested. Preoperative pulmonary function test was done for measurement of the basal peak expiratory flow rate (PEFR) (L/min), forced expiratory volume 1 (FEV1) (L), forced vital capacity (FVC) (L), and FEV1/FVC. Pregnant and lactating patients, patients who suffer moderate or severe obstructive sleep apnea, psychologically unstable, have neuromuscular or skeletal diseases, or have a history of congestive heart failure or hepatic or renal failure, were excluded from the study.
The randomization of the study was carried out by the aid of computer-generated software, results introduced in closed sealed envelopes containing cards presented to each patient to choose from. Patients were randomly distributed in two groups according to the maintenance dose of muscle relaxants given: deep neuromuscular block group and moderate neuromuscular block group.
Monitors were attached to all patients once they were admitted to the operating theatre, with a pulse oximeter, non-invasive blood pressure monitor, and a 3-lead ECG. An 18-gauge cannula was inserted for each patient to provide intravenous access for an infusion of lactated ringer solution 10 ml/kg, as a fluid preload.
Quantitative neuromuscular function was monitored using an acceleromyograph (TOF-watch-SX, MSD BV, Oss, Netherlands) that measures the adductor pollicis muscle response. Two electrodes were placed over the course of the ulnar at the radial side of the flexor carpi ulnaris muscle 1 cm proximal to the wrist joint. The contractions of the ipsilateral adductor pollicis muscle (causing adduction of the thumb) were detected by attaching a sensor to the tip of the thumb and placing it in a flexible adaptor to generate preload. TOF-watch-SX was calibrated and stabilized after induction of general anesthesia and before rocuronium administration, according to manufacturer specifications. Neuromuscular block was assessed after endotracheal intubation at 15-second intervals.
A well-fitted face mask was used for adequate preoxygenation of all patients, with 80% oxygen for 5 minutes. Anesthesia was induced using fentanyl 1.5 ug/kg (ideal body weight) and propofol 2 mg/kg (lean body weight). The neuromuscular monitor was then stabilized through a 50-Hz tetanic stimulation application for 5 s, with calibration of the TOF-watch-SX and documentation of a series of TOF measurements for >2 min, until a stable baseline was obtained (<5% variation in the TOF ratios). An intubating dose of rocuronium (Esmeron®, 10 mg/ml, N.V. Organon, Oss, Netherlands) 0.6 mg/kg (lean body weight) was then injected, and a cuffed endotracheal tube of suitable size was inserted when TOF count reached 1 or less. The patients were then attached to a mechanical ventilator (Anesthesia machine: GE Datex-Ohmda Avance CS2, USA) using a flow rate of 1 L/min composed of 1:1 oxygen to air, with adjustment of the ventilation parameters to keep end-tidal CO2 between 32 and 36 mmHg. Ventilator parameters were (Pressure-controlled volume guarantee mode, tidal volume of 6 ml/kg, respiratory rate: 12/min, I:E ratio: 1:2, maximal airway pressure (Pmax) 40 cmH20, and optimal PEEP which was reached by stepwise titration to maintain recruitment of the basal alveoli). Maintenance of anesthesia was conducted through propofol total intravenous anesthesia (150–200 ug/kg/min) to maintain the values of bispectral index at 40–50, incremental doses of i.v. fentanyl (1 ug/kg/hr), and the determined maintenance dose of rocuronium according to the group of patients.
In the deep block group, rocuronium infusion at a dose of 0.6 mg/kg/hr (lean body weight) was started after endotracheal intubation, to maintain a TOF count of 0, with PTC > 1. Rocuronium (100 mg) was prepared in 50 ml normal saline 0.9% syringe pump. The moderate block group, on the other hand, received repeated top-up doses 0.1 mgt/kg of rocuronium to keep the TOF count at 1–2. The total dose consumption of rocuronium and fentanyl was calculated and recorded.
The laparoscopic interventions were conducted by the same surgeon with CO2 pneumoperitoneum set at 15 mmHg using a flow of 5 L/min. An increase in intra-abdominal pressure above 15 CmH2O, not related to pressure exerted by the surgeon or instruments on the abdominal wall, was recorded. Any abdominal muscle movement or spontaneous diaphragm movement during the operation reported by the surgeon or anesthesiologist was recorded. The anesthesiologist who was involved in anesthesia of the patients was not blinded to the groups and did not participate in the collection of the data or any measurements. Both the patients and the surgeon were blinded to the groups. The surgeon was kept blind by use of a syringe pump connected to the patient and labeled to contain rocuronium; for patients in the deep block group, the syringe contained actual rocuronium, while in the moderate block group it contained normal saline. Neuromuscular monitoring and its interpretation were also kept hidden from the surgeon.
To prevent nausea and vomiting postoperatively, dexamethasone 4 mg was given after induction of general anesthesia, and ondansetron 4 mg was given at the end of surgery. Thirty minutes before the end of the surgery, pethidine 50 mg was administered intravenously. At the end of the surgery, propofol infusion was stopped. Rocuronium infusion was also stopped in the deep block group when the surgeon started closing the wound. When the TOF count reached 3 or 4, the muscle relaxation was reversed using sugammadex 2 mg/kg for the deep block group and neostigmine 0.05 mg/kg plus atropine 0.01 mg/kg for the moderate block group.
After awake tracheal extubation, the patients were transported to the PACU for postoperative monitoring. Oxygen supplementation was provided via a nasal cannula at a flow of 2–3 L/min, and paracetamol 1 g/6 hr i.v. infusions were given for postoperative analgesia. Pethidine 50 mg was given as rescue analgesia when the postoperative pain score was higher than 3. Postoperative pain was evaluated on admission to PACU; 15 min, 30 min, 60 min, and 90 min later; and on discharge from PACU using the visual analogue scale (VAS), ranging from 0 (no pain) to 10 (worst level of pain). The time spent in PACU and total consumption of rescue analgesia there was recorded. “The patients were discharged from the PACU when their modified Aldrete scale reached 10.”
The quality of surgical space conditions was the primary outcome. Before the end of surgery, the surgeon was asked to evaluate the surgical space conditions using a special 5-point rating scale [Citation14]: 1 = extremely poor, 2 = poor, 3 = accepted, 4 = good, and 5 = optimal. Intraoperative pulmonary mechanics and postoperative pulmonary functions were secondary outcomes. The pulmonary mechanics included peak airway pressure (Ppeak), plateau pressure (Pplat), mean airway pressure (Pmean), and dynamic compliance of the respiratory system (Cdyn), monitored in both groups immediately after endotracheal intubation and before starting the maintenance dose of rocuronium (T1), 30 minutes after insufflations (T2), and at the end of surgery (T3).
An electronic portable peak flowmeter was used for assessment of the pre- and postoperative pulmonary functions. The mean of three readings of PEFR (L/min), FEV1 (L), FVC (L), and FEV1/FVC had been recorded pre- and postoperatively. These measurements were recorded while the patients were in a semi-sitting position (45°) in the anesthesia clinic, during the preoperative visit and in the PACU postoperatively. In postoperative measurements of pulmonary functions, the effect of sedation is excluded first by the aid of the modified observer’s assessment of alertness [Citation18]. The time elapsed between reversal of muscle relaxant (TOF 3 or 4) and performing postoperative pulmonary function assessment was recorded. Patients who required postoperative non-invasive ventilation support by CPAP or BiPAP or required re-intubation were also reported.
3. Statistical analysis
The sample size was calculated based on the quality of surgical space conditions. Based on the results of a previous study (pooled standard deviation of the two groups = 1.051) [Citation8], at least 21 patients were needed from each group to detect a mean difference of 1.2 in the surgical rating scale at α value of 0.05 and power of study 95%. The SPSS 17 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the collected data. The Kolmogorov-Smirnov test was performed to check the assumption of normality. The quantitative parameters that normality distributed were expressed as mean ± standard deviation (SD) and analyzed using the Student t-test. Quality of surgical space conditions was expressed as a median with interquartile range and analyzed for the studied groups using the Mann-Whitney U test. The categorical data were expressed as a number and a percentage and compared via the Chi-square test or Fisher’s exact test as appropriate. A significant change was considered when the P value was less than 0.05.
4. Results
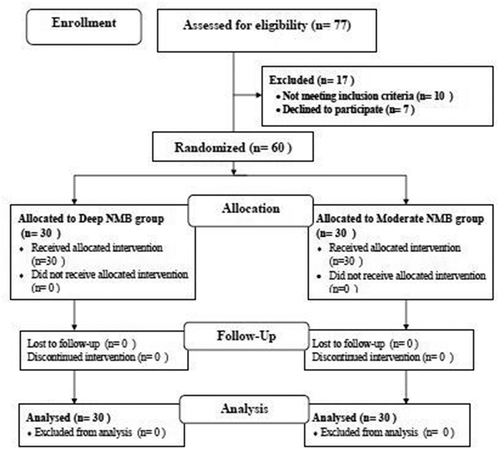
Seventy-seven patients were assessed for eligibility to participate in this study. Seventeen were excluded (7 refused to participate in the research and 10 were not meeting the inclusion criteria of the study: 6 suffered moderate to severe obstructive sleep apnea, 2 had renal impairment, and 2 had liver impairment). The remaining 60 patients were equally and randomly distributed in two groups (30 patients each) ().
The statistical analysis of the basic criteria of the patients that included age, gender, and body mass index showed a statistically insignificant difference between the two groups (P = 0.218, 0. 785, and 0.126, respectively). The type and duration of surgery were also statistically indifferent between the two studied groups (P = 0. 789 and 0.313, respectively) ().
Table 1. Demographic data of the studied patients.
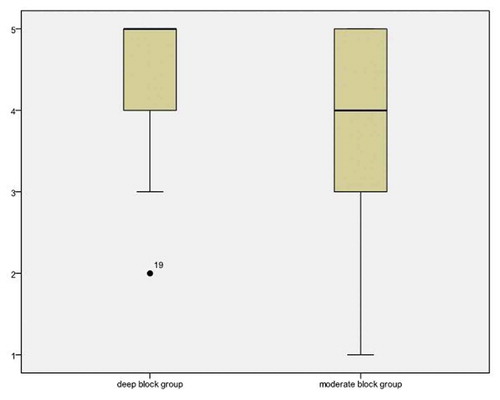
The surgeon did not find a significant difference between the surgical exposure scores of the two groups (5 [Citation4,Citation5] in the deep neuromuscular block group versus 4 [Citation3–Citation5] in the moderate neuromuscular block group, with 95% confidence interval 0–1 and P = 0.243) (). Besides, there was an insignificant difference between the two groups in the distribution of surgical exposure score on the 5-point scale (0/1/3/7/19 in the deep block group and 1/2/5/8/14 in the moderate block group, with P = 0.618). There was also a statistically insignificant difference between the two groups regarding the increase in intra-abdominal pressure above 15 CmH2O, not related to pressure exerted by the surgeon or instruments on the abdominal wall, and intraoperative abdominal or diaphragmatic movements (P = 0.299 and 0.424, respectively) ().
Table 2. Intraoperative criteria of both groups.
The measurements of the intraoperative pulmonary mechanics that included Ppeak, Pplat, Pmean, and Cdyn revealed a statistically insignificant difference between the two groups across all time intervals (P > 0.05) ().
Table 3. Intraoperative pulmonary mechanics in the studied groups.
The preoperative pulmonary function tests, including PEFR, FEV1, FVC, and FEV1/FVC, in the two groups, were comparable (P = 0.337, 0.227, 0.258, and 0.467). There was also a statistically significant decrease in the mean values of postoperative PFTs compared to preoperative values in both groups. However, there was an insignificant difference between the two groups in the mean values of postoperative PFTs (P = 0.691, 0.507, 0.293, and 0.828) ().
Table 4. Preoperative and postoperative pulmonary functions in the two groups.
The time elapsed between the reversal of muscle relaxation and performing postoperative pulmonary functions in the two groups was comparable (P = 0.245). There was a statistically significant increase in the mean value of the total dose consumption of rocuronium in the deep neuromuscular block group compared to the moderate neuromuscular block group (P < 0.0001). However, the mean value of the total dose consumption of fentanyl in the two groups was comparable (P = 0.221) ().
Postoperatively, there was an insignificant statistical difference between the two groups in the time spent in the PACU (P = 0.602). Postoperative pethidine consumption was also indifferent between the two groups (P = 0.611). In addition, postoperative VAS was comparable, until discharge from PACU (P > 0.05). Moreover, there was an insignificant difference between both groups in the incidence of postoperative nausea and vomiting or the need for postoperative respiratory support by BiPAP or CPAP (P = 0.612 and 1.00, respectively) ().
Table 5. Postoperative criteria of the two groups.
5. Discussion
Anesthetic management of morbidly obese patients undergoing laparoscopic surgeries is faced with many challenges. These include the optimal degree of the neuromuscular blockade that should be used. Conflict arises mainly from the degree of surgical exposure and the effect on perioperative respiratory functions.
In this clinical study, deep neuromuscular block did not improve the quality of surgical exposure conditions during laparoscopic bariatric surgery, compared to moderate block. The intraoperative pulmonary mechanics, the change in postoperative pulmonary function tests, the incidence of postoperative complications, and postoperative pain were insignificantly different between the deep neuromuscular block and the moderate neuromuscular block groups.
The response of different muscles to neuromuscular blocking agents is not uniform; the abdominal muscles and diaphragm recover more rapidly from neuromuscular block than the adductor pollicis muscle [Citation19,Citation20]. The diaphragmatic movement remains possible even with deep neuromuscular block [Citation21]. Movement of the diaphragm or abdominal muscles is possible even though there is no response to TOF stimulation at the adductor pollicis muscle. These movements were evaluated in the current study by recording the increase in intra-abdominal pressure above 15 cmH2O and any intraoperative spontaneous diaphragmatic or abdominal muscle movement noted by the anesthesiologist or surgeon. There was an insignificant difference between the deep and moderate neuromuscular block groups regarding both.
Baete et al. [Citation8] compared deep with moderate neuromuscular block in 60 adult morbidly obese patients presented for laparoscopic bariatric surgeries. They concluded that the use of deep neuromuscular block was not associated with increased surgeon satisfaction of surgical exposure, improved control of intra-abdominal pressure, or decreased mean duration of surgery. Postoperative pulmonary functions were equally lower postoperatively than preoperatively, with both deep and moderate neuromuscular block.
Barrio et al. [Citation22] in their study also revealed that deep neuromuscular block had no added effect on surgical exposure conditions, and standard pneumoperitoneum pressure had a greater effect on improving surgical conditions. On the other hand, Torensma et al. [Citation23] concluded that deep neuromuscular block improved surgical conditions with less postoperative pain, compared to moderate neuromuscular block, during bariatric surgery. The inconsistency between their results and the results obtained in the current study may be due to their use of different methods of delivering the maintenance dose of muscle relaxation.
Martini et al. [Citation14] in a randomized study evaluated the effect of deep and moderate neuromuscular block in 24 adult patients presented for extra-peritoneal laparoscopy, for prostatectomy or nephrectomy. They found that deep neuromuscular block improved the quality of surgical conditions and did not significantly affect postoperative pulmonary functions. The discrepancy between the current study results and those of Martini may be explained by evaluation of retroperitoneal surgical field, which is surrounded by more skeletal muscles that require larger doses of muscle relaxants. They also evaluated the surgical field in two different surgeries managed by different surgeons, which may significantly affect the surgical rating score. Moreover, Blobner et al. [Citation24] evaluated the effect of deep neuromuscular block on surgical exposure in patients presented for laparoscopic cholecystectomy. They demonstrated that deep neuromuscular block was associated with an increase in quality of surgical exposure, through improving visibility, and a decrease in involuntary movements. This difference from the findings of the current study may be due to their comparison between deep neuromuscular block and no block at all.
The single-center randomized controlled study of Dubois et al.(13 found that deep blockade significantly improved the surgical field and reduced the unaccepted surgical conditions. They compared the deep neuromuscular block with the shallow one, which may explain the difference between their results and those of the current study. Moreover, Anne et al. [Citation12] conducted a randomized assessor-blinded study on 48 patients undergoing elective low-pressure laparoscopic cholecystectomy. Patients were randomly distributed to two groups: deep block group and moderate block group. Surgical exposure was found to be better with deep neuromuscular block, which differs from the results of the current study. This difference may be attributed to the use of low-pressure laparoscopy (8 mmHg), which may require more muscular relaxation.
Intraoperative respiratory mechanics and the decrease of postoperative pulmonary function tests were insignificantly different between the deep and moderate neuromuscular block groups. The morbid obese patients, especially those undergoing bariatric surgery, are at high risk of perioperative pulmonary adverse effects, including atelectasis, hypoxemia, postoperative pneumonia, and respiratory failure [Citation25,Citation26]. In addition to the significant physiological alteration of respiratory physiology and arterial oxygenation associated with morbid obesity [Citation27], CO2 pneumoperitoneum during laparoscopic bariatric surgeries increases the intra-abdominal pressure. The cephalic shift of the diaphragm also results in an increase in intrathoracic pressure, with a reduction in the lung volume and compliance with early airway closure and atelectasis in the dependent parts [Citation28–Citation30].
Reduction in postoperative pulmonary tests regardless the degree of muscle relaxation was recorded in morbidly obese patients undergoing laparoscopic bariatric surgery [Citation8] and in non-obese patients undergoing laparoscopic cholecystectomy [Citation24].
6. Limitation of this study
Limitation of this study mainly arose from the use of standard pressure laparoscopy only (15 mmHg) without comparing it to low-pressure laparoscopy. The use of different reversal agents of muscle relaxation in the two groups was also a limitation to the study.
7. Conclusion
Finally, it can be concluded that deep neuromuscular block in morbid obese patients undergoing laparoscopic bariatric surgeries, compared with moderate block, did not improve the quality of surgical exposure conditions. Moreover, the blockade effect on the intraoperative pulmonary mechanics, postoperative pulmonary functions, postoperative pain, and incidence of postoperative complications was nearly the same.
Authors contribution
Mohamed M. Abu Yazed contributed to the formulation of the study design, analysis, interpretation of the data, and final revision and submission.
Sameh Abdelkhalik Ahmed contributed in the study design, collection of the data, drafting, and revising the article. Also, he gave final approval to the final format of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Griffith HR, Johnson GE. The use of curare in general anesthesia. Anesthesiol J Am Soc Anesthesiologists. 1942;3(4):418–420.
- Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubationa randomized controlled trial. Anesthesiol J Am Soc Anesthesiologists. 2003;98(5):1049–1056.
- Dubois PE, Mulier J. A review of the interest of sugammadex for deep neuromuscular blockade management in Belgium. Acta Anaesthesiol Belg. 2013;64(2):49–60.
- Debaene B, Plaud B, Dilly MP, et al. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. J Am Soc Anesthesiologists. 2003;98(5):1042–1048.
- Berg H, Viby‐Mogensen J, Roed J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications: a prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41(9):1095–1103.
- Gallagher SF, Haines KL, Osterlund LG, et al. Postoperative hypoxemia: common, undetected, and unsuspected after bariatric surgery. J Surg Res. 2010;159(2):622–626.
- Saliman JA, Benditt JO, Flum DR, et al. Pulmonary function in the morbidly obese. Surg Obes Relat Dis. 2008;4(5):632–639.
- Baete S, Vercruysse G, Vander Laenen M, et al. The effect of deep versus moderate neuromuscular block on surgical conditions and postoperative respiratory function in bariatric laparoscopic surgery: a randomized, double blind clinical trial. Anesth Analg. 2017;124(5):1469–1475.
- Ghoneim MM, Weiskopf RB. Awareness during anesthesia. Anesthesiol J Am Soc Anesthesiologists. 2000;92(2):597.
- Plaud B, Debaene B, Donati F, et al. Residual paralysis after emergence from anesthesia. J Am Soc Anesthesiologists. 2010;112(4):1013–1022.
- Kopman AF, Naguib M. Laparoscopic surgery and muscle relaxants: is deep block helpful? Anesth Analg. 2015;120(1):51–58.
- Staehr-Rye AK, Rasmussen LS, Rosenberg J, et al. Surgical space conditions during low-pressure laparoscopic cholecystectomy with deep versus moderate neuromuscular blockade: a randomized clinical study. Anesth Analg. 2014;119(5):1084–1092.
- Dubois PE, Putz L, Jamart J, et al. Deep neuromuscular block improves surgical conditions during laparoscopic hysterectomy: a randomised controlled trial. Eur J Anaesthesiol. 2014;31(8):430–436.
- Martini C, Boon M, Bevers R, et al. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br J Anaesth. 2014;112(3):498–505.
- Jones RK, Caldwell JE, Brull SJ, et al. Reversal of profound rocuronium-induced blockade with sugammadexa randomized comparison with neostigmine. J Am Soc Anesthesiologists. 2008;109(5):816–824.
- Blobner M, Eriksson LI, Scholz J, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27(10):874–881.
- Geldner G, Niskanen M, Laurila P, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67(9):991–998.
- Cohen LB, DeLegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133(2):675–701.
- Kirov K, Motamed C, Dhonneur G. Differential sensitivity of abdominal muscles and the diaphragm to mivacuriuman electromyographic study. Anesthesiol J Am Soc Anesthesiologists. 2001;95(6):1323–1328.
- Donati F, Meistelman C, Plaud B. Vecuronium neuromuscular blockade at the adductor muscles of the larynx and adductor pollicis. Anesthesiology. 1991;74(5):833–837.
- Fernando P, Viby‐Mogensen J, Bonsu A, et al. Relationship between posttetanic count and response to carinal stimulation during vecuronium‐induced neuromuscular blockade. Acta Anaesthesiol Scand. 1987;31(7):593–596.
- Barrio J, Errando CL, García-Ramón J, et al. Influence of depth of neuromuscular blockade on surgical conditions during low-pressure pneumoperitoneum laparoscopic cholecystectomy: a randomized blinded study. J Clin Anesth. 2017;42:26–30.
- Torensma B, Martini CH, Boon M, et al. Deep neuromuscular block improves surgical conditions during bariatric surgery and reduces postoperative pain: a randomized double blind controlled trial. PLoS One. 2016;11(12):e0167907.
- Blobner M, Frick CG, Stäuble RB, et al. Neuromuscular blockade improves surgical conditions (NISCO). Surg Endosc. 2015;29(3):627–636.
- Eichenberger A-S, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95(6):1788–1792.
- Gupta PK, Gupta H, Kaushik M, et al. Predictors of pulmonary complications after bariatric surgery. Surg Obes Relat Dis. 2012;8(5):574–581.
- Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210.
- Andersson LE, Bååth M, Thörne A, et al. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiol J Am Soc Anesthesiologists. 2005;102(2):293–299.
- Strang CM, Hachenberg T, Fredén F, et al. Development of atelectasis and arterial to end-tidal PCO2-difference in a porcine model of pneumoperitoneum. Br J Anaesth. 2009;103(2):298–303.
- Duggan M, Kavanagh BP. Atelectasis in the perioperative patient. Curr Opin Anesthesiol. 2007;20(1):37–42.