ABSTRACT
Objectives
The study compared the effect of pre-emptive serratus plane block, with postoperative continuous drug delivery: into the serratus plane (bupivacaine 0.125% CDB), or around the wound (lidocaine 5% patches LP) on acute nociceptive and neuropathic pain after mastectomy.
Methods
This randomized-controlled blinded study was conducted on 43 women scheduled for mastectomy for breast cancer, under standard general anaesthesia and pre-emptive serratus plane block. Patients were randomly assigned to 2 groups according to postoperative analgesia: Group (S) received a 6 ml hourly doses of bupivacaine (0.125%) for 24 hrs using an epidural catheter inserted into the serratus plane and Group (L) received 2 LP around the wound for 12 hrs/24 hrs. IV morphine (3 mg) was given to patients with visual analogue scale >3 and repeated at 10 min intervals if needed.
Measurements
Primary outcome was visual analogue scale (VAS) for nociceptive pain at rest and arm movement for 24 hrs postoperatively. Secondary outcomes included incidence, characters and severity of acute neuropathic pain, using DN4 questionnaire and Neuropathic Pain Scale (NPS) for 4 weeks. Hypothesia to touch and temperature, mechanical allodynia and hyperalgesia were assessed between T2-T6 compared to the other side for 4 weeks. Patient satisfaction was measured by satisfaction score.
Results
There was no significant difference between the two groups regarding VAS at rest and movement, incidence, duration and sites of neuropathic pain and its effect on sleep, mood and work. The intensity of numbness measured by NPS was significantly less in Group L than Group S in the third postoperative week (P ≤ 0.05). Patients ‘satisfaction with postoperative pain relief was higher in Group L (P ≤ 0.05).
Conclusion
LP are as effective in reducing acute nociceptive pain as continuous bupivacaine delivery into the serratus plane. They are superior in reducing numbness and favoured by patients postmastectomy.
1. Introduction
Postoperative pain is encountered by 80% of patients undergoing surgical procedures, 75% of them reported moderate to severe pain [Citation1]. Inadequate control of postoperative pain and in particular after breast surgery for cancer gives a negative impact on functional recovery and quality of life and presents a risk for chronic postsurgical pain [Citation2,Citation3].
The American Pain Society recommends a multimodal approach for management of postmastectomy pain (PMP) [Citation4]. The multimodal approach includes systemic analgesics, local-anaesthetic-based regional analgesia techniques and non-pharmacologic therapies [Citation5]. Several factors affect analgesic outcomes; including the timing of analgesic delivery, as well as quality and duration of analgesics. For optimum control of PMP, analgesics should be initiated preoperatively and extended perioperatively to reduce acute pain severity and incidence of chronic PMP [Citation6]. Regional analgesia, as a part of multimodal analgesia for breast surgery for cancer, prevents receptors sensitization and windup potentiation, evokes opioid sparing and reduces immunological suppression [Citation7]. Furthermore, the use of local anaesthetics itself is proposed to have a suppressive effect on tumour cells by blocking upregulated Na channels on the cell surface [Citation8].
Serratus anterior plane block (SAPB) and Lidocaine 5% patches (LP) are two regional anaesthetic techniques used for management of postoperative pain specifically the neuropathic type [Citation9].
The current study compared the effect of LP to that of continuous delivery of bupivacaine (CDB), into the serratus plane, on acute nociceptive and neuropathic pain after mastectomy.
2. Patients and methods
After taking Ethical Committee approval and obtaining patients informed consent, this randomized prospective-blinded study was conducted on 43 female patients undergoing mastectomy for breast cancer. . The sample size, 20 patients in each group, was calculated using Medcalc program version 8.1.0.0 to detect a 30% mean difference in acute postoperative pain between the studied groups (measured by the visual analogue scale VAS) at 5% significance level (alpha) and 80% power.
All patients were American Society of Anesthesiologists physical status II (ASA II), admitted to the Surgical Department, Medical Research Institute Hospital, Alexandria University for mastectomy with axillary clearance. Exclusion criteria included history of previous breast surgery, heart block, neurological, neuromuscular or psychiatric disease, chronic pain, opioid dependence, preoperative chemotherapy and any contraindication to serratus block or to the studied drugs.
Ultrasound (U/S) guided preoperative SAPB was provided to patients in both groups. Postoperative analgesia was maintained for 24 hours, either through CDB into the plane between the serratus anterior and latissimus dorsi muscles or through the application of 2 LP 5% to the skin around the wound for 12 hours postoperatively.
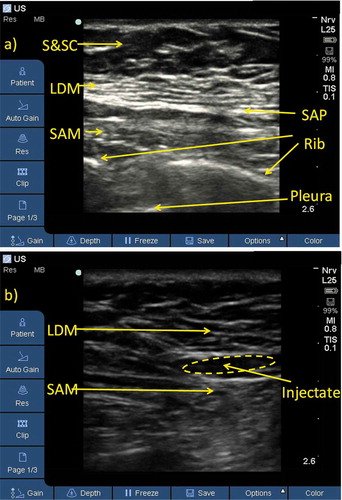
2.1. Pre-emptive serratus plane block
After premedication with intravenous (IV) midazolam (0.02 mg/kg) and fentanyl (0.5 μg/kg), each patient was asked to lie in lateral position with her arm forwards. A linear U/S transducer (10–12 MHz) of SonoSite (S nerve, 2D, Inc., USA) U/S machine was used to scan the ipsilateral chest wall at the level of the 4th-5th ribs between the mid and posterior axillary lines in a sagittal oblique plane (). After identification of the latissimus dorsi and serratus muscles, a 22-gauge needle was introduced from the lower end of the probe, using an in-plane approach, towards the fascial plane between the two muscles, and 0.4 ml/kg of 0.25% bupivacaine was injected. Ipsilateral sensory block was assessed every 3 minutes by loss of cold sensation to an ice pack. The adequacy of the sensory block (T2-T6), including the ipsilateral axilla was confirmed before the induction of anaesthesia.
2.2. Anaesthesia
General anaesthesia was induced with IV fentanyl (1 μg/kg), propofol (1–2 mg/kg) and rocuronium (0.6 mg/kg) to facilitate tracheal intubation. Anaesthesia was maintained with isoflurane (1–2%), air/oxygen mixture. Incremental doses of rocuronium (0.1 mg/kg) were given to maintain the train of four (TOF) count at 2 using the nerve stimulator module (TOF watch – Organon-Ireland). Anaesthesia was discontinued and residual neuromuscular block was reversed by neostigmine (0.04 mg/kg). The trachea was extubated and patients were transferred to the postoperative anaesthesia care unit (PACU) for the next 24 hours.
2.3. Postoperative analgesia
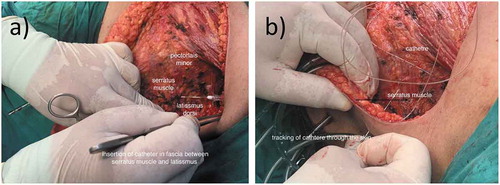
Patients in Group S received CDB using a closed continuous infusion system that includes an epidural catheter connecting to 300 ml elastomeric pump with silicon balloon reservoir. The catheter was inserted under vision during surgery between serratus anterior and latissimus dorsi muscles (). A 6 ml hourly dose of bupivacaine (0.125%) was delivered to the patient postoperative for 24 hours through the catheter. For patients in Group L, 2 LP were applied around surgical wound for 12 hours to provide regional analgesia for 24 hours postoperative. IV morphine (3 mg) was given, on demand, to any patient, in both groups, with VAS > 3 and repeated at 10 min intervals if needed.
2.4. Measurements
The primary outcome was VAS for acute nociceptive pain after local application of LP or CDB into the serratus plane. Secondary outcomes measured the incidence and severity of acute neuropathic pain throughout four postoperative weeks, patient satisfaction and complications for both analgesic techniques.
2.5. Measuring parameters/tools
VAS: measured nociceptive pain at rest and ipsilateral arm movement every hour for the first 4 postoperative hours then every 4 hours for 24 hours.
Postoperative morphine consumption was calculated for 24 hours.
DN4 (Douleur Neuropathique 4) questionnaire (10 Qs with cut-off value 4/10) [Citation10]: detected postoperative neuropathic pain weekly for 4 weeks.
Neuropathic Pain Scale (NPS): evaluated the character and intensity of neuropathic pain weekly for 4 weeks. The NPS describes how people may experience pain sensations differently and explains how unpleasantness differs from intensity. The scale presents 10 domains of pain, including two items that assess global pain intensity and pain unpleasantness and eight items that assess the specific qualities or locations of neuropathic pain. Patients were asked to rate each quality of pain (sharp, hot, dull, cold, numb, electric-like, burning, itchy and raw skin) on a scale of 0 to 10, where 0 = no pain and 10 = extreme severity [Citation11].
The onset, duration and distribution of neuropathic pain were recorded in addition to its interference with work, mood and sleep.
The patient satisfaction questionnaire was recorded 24 hours postoperative. It is a 5 point Likert scale (0–4point) (0 = strongly dissatisfied, 1 = dissatisfied, 2 = neutral, 3 = satisfied, 4 = strongly satisfied) assessed the satisfaction with postmastectomy medical care including information delivered to patients about postmastectomy pain (types and assessment), doctors’ attention to patients’ questions about pain and satisfaction with the method used for postoperative pain relief [Citation12].
Complete neurological examination was performed weekly for 4 postoperative weeks, on the ipsilateral arm, axilla and chest wall between dermatomes T2-T6 in comparison to other side. Hypothesia (absent sensation to blunt needle or cold ice pack), mechanical allodynia (pain on slight touch or repeated brushing) and hyperalgesia (exaggerated response to pin prick sensation or cold ice pack) were assessed and recorded.
2.6. Statistical analysis
Statistical analysis was performed using SPSS version 20. Normality testing was done through the Kolmogorov–Smirnov test. Normally distributed quantitative variables were described by the mean and standard deviation, while not normally distributed data were described through the median and range. Qualitative variables were described by their frequencies and %. Comparisons between the two studied groups were done through the independent t-test for normally distributed quantitative variables, while the Mann–Whitney test was used for not normally distributed variables. The Chi-squared test or the Fisher’s exact test was used to compare for qualitative data. Statistical significance was set at p ≤ 0.05.
3. Results
Patients’ characteristics and duration of surgery were comparable in both studied groups ().
Table 1. Patients’ characteristics and duration of surgery.
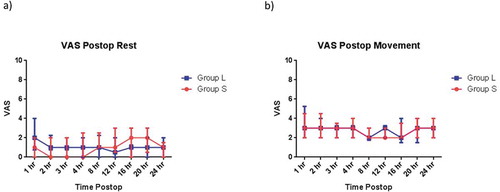
The visual analogue scale for pain at rest and movement and postoperative morphine consumption, during the first 24 hours postoperatively, did not differ significantly between the studied groups ().
Figure 4. Postoperative VAS (median, IQR) (a) at rest and (b) with arm movement for Group S (red squares) and Group L (blue circles).
DN4 questionnaire measured similar incidence of postoperative neuropathic pain among patients in both studied groups. No significant differences were detected between the two groups regarding the onset, duration and site of acute postmastectomy pain. The most frequent site for neuropathic pain was the ipsilateral arm. Neuropathic pain influenced work, sleep and mood comparably in both groups ().
Table 2. Douleur Neuropathique 4, site, onset and duration of neuropathic pain throughout 4 weeks after mastectomy.
The unpleasantness and pain intensity measured by neuropathic pain scale showed no significant differences between the two studied groups. The intensity of numbness was significantly less in Group L than Group S at the third postoperative week. Patients’ satisfaction scale with medical care and method of pain relief was higher in Group L ().
Table 3. Neuropathic pain scale (NPS) (0–10 cm) throughout 4 weeks after mastectomy and patients ‘satisfaction (0–4 point) with medical care and method of postoperative pain relief.
None of the patients in either of the studied groups was strongly dissatisfied, dissatisfied or neutral. Patients in Group L did not report any complications related to LP, five patients in Group S complained of stitching pain, with arm movement, at the site of the catheter insertion.
No significant differences in sensory evaluation between the studied groups. Hypothesia to touch and cold at ipsilateral T2-T6 dermatomes were the most frequent neuropathic pain signs encountered during sensory examination ().
Table 4. Neuropathic sensory changes at different thoracic dermatomes throughout 4 weeks after mastectomy.
4. Discussion
PMP is a mixed type of pain, including nociceptive, musculoskeletal and neuropathic pain, that could persist for a long time after surgery and influence quality of life badly [Citation3].
The best strategy to manage PMP is to prevent its occurrence or attenuate its intensity through delivering of pre-emptive multimodal analgesia [Citation13]. The current study compared two techniques of postoperative analgesia, as part of a multimodal approach for PMP. The effect of LP was compared to postoperative CDB into the serratus plane on acute nociceptive and neuropathic pain after mastectomy. The result of the current study showed that LP on the sides of the surgical incision showed similar postoperative analgesic effect to CDB in the serratus plane throughout the first 24 hours after surgery.
Fascial plane blocks, including Serratus anterior plane block (SAPB), have been reported for perioperative analgesia in breast surgeries, especially under ultrasound guidance [Citation14]. SAPB showed growing evidence in reducing pain scores and opioid consumption after breast surgery for cancer [Citation15]. It induces wide thoracic sensory loss as a result of suppression of conduction of the lateral cutaneous branches of the intercostal nerves, long thoracic nerve and thoracodorsal nerves [Citation16]. In one study, it provides a similar analgesic effect to thoracic paravertebral block that is considered the gold standard regional analgesic technique for breast surgery [Citation17]. It was comparable to thoracic epidural analgesia for thoracic surgery in cancer patients in another study [Citation18].
The postoperative analgesic effect of LP as a part of multimodal analgesia was discussed and encouraged in a set of studies. The topical LP 5% induces its effect by penetrating the skin and blocking sodium channel receptors, on the surface neuronal membranes, which are upregulated after tissue injury or trauma. Bound lidocaine prevents entry of sodium ions and reduces abnormal ectopic discharges produced by nociceptors; therefore interrupting the conduction of the pain [Citation19]. The design of the LP enables sufficient lidocaine delivery to block sodium channels in C and A delta fibres but not enough to block sodium channels on large myelinated Ab fibres thus no sensory loss is experienced [Citation19]. Furthermore, topical administration of lidocaine limits the incidence of side effect, where systemic absorption is minimal.
In the previous study, LP versus placebo patch was used with IV morphine patient-controlled analgesia (PCA) and ketorolac every 6 hours for postoperative pain relief after radical retropubic prostatectomy, where patients reported significantly less pain at rest and on coughing and significantly better pain relief in LP group. Furthermore, pain interfering with walking or deep breathing and mood was significantly less than the patients in the placebo group [Citation20]. LP was used effectively to reduce pain after laparoscopic surgeries; Kwon et al. reported significantly less pain score in patients received LP as a part of multimodal analgesia at different intervals for 36 hours after laparoscopic gynaecologic surgery compared to placebo [Citation21]. Furthermore, a superior analgesic effect of LP over no patch was reported for laparoscopic hernia repair [Citation22]. A recent randomised controlled study conducted on 48 patients for total knee replacement investigated the analgesic effect of LP versus no patch, as an adjuvant to standard analgesics, for 28 postoperative days. The study concluded that LP was effective in reducing pain and decreasing tramadol consumption during the period of the study, and added an analgesic value when used with other multimodal analgesic modalities [Citation23].
Despite the aforementioned favourable results, not all researches showed positive postoperative analgesic effects of LP. A study investigated the effect of LP on post-knee arthroscopy pain failed to demonstrate any additional pain relief after the application of LP 5% [Citation24]. Another randomized single-blinded study compared LP versus placebo for 6 months after robotic cardiac surgery showed that, no significant reduction in acute or persistent pain, between the studied groups. Patients in their study received postoperative IV fentanyl PCA for 3 days followed by oral opioid in both studied groups [Citation25]. A meta-analysis of five randomized trials, comparing the effect of LP to control (no treatment/placebo) for acute pain management, including post-surgical pain, showed that the application of LP may not be an effective adjunct for postoperative pain and the study called for further large, well-designed studies to get an evidence [Citation26]. The contrary results reported could be explained by the difference in surgical procedures, as well as the unique opioid-based anaesthesia for cardiac surgery which could mask the additive effect of postoperative LP.
The current study used DN4 to diagnose postmastectomy neuropathic pain for 4 weeks postoperatively. The number of patients who reported neuropathic pain was nearly similar in both groups. No significant differences were found in the incidence, onset, duration and distribution of postmastectomy neuropathic pain in four locations; axilla, arm, anterior and posterior chest wall. Moreover, pain interfering with work, sleep and mood showed no significant difference between the studied groups.
In consistence with the present result, Bouhassira and colleagues reported a high incidence of acute postoperative neuropathic pain after particular surgeries including breast surgery. The study showed that 39% of patients suffered from acute postoperative neuropathic pain continued to have pain of neuropathic component 2 months after surgery [Citation27].
In the current study, composite neuropathic pain scores were used to measure unpleasantness, intensity and characters of neuropathic pain. These parameters were comparable between the two studied groups except for the numbness that was the most common neuropathic pain character encountered by patients in both groups but its intensity at the third postoperative week was significantly less in Group L than Group S.
The efficacy of SAPB and LP as a part of multimodal analgesia for acute and chronic neuropathic pain was documented after breast cancer surgery. In a series of cases, SAPB was performed frequently with other pharmacological and non-pharmacological treatment for women reporting severe neuropathic pain after breast therapy for cancer. They experienced a marked reduction in their pain score and improvement in functional status and ability to perform daily activities [Citation28]. LP was approved, by the United States Food and Drug Administration in 1999, for the relief of pain of postherpetic neuralgia. Multiple studies showed that the LP 5% may be effective in alleviating neuropathic pain in patients with postmastectomy syndrome, diabetic polyneuropathy and complex regional pain syndrome [Citation29]. The superiority of LP over CDB in serratus plane on the intensity of numbness at third postoperative week could be explained by the synergistic analgesic effect of LP with pre-emptive SAPB. LP by its direct stabilizing effect on the upregulated nociceptors added more beneficial effect to pre-emptive SPB in attenuating nociceptors transmission, pain conduction and central sensitization. This desired effect was maximally noticed and reported by patients when they recovered and commenced their regular daily activities.
In the current study, patient satisfaction with medical care and the method of postoperative pain relief were measured by Likert scale, which is a bipolar scale where 0 = strong dissatisfaction and 5 = strong satisfaction [Citation12]. All patients involved in the present study were satisfied or strongly satisfied with medical care and the method of postoperative pain relief but the satisfaction score was higher in Group L than in Group S. The higher satisfaction scale in Group L could be explained by the simplicity and safety of LP, as no reporting of any complication after its application, on the other side, five patients in Group S reported stitching pain at the site of catheter insertion, in particular with arm movement.
Hypothesia to touch and coldness were the most frequent signs detected at the chest wall, axilla and ipsilateral arm during sensory examination in both studied groups. No difference in the sensory evaluation was recorded between the studied groups. The presence of sensory dysfunction after simple mastectomy was reported by Passavanti and colleagues in a retrograded study which measured allodynia around the wound, negative pin-prick test in the breast area and negative pin-prick test at the ipsilateral upper limb in 85%, 75% and 46% of patients, respectively. These sensory deficits were explained by the surgical traumatization of numerous nerves innervating the breast, the possibility of neuroma formation and chronic compression.
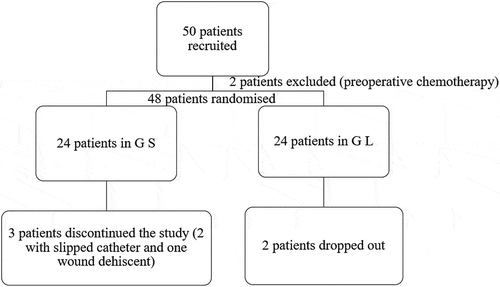
One of the limitations of the current study is the small sample size. Forty-eight patients were recruited to participate in the study; however, three patients dropped out of Group S (two due to slipped catheter and one due to wound dehiscence), and two patients dropped out of Group L. Another limitation was the follow-up period. Although patients were regularly followed up for 4 weeks post-operatively, a longer period of follow-up (12 or more weeks) is needed to determine the development of chronic PMP.
5. Conclusions and recommendations
LP, as a part of multimodal analgesia, is as effective as CDB into the serratus plane in reducing postmastectomy nociceptive pain, superior in reducing numbness and favoured by patients after mastectomy.
Further studies are needed to collect large samples from multicenter with longer follow-up periods.
Acknowledgments
The authors would like to acknowledge the effort and contribution of Dr Asmaa Abdelhameed Ahmed Hassan, Lecturer, Department of Biostatistics, Medical Research Institute, Alexandria University, Egypt.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540, table of contents.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Jung BF, Ahrendt GM, Oaklander AL, et al. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1–2):1–13.
- Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–157.
- Wolfe RC. Multimodal analgesia in the perioperative setting. J PeriAnesthesia Nurs. 2018;33(4):563–569.
- Lavand’homme P. From preemptive to preventive analgesia: time to reconsider the role of perioperative peripheral nerve blocks? Reg Anesth Pain Med. 2011;36(1):4–6.
- Le-Wendling L, Nin O, Capdevila X. Cancer recurrence and regional anesthesia: the theories, the data, and the future in outcomes. Pain Med. 2016;17(4):756–775.
- Niwa H, Rowbotham DJ, Lambert DG, et al. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth. 2013;27(5):731–741.
- Cheville AL, Sloan JA, Northfelt DW, et al. Use of a lidocaine patch in the management of postsurgical neuropathic pain in patients with cancer: a phase III double-blind crossover study (N01CB). Support Care Cancer. 2009;17(4):451–460.
- Spallone V, Morganti R, D’Amato C, et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetic Med. 2012;29(5):578–585.
- Jensen MP, Friedman M, Bonzo D, et al. The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clin J Pain. 2006;22(1):97–103.
- Boone H, Boone D. Analyzing likert data. J Ext. 2012;50(2):1–5.
- Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–634.
- Garg R, Bhan S, Vig S. Newer regional analgesia interventions (fascial plane blocks) for breast surgeries: review of literature. Indian J Anaesth. 2018;62(4):254–262.
- Rahimzadeh P, Imani F, Faiz SHR, et al. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a randomised clinical study. Turk J Anaesthesiol Reanim. 2018;46(5):388–392.
- NYSORA. Pectoral and serratus plane blocks. 2018. Available from: https://www.nysora.com/techniques/truncal-and-cutaneous-blocks/pectoralis-serratus-plane-blocks/ Access date: May 2018.
- Gupta K, Srikanth K, Girdhar KK, et al. Analgesic efficacy of ultrasound-guided paravertebral block versus serratus plane block for modified radical mastectomy: a randomised, controlled trial. Indian J Anaesth. 2017;61(5):381–386.
- Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31(1):152–158.
- Krumova EK, Zeller M, Westermann A, et al. Lidocaine patch (5%) produces a selective, but incomplete block of Adelta and C fibers. Pain. 2012;153(2):273–280.
- Habib AS, Polascik TJ, Weizer AZ, et al. Lidocaine patch for postoperative analgesia after radical retropubic prostatectomy. Anesth Analg. 2009;108(6):1950–1953.
- Kwon YS, Kim JB, Jung HJ, et al. Treatment for postoperative wound pain in gynecologic laparoscopic surgery: topical lidocaine patches. J Laparoendosc Adv Surg Tech Part A. 2012;22(7):668–673.
- Saber AA, Elgamal MH, Rao AJ, et al. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. Int J Surg. 2009;7(1):36–38.
- Sadigursky D, de Castro Oliveira M, Macedo JGG, et al. Effectiveness of lidocaine patches for pain treatment after total knee arthroplasty. Med Express (Sau Paulo). 2017;4:6.
- Khanna M, Peters C, Singh JR. Treating pain with the lidocaine patch 5% after total knee arthroplasty. Pm & R. 2012;4(9):642–646.
- Vrooman B, Kapural L, Sarwar S, et al. Lidocaine 5% patch for treatment of acute pain after robotic cardiac surgery and prevention of persistent incisional pain: a randomized, placebo-controlled, double-blind trial. Pain Med. 2015;16(8):1610–1621.
- Bai Y, Miller T, Tan M, et al. Lidocaine patch for acute pain management: a meta-analysis of prospective controlled trials. Curr Med Res Opin. 2015;31(3):575–581.
- Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36.
- Takimoto K, Nishijima K, Ono M. Serratus plane block for persistent pain after partial mastectomy and axillary node dissection. Pain Physician. 2016;19(3):E481–6.
- Devers A, Galer BS. Topical lidocaine patch relieves a variety of neuropathic pain conditions: an open-label study. Clin J Pain. 2000;16(3):205–208.