ABSTRACT
Background
Recovery from general anesthesia after endoscopic sinus surgery can abruptly become dangerous and having serious complications leading to lost intravenous cannulas, disconnected cables, self extubation, physical injury, increased pain and hemorrhage. This study aimed to evaluate the effects of adding ketamine to dexmedetomidine on smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery.
Methods
A prospective double-blind randomized controlled study. Ten minutes before ending of surgery, 94 patients were randomly divided into two equal groups: Group (KD) (n = 47): received ketamine 0.5 mg/kg and dexmedetomidine 0.5ug/kg completed to 10 ml 0.9% normal saline and Group (C) (n = 47): received 10 ml 0.9% normal saline.
Result
The extubation, recovery and discharge times were statistically significant prolonged in KD group (6.82 ± 0.76, 8.68 ± 0.81 and17.63 ± 4.8) min compared to C group (4.34 ± 0.61, 6.3 ± 0.72 and14.8 ± 2.7) min respectively. The median Ricker Sedation-Agitation scale (RSAS) score at the recovery time, at admission to PACU, at PACU and at discharge from PACU was statistically significant lower in KD group compared to C group (p < 0.0001). The mean Visual Analogue Scale (VAS) score on arrival to PACU, discharge from PACU, one hour, 2hs, 4hs, 8hs, 12hs and 24hs postoperative and pethidine requirements were statistically significant lower in KD group compared to C group (p < 0.0001). No statistically significant difference in side-effects between groups.
Conclusion
Adding 0.5 mg ketamine to 0.5ug dexmedetomidine provides smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery.
1. Introduction
Endoscopic sinus surgery is considered the most effective surgery for treatment of rhinosinusitis. General anesthesia is essential to provide optimal operating conditions. Awake extubation after endoscopic sinus surgeries is preferred as the airway is contaminated by blood and the nasal airway is blocked by surgical packs that cause sense of suffocation. Awake extubation and nasal packs are triggering causes of adverse effects on recovery from anesthesia [Citation1,Citation2].
Isoflurane is a rapid emergence inhaled anesthetic drug with minimal muscle relaxant effect. Thus, it is usually used in most nasal surgeries anesthesia but often linked with high incidence of emergence agitation [Citation3].
Dexmedetomidine is a selective α2 adrenoceptor agonist. It has sedative, hypnotic, anxiolytic, analgesic, sympatholytic properties and opioid sparing effect without respiratory depression. Ketamine is N-methyl- D-aspartate receptor antagonist; it has anesthetic, sedative and analgesic effects. Some studies had shown the protective effects of adding ketamine to dexamedetomidine on smooth recovery in children [Citation4–Citation6], meanwhile, scanty studies in adults.
Addition of low dose of ketamine to dexmedetomidine is supposed to produce less toxicity compared to each drug alone by decreasing the required doses, this can decrease some of the pitfalls occurring when use dexmedetomidine as a sole agent [Citation7]. To the best of our knowledge this study was the only one that was conducted to evaluate the effects of adding ketamine to dexmedetomidine on smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery.
2. Patients and method
This study was approved by the university’s Institutional Review Board (IRB #6017-9-3-2019) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment.
A prospective double-blind randomized controlled clinical study, conducted on 94 patients from March 2019 to February 2020.
The patients included in this study were of either sex, age between 21 and 60 years, belonging to American Society of Anesthesiologist (ASA) I, II physical status with body mass index (BMI) 25–35 kg/m2 scheduled to elective endoscopic sinus surgery under general anesthesia. Patients with chronic pain, altered mental status or with severe hepatic, cardiovascular, renal and neurologic disorders or on psychoactive, B agonist and painkiller drugs and pregnant women were excluded from this study.
2.1. General anesthesia
After routine preoperative evaluation, the standard monitors were attached to the patients: electrocardiogram, non-invasive blood pressure and pulse oximeter and baseline parameters were recorded {Heart rate (HR), mean arterial pressure (BP) and peripheral oxygen saturation (SPO2)}. For all patients, intravenous (IV) line was inserted and IV fluid had been started. Anesthesia induced with IV fentanyl 2 ug/kg and IV propofol 2 mg/kg, endotracheal tube placement was facilitated with 0.5 mg/kg IV atracurium. Following endotracheal intubation, anesthesia was maintained on 2% isoflurane in 100% O2, incremental doses of atracurium 0.1 mg/kg and fentanyl 1 ug/kg. Ventilation was adjusted to maintain the ETCO2 (end tidal CO2) at 30 to 35 mmHg. Ten minutes before the end of surgical procedure, patients were randomized into two equal groups by computer generated random number sequence:
Group (KD) (n = 47): Patients were received single intravenous bolus of mixture of ketamine 0.5 mg/kg and dexmedetomidine 0.5ug/kg completed to 10 ml 0.9% normal saline in one syringe.
Group (C) (control group) (n = 47): Patients were received single intravenous bolus of 10 ml 0.9% normal saline.
After ending of the surgery, the inhalational anesthetic will be turned off, and the muscle relaxant will be reversed by a combination of neostigmine 0.05 mg/kg plus atropine 0.01 mg/kg. The patient will be extubated after reach sufficient spontaneous breathing (>12 breath/minute) facial grimaces, gag reflex and purposeful motor movements [Citation8], and then transferred to the post anesthesia care unit (PACU).
In PACU, the patient on standard monitors and the outcome assessor (the anesthesiologist who is blind to the study) will assess the outcomes.
2.2. Assessment
Extubation time in minutes: The time from discontinuation of isoflurane to extubation.
Recovery time in minutes: The time from discontinuation of isoflurane to first response to verbal command.
Discharge time in minutes: The time from arrival to post-anesthesia care unite (PACU) to discharge to the ward according to modified Alderte score [Citation9] ().
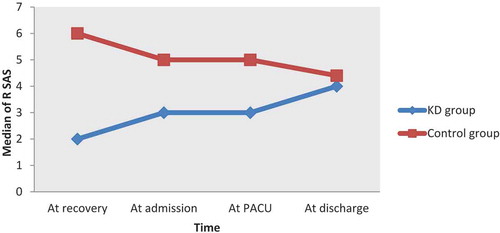
The level of emergence agitation is assessed by Ricker Sedation-Agitation scale (RSAS) [Citation10] at the recovery time, at admission to PACU, at PACU and at discharge from PACU. RSAS is seven scales; 1 = Unarousable (minimal or no response to noxious stimuli, not follow commands), 2 = Very sedated (arouses to physical stimulus but not follow the commands), 3 = Sedated (difficult to arouse but awaken to verbal stimulus or gentle shaking), 4 = Calm, cooperative (calm, awakens easily and follows commands), 5 = Agitated (anxious or restless, try to sit down and calms down to verbal instructions), 6 = Very agitated(does not calm despite frequent verbal reminding of limits, require physical restraint, biting the endotracheal tube), 7 = Dangerous agitation (pulling at endotracheal tube, thrashing side to side and striking at staff). Agitation was defined as ASAS score ≥ 5. Patients with score > 5 will be given 2 mg midazolam.
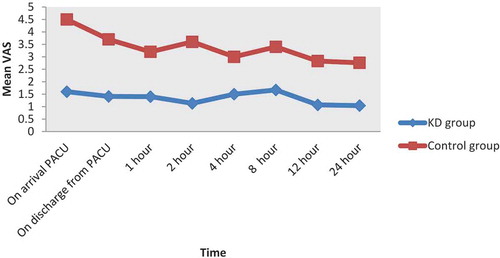
Postoperative pain will be evaluated using Visual Analogue Scale (VAS) [Citation11]. A commonly used scale is a 10-cm line labeled with “worst pain imaginable” on the right border and “no pain” on the left border. The patient is instructed to make a mark along the line to represent the intensity of pain currently being experienced. It was assessed on arrival to PACU, discharge from PACU, one hour, 2hs, 4hs, 8hs, 12hs and 24hs postoperative. Paracetamol 1 gm IV was given every 6 hours as a protocol for pain management, and IV pethidine 50 mg (rescue analgesic) was given if VAS≥3.
Any Side effects as: nausea or vomiting, or any other complication such as hypotension (mean arterial blood pressure decreases by >20% of basal reading), bradycardia (HR decreases by >20% of basal reading) or respiratory depression (RR < 8 breath/min).
Table 1. Modified Aldrete score.
2.3. Sample size
Assuming that the mean ± SD of postoperative pain score was 3.1 ± 1.7 in control group versus 2.2 ± 1.4 in dexmedetomidine group [Citation1]. So the total sample size is 94 cases (47 in each group) using Open Epi Info with confidence interval 95% and power of test is 80%.
2.4. Statistical analysis
All data were tabulated and statistically analyzed using SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation (SD) and analyzed by independent sample t test. While, qualitative data were expressed as number and percentage and were analyzed by Chi-Square test (χ 2). P-value was considered significant if ˂0.05 and highly significant if ˂0.001.
3. Results
One hundred patients were prepared for the study. However, four patients refuse to complete the study after their consent and for two patients their surgeries were cancelled. So, 94 patients were randomly divided among the two groups (47 patients for each) (). All the 94 participants in the two groups of the study were comparable regarding the age, sex, BMI, ASA physical status and the duration of surgery ().
Table 2. Patient’s characteristics and duration of surgery.
The extubation, recovery and discharge times were statistically significant prolonged in KD group (6.82 ± 0.76, 8.68 ± 0.81 and17.63 ± 4.8) min compared to group C (4.34 ± 0.61, 6.3 ± 0.72 and14.8 ± 2.7) min (p < 0.001) ().
Table 3. Comparison of the recovery data between groups.
The median distribution of RSAS score was statistically significant lower in KD group compared to Control group (p < 0.001) at the measured time points ().
Figure 2. Median for Riker sedation – agitation scale (RSAS) scores in KD group and control group at measured time points.
The total pethidine dose requirement postoperative was statistically significant lower in group KD (86.4 ± 13.46 mg) compared to group C (228.7 ± 12.8) (p < 0.001) (). As the mean of visual analogue scale score for pain in all recorded times was statistically significant lower in the KD group compared to C group (p < 0.001) ().
Figure 3. Mean visual analogue scale (VAS) scores in KD group and control group at measured time points.
There were no statistically significant side-effects between groups. Only one patient (2.12%) had vomiting in KD group compared to three patients (6.38%) in the control group with no statistically significant difference (p = 0.3) ().
4. Discussion
No ideal drug provides effective analgesia and sedation with stable hemodynamics, short recovery time and less side effects after general anesthesia so the anesthetists need to discover the ideal combination [Citation12]. In this study, we evaluate the effects of adding ketamine to dexmedetomidine on smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery.
In the present study, the extubation, recovery and discharge times were statistically significant prolonged in KD group compared to C group. Also, the median RASS score of agitation was statistically significant lower in KD group compared to C group. These findings were well correlated with Xu et al. [Citation1] and Jain et al. [Citation13] who reported prolongation of the extubation, recovery and discharge times when evaluating the effects of dexmedetomindine on recovery from general anesthesia but on contrary to Prasad et al. [Citation5] study as the time of discharge were significantly shorter in dexmedetomidine group compared to control group. The longer time of discharge in KD group in our study was related to augmentation of the sedative effect of dexmedetomidine by ketamine.
Ketamine dissociates the cortex from limbic system and has amnestic effects, analgesic effects at high doses and hypnotic effects at sub-anesthetic doses [Citation14]. Besides many previous studies reported that ketamine reduced the incidence of emergence agitation (EA) when compared to placebo [Citation15,Citation16] although, a lot of those studies were done on pediatrics. Recently, Demir and Yuzkat [Citation8] investigated the effect of sub-anesthetic doses of ketamine on EA in adult patients undergoing rhinoplasty reported that EA incidence was significantly reduced just after extubation and during follow-ups in PACU.
Meanwhile, Garg et al. [Citation17] revealed that dexmedetomidine was effective in reducing the incidence of EA by 89.5% measured by RSAS when investigating efficacy of dexmedetomidine for prevention of EA in patients posted for nasal surgery.
Hadi et al. [Citation4] reported that low-dose ketamine 0.15 mg/kg followed by dexmedetomidine 0.3ug/kg i.v (KETODEX), about 10 min before the end of surgery, significantly lower the severity of post sevoflurane EA compared to control group in children undergoing adenotonsillectomy. Also, an observational study done by Yerramilli et al. [Citation6] concluded that dexmedetomidine-ketamine were ideal combination providing smooth recovery with one improving the side-effects of the others for day care anesthesia in pediatric surgery and those were in context with the results of the current study.
Post-operative pain is one of the main triggering factors of emergence agitation [Citation18]. In this study, mean VAS score and pethidine requirements were significantly lower in KD group compared to C group. Our findings were in accordance with Hadi et al. [Citation4] study.
There were no significant side-effects between groups in this study except only one patient had postoperative vomiting in KD group and three patients in control group. This can be related to small dose of ketamine (<1 mg/kg) which has little effect on the blood pressure, heart rate, incidence of post-operative hallucination and less respiratory depression [Citation19–Citation21]. Similarly, dexmedetomidine produces dose-dependent sedation, analgesia and sympatholysis without any effect on respiratory function [Citation22].
5. Limitation
We did not estimate the preoperative anxiety and intraoperative depth of anesthesia by Bispectral Index (BIS) although they are risk factors for smooth recovery but, did not affect our results as we investigated the effects of adding ketamine to dexmedetomindine at the end of the surgery on smooth recovery profile from endoscopic sinus surgery whatever the cause. So, we recommend further studies.
6. Conclusion
Adding 0.5 mg ketamine to 0.5ug dexmedetomidine provides smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery.
Disclosure statement
There is no conflict of interest
References
- Xu K, Pan Yand Zhu M. Effect of dexmedetomidine on the recovery profiles from general anesthesia in patients undergoing endoscopic sinus surgery. Int J Clin Exp Med. 2016;9(5):8405–8410.
- Kim SY, Kim JM, Lee JH, et al. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;2:222–228.
- Singh R, Kharbanda M, Sood N, et al. Comparative evaluation of incidence and post-operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anesthesia. Indian J Anaeth. 2012;56:156–161.
- Hadi SM, Saleh AJ, Tang YZ, et al. The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int J Pediatr Otorhinolaryngol. 2015;79(5):671–676.
- Prasad K, Sophia P, Lakshmi BS. Bolus doses of ketofol versus dexmedetomidine for the prevention of emergence agitation in children: A prospective randomized controlled clinical trial. Int J Sci Stu. 2017;5:171–176.
- Yerramilli RN, Aavula M. Ketamine and dexmedetomidine for day care anesthesia in pediatric surgry. J Evolution Med. Dent. Sci. 2016;5:5257–5260.
- Kurhekar P, Vinod K, Rajarathinam B, et al. Randomized comparison between dexmedetomidine and midazolam for prevention of emergence agitation after nasal surgeries. Saudi J Anaesth. 2018;12(1):61–66.
- Demir CY, Yuzkat N. Prevention of emergence agitation with ketamine in rhinoplasty. Aesth Plast Surg. 2018;42:847–853.
- Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89–91.
- RR R, JT P, GL F. Prospective evaluation of sedation –agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329.
- McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–1019.
- Green SM, Roback MG, Kennedy RM, et al. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57(5):449–461.
- Jain V, Chaturved A, Pandia MP, et al. Effect of dexmedetomidine on recovery profile of patients undergoing anterior cervical discectomy and fusion. J Anesthesiol Clin Pharmacol. 2019;35:92–98.
- Bhat R, Santhosh MC, Annigeri VM, et al. comparison of intranasal dexmedetomidine and dexmedetomidine-ketamine for premedication in pediatric patients: a randomized double-blind study. Anesth Essays Res. 2016;10(2):349–355.
- Wang X, Deng Q, Liu B, et al. preventing emergence agitation using ancillary drugs with sevoflurane for pediatric anesthesia: a network meta-analysis. Mol Neurobiol. 2017;54(9):7312–7326.
- Fang XZ, Gao J, Ge YL, et al. Network meta-analysis on the efficacy of dexmedetomidine, midazolam, ketamine, propofol and fentanyl for the prevention of sevoflurane- related emergence agitation in children. Am J Ther. 2016;23(4):e1032–e1042.
- Garg A, Kamal M, Mohammed S, et al. Efficacy of dexmedetomidine for prevention of emergence agitation in patients posted for nasal surgery under desfluraneanaesthesia: A prospective double-blinded randomized controlled trial. Indian J Anaesth. 2008;62(7):524–530.
- De Silva LM, Braz LG, MÓdolo NS. Emergence agitation in pediatric anesthesia: current features. J Pediatr (Rio J). 2008;84(2):107–113.
- Jahangir SM, Islam F, Aziz L. Ketamine infusion for postoperative analgesia in asthmatics: a comparison with intermittent meperidine. Anesth Analg. 1993;76(1):45–49.
- Da Conceicao MJ, Bruggemann DA, Da Conceicao D, et al. Effectof an intravenous single dose of ketamine on postoperative pain in tonsillectomy patients. Pediatr Anesth. 2006;16:962–967.
- Butkovic D, Kraklik S, Matolic M, et al. Comparisonof a preincisional and postincisional small dose of ketamine for postoperative analgesia in children. Bratisl Lek Listy. 2007;108(4–5):184–188.
- Talke PO, Caldwell JE, Richardson CA, et al. The effect of dexmedetomidine on neuromuscular blockade in human volunteers. Anesth Analg. 1999;88(3):633–639.