ABSTRACT
Background
Lung ultrasound could identify increased levels of extra-vascular lung water (EVLW) in severe preeclampsia (PE) also; increased optic nerve sheath diameter (ONSD) can indirectly reflect intracranial edema that could be a part of PE generalized edema. Our study aimed to evaluate the correlation between ONSD and EVLW in severe PE and to calculate ONSD cutoffs for prediction of pulmonary congestion severity degrees.
Methods
This prospective cohort study was conducted on 54 consecutively admitted singleton pregnant females complicated with severe preeclampsia from October 2019 to April 2020. Lung ultrasound for quantification of The Echo Comet Score (ECS) and optic nerve ultrasound assessments were performed for all enrolled parturients within 24 h before delivery and at 24 h post-delivery.
Results
Statistically high significant correlation was found between ECS and ONSD measurements both before and after delivery (r2 = 0.960, p < 0.001 and r2 = 0.847, p < 0.001 for before and after delivery, respectively). Statistically, both ECS and ONSD were highly significant lower at 24 h after delivery compared to the before delivery. The albumin level was statistically highly significant low in patients with severe pulmonary congestion. ONSD measured within 24 hours before delivery with 5.6–6.4 mm in diameter could predict moderate pulmonary congestion with 94.12% sensitivity and 100% specificity and ONSD > 6.4 mm predict severe pulmonary congestion with 100% sensitivity and 94% specificity.
Conclusion
ONSD is significantly correlated with EVLW in severe preeclampsia and it can predict the degree of pulmonary congestion with cutoff >6.4 mm predicts severe pulmonary congestion.
1. Introduction
Severe preeclampsia (PE) is a progressive multisystem pregnancy disorder. It is considered the second leading cause of maternal death worldwide. Usually, it is diagnosed by the new-onset hypertension and either proteinuria or end-organ dysfunction in the second half of pregnancy [Citation1].
Acute Pulmonary edema is potentially lethal and is the most common cardiopulmonary complication of preeclampsia [Citation2]. Thus, meticulous fluid management of these patients is crucial but it is often difficult because the underlying endolethial damage leads to water, electrolytes, and plasma leakage from the intravascular space which produce significant fluid shifts into the interstitial space resulting in peripheral and/or central (pulmonary and central nervous system) edema. Also, there is a potential for hypovolemia due to the depletion of intravascular volume. Under-resuscitation of preeclampsia patients impairs organ perfusion; while, on the other hand, fluid overload leads to tissue edema and aggravates pulmonary edema. Therefore, fluid administration must be assessed to preserve organ perfusion, while preventing lung congestion and pulmonary edema [Citation3–5].
Early detection of lung congestion would allow early and optimal management of these patients. Lung ultrasound was reported as a useful diagnostic tool which could identify increased levels of extra-vascular lung water (EVLW) in severe PE before clinical signs of pulmonary edema appear [Citation6,Citation7]. Consequently, lung ultrasound could guide fluid management and identify those in need for diuretic therapy among severe PE patients [Citation8]. Though it is considered accurate, safe, and non-invasive valuable tool, its use could be limited by the need to several measurements that could be time consuming.
Changes in the optic nerve sheath diameter (ONSD) detected by ultrasound are considered an important manifestation of increased intracranial pressure (ICP). The normal optic nerve sheath diameter measures up to 5.0 mm and an average ONSD more than 5 mm is considered abnormal and elevated intracranial pressure should be suspected [Citation9]. Cerebral edema have been demonstrated in 71% to 100% of magnetic resonance imaging in preeclampsia patients [Citation10] and an increase in ONSD has been described in preeclamptic females compared to healthy pregnant females [Citation11-13]. Therefore, Increased ONSD can indirectly reflect the state of intracranial edema that could be a part of generalized edema of preeclampsia [Citation14] and it could be a possible marker of generalized tissue edema and fluid overload in these patients.
Chen et al., studied ONSD and the intravascular volume status of patients after cardiac surgery and found that changes in ONSD can dynamically mirror changes in volume status in patients with post-operative cardiac surgery [Citation15] however more data on the correlation between ONSD and markers of fluid status are needed.
We hypothesized that changes in ONSD which is quick, safe, and repeatable bedside test are correlated with EVLW detected by ultrasound as a volume status marker in severe PE patients and could be recommended as a guide for volume assessment and management is these patients.
2. Patients and methods
2.1. Study design and population
This prospective observational cohort study was carried out on 54 singleton pregnant females complicated with severe preeclampsia aged 18–40 years old who were admitted to Surgical Intensive Care Unit, Zagazig University Hospitals between October 2019 to April 2020 and informed written consent from patients or their legal guardian was obtained before enrollment in the study. The institutional review board approval (The research ethical committee of Faculty of Medicine, Zagazig University) was obtained with the reference number (ZU-IRB#: 6074–26-4-2020) and our study was retrospectively registered under clinicaltrials.gov (NCT04367519).
Patients who refuse to participate in the study and those who were diagnosed with mild preeclampsia as well as patients with a history of prior ocular trauma or surgery, presence of ocular wound; preexisting heart disease, known pulmonary disorders, interstitial lung disease, and pneumonia were excluded from our study.
Before enrollment into the study, all the patients were fully examined by an obstetrician who was not involved in the study to diagnose those with severe preeclampsia. As per our hospital’s protocol, all patients diagnosed with severe preeclampsia were admitted and managed in intensive care unit pre-delivery and for at least 24 hours post-delivery. Consecutively admitted patients to Surgical Intensive Care Unit during the study period fulfilling the inclusion criteria were enrolled. Patient management was according to the existing protocols and no change was done by the investigators during the study period.
Preeclampsia was diagnosed when systolic blood pressure (SBP) >140 mmHg and/or diastolic blood pressure (DBP) >90 mmHg presenting after 20 weeks of gestation with significant proteinuria and severe preeclampsia was determined by the presence of one or more of the following severity features of the American College of Obstetricians and Gynecologists (ACOG) [Citation16]: if systolic blood pressure (SBP) ≥160 mmHg or diastolic blood pressure (DBP) ≥110 mmHg measured on two occasions at least 4 hours apart, thrombocytopenia (platelet count less than 100,000 * 109/L), impaired liver function diagnosed by elevated liver enzymes to twice the upper normal concentration limit, severe persistent not explained epigastric or right upper quadrant pain not responding to medications, renal insufficiency determined by serum creatinine concentration >1.1 mg/dL or doubling of the serum creatinine concentration in the absence of other renal disease, pulmonary edema, new-onset headache not responding to medications and not explained by alternative diagnoses, and/or visual disturbances.
All patients were cared with continuous blood pressure monitoring, fluid intake and urine output was assessed every hour, and blood investigations was repeated at least every 24 hours to monitor liver and kidney functions, electrolytes, complete blood count. Antihypertensive treatment to maintain systolic blood pressure <160 mmHg and diastolic blood pressure <110 mmHg according to the standard unit protocol and Eclampsia prophylaxis with Magnesium sulfate infusion as 4 gm intravenous loading dose, followed by 1 gm/hour infusion for at least 24 hours was initiated on ICU admission. Intravenous and oral fluid intake was minimized just to maintain euvolumia.
Ultrasound assessment was performed for all enrolled parturients within 24 h before delivery and at 24 h post-delivery.
2.2. Performance of lung ultrasound
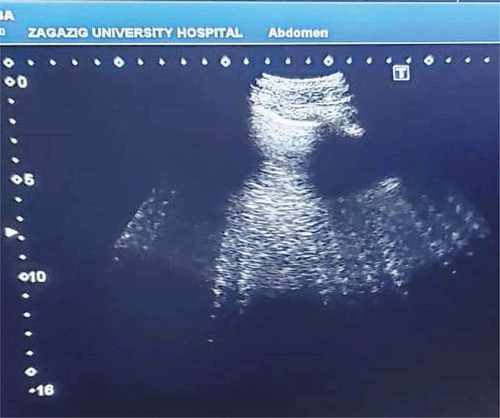
Lung ultrasound (LUS) assessment was performed with the patient in supine position by TOSHIBA Xario 100 ultrasound machine with 3–6 MHz curvilinear probe using the standard technique for quantification of The Echo Comet Score (ECS). The 28-rib interspaces technique which divides the chest wall in 12 areas on the left (from the second to the fourth intercostal space) and 16 areas on the right (from the second to the fifth intercostal space). Each intercostal space is scanned in four different positions (para-sternal, midclavicular, anterior axillary, and mid-axillary). Within each window; B-lines were identified and counted. The number of summed B-lines generates the ECS diagnosing the amount of EVLW (). B-lines are multiple hyperechoic reflections arising from the pleural line, having narrow base and spreading away from the transducer towards the bottom of the screen with a to-and-fro movement synchronized with lung sliding and without fading (). Up to 2 B-lines per single intercostal space, or up to 5 in the comprehensive scan can be a normal finding [Citation17–19].
Table 1. Echo comet score of B-lines [Citation19].
2.3. Ultrasound measurement of optic nerve sheath diameter
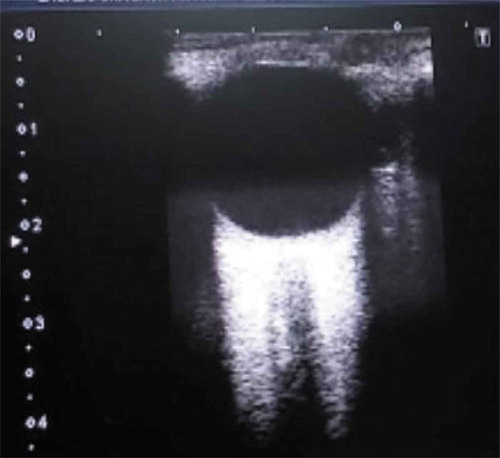
Ocular ultrasonography was performed with the patients placed in supine position with closed eyes. A layer of ultrasound gel was applied over the closed upper eyelid and the liner high-frequency probe 7–12 MHz of TOSHIBA Xario 100 ultrasound machine was placed on the temporal area of the eyelid with the hand holding it resting on the forehead of the patient, to prevent unnecessary pressure being exerted on the eye. The probe was then adjusted to a suitable angle in order to display the entry of the optic nerve into the globe. ONSD is then measured 3 mm behind the globe in the transverse plane perpendicular to the optic nerve () [Citation11]. For each eye, one measurement was made and the reported ONSD corresponds to the mean of the two values obtained for each patient.
2.4. Sample size calculation
Our study aimed to determine the correlation between ONSD and EVLW and to find out the cutoffs for ONSD to be able to classify pulmonary congestion in severe preeclampsia patients into three degrees of severity, i.e., mild, moderate, and severe. To be able to define the cutoff between mild and moderate we supposed that there is a significant difference between both groups regarding ONSD. From our pilot study, ONSD (mean and SD) was 5.40 ± 0.35 mm and 6.44 ± 0.26 mm in mild and moderate groups, respectively, at power of 0.8 and level of significance of 0.05 with 3 patients in each group. Also, to be able to define the cutoff between moderate and severe we supposed that there is a significant difference between both groups regarding ONSD. From our pilot study, ONSD (mean and SD) was 6.44 ± 0.26 mm and 6.94 ± 0.20 mm in moderate and severe groups, respectively, at power of 0.8 and level of significance of 0.05 with 5 patients in each group.
As our design is cohort so to be able to get at least five severe preeclampsia patients with pulmonary edema and in view that acute pulmonary edema may develop in up to 5% of preeclampsia patients [Citation20]. The appropriate sample size is 54 patients at power of 0.8 and level of significance of 0.05.
2.5. Statistical analysis
Data collected throughout history, basic clinical examination, laboratory investigations and ultrasound measurements were coded, and entered using Microsoft Excel software then they were imported and analyzed using Statistical Package for the Social Sciences software for windows (SPSS version 22.0). According to the data type qualitative data and categorical variables were expressed as number (percentage), quantitative data and continuous variables were represented by mean ± SD, the following tests were used to test differences for significance; Mann Whitney, Wilcoxon Signed Ranks test, and Kruskal Wallis test. Correlation by using Pearson’s correlation test to evaluate the relationship between ECS and ONSD values. P-value was set at <0.05 for statistical significant results and P-value <0.001 for high statistical significant results. To determine the ONSD cutoffs for predicting pulmonary congestion severity, receiver operator characteristic (ROC) curves were used. For each ROC curve, the sensitivity, specificity, positive and negative predictive values were calculated as well as the optimal ONSD cutoff point with maximal sensitivity and specificity for predicting mild, moderate, and severe pulmonary congestion.
3. Results
During the study period fifty four severe preeclampsia parturients with mean age 28.31 ± 5.21 years and mean gestational age 34.81 ± 1.68 weeks were included. Baseline clinical, demographic and laboratory data are presented in . All participants had one or more of severe preeclampsia features and all of them had lung congestion. Hypertension (systolic blood pressure (SBP) ≥160 mmHg or diastolic blood pressure (DBP) ≥110 mmHg) was the commonest severity feature (94.4%, n = 51) and it was found as the only feature of severity in 30 parturients (55.6%) while hypertension and visual disturbances was found in 20 parturients (37%) one of these patients was presented with symptomatic pulmonary edema (1.9%) as well as only one parturient suffered hypertension with thrombocytopenia (1.9%). The three participants in whom hypertension was not a sign of severity, severe preeclampsia was diagnosed by thrombocytopenia with liver impairment in two parturients (3.7%) and with renal impairment in one parturient (1.9%) ().
Table 2. Patients’ characteristics, severe clinical features of preeclampsia and laboratory results on admission.
For all participants the ultrasound measurements were performed within 24 h before delivery and at 24 h after delivery. Statistically, both ONSD and Echo Comet Scores were highly significant lower at 24 h after delivery compared to the before delivery measurements ().
Table 3. ECS and ONSD mean values before and after delivery for all participants.
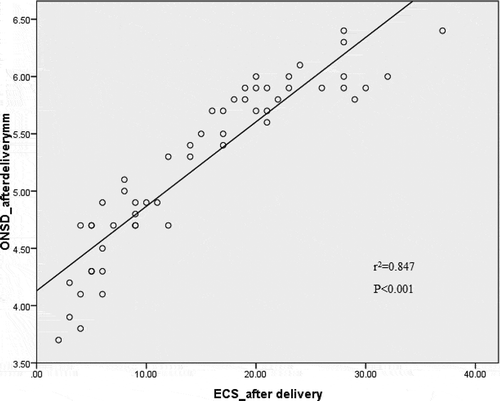
Statistically high significant correlation was found between ECS and ONSD measurements both before and after delivery (rCitation2 = 0.960, p < 0.001 and r2 = 0.847, p < 0.001 for before and after delivery, respectively) (, ).
Figure 3. Correlation between optic nerve sheath diameter and extra-vascular lung water by ultrasound measurements before delivery. ONSD, optic nerve sheath diameter; ECS, echo comet score.
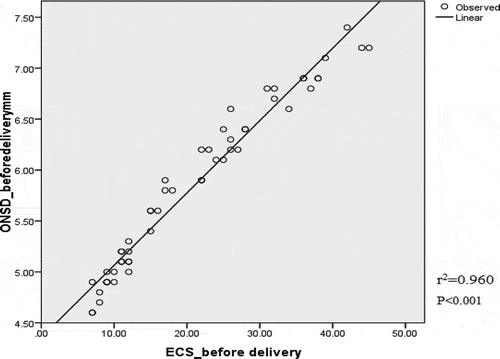
Figure 4. Correlation between optic nerve sheath diameter and extra-vascular lung water by ultrasound measurements at 24 h after delivery. ONSD, optic nerve sheath diameter; ECS, echo comet score.
According to the lung ultrasound Echo Comet Score values measured before delivery the enrolled cohort were classified into three degrees of pulmonary congestion severity: mild congestion group (n = 24 patients, 44.4%), moderate group (n = 17 patients, 31.5%), and severe group (n = 13 patients, 24.1%). There were no statistical significant differences among the three groups regarding the age, weight, height, and gestational age as well as the laboratory results on admission except the albumin level was statistically highly significant low in patients with severe pulmonary congestion while there was high statistical significant difference among the three groups regarding the before and after delivery mean values of both ECS and ONSD ().
Table 4. Characteristics and laboratory results of the three pulmonary congestion severity degree patients.
To be able to differentiate between patients with mild, moderate, and severe pulmonary congestion using the ONSD rather than the more complicated ECS measurement, statistical significant difference was found between mild and moderate groups regarding ONSD before delivery and a cutoff point was estimated to be 5.6 mm below it, patients have mild pulmonary congestion and above it, they have moderate degree of pulmonary congestion () also, statistical significant difference was found between moderate and severe groups and a cutoff point was estimated to be 6.4 mm below it, patients have moderate pulmonary congestion and above it, they have severe degree of pulmonary congestion () ().
Figure 5. ROC curve of before delivery ONSD for detection of mild versus moderate pulmonary congestion.
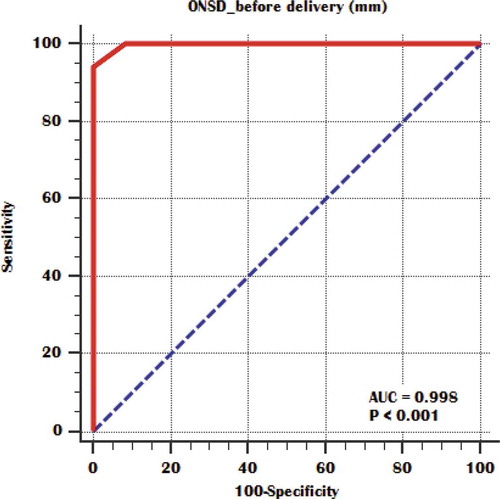
Figure 6. ROC curve of before delivery ONSD for detection of moderate versus severe pulmonary congestion.
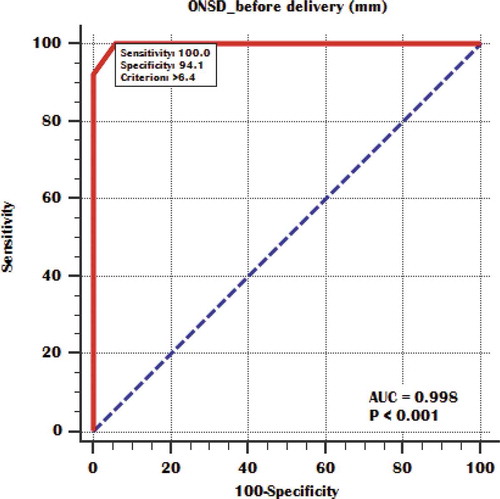
Table 5. Cutoffs of before delivery ONSD for detection of mild, moderate and severe pulmonary congestion.
4. Discussion
Our study main aim was to evaluate the correlation of ONSD with EVLW detected by ultrasound as a volume status marker in severe preeclampsia patients and to calculate ONSD cutoff points for prediction of different pulmonary congestion severity degrees in these patients. Our results demonstrated that ONSD significantly correlates with EVLW amount in severe preeclampsia patients both before and after delivery and it could indicate the volume status and guide the fluid management in these patients. We found that ONSD measured within 24 hours before delivery with 5.6–6.4 mm in diameter could predict moderate pulmonary congestion with 94.12% sensitivity and 100% specificity as well as a more dilated optic nerve sheath of more than 6.4 mm in diameter could diagnose severe pulmonary congestion in severe preeclamptic patients.
Supporting our results that ONSD could indicate the volume status, the results of Chen et al., study which demonstrated that in patients after cardiac surgery changes of ONSD were related to changes of central venous pressure and inferior vena cava diameter as well as the cumulative fluid balance and they reported that ONSD measurement could help in cerebral edema assessment and its changes dynamically mirror volume status changes in patients post-cardiac surgery [Citation15]. Also, our findings are in accordance with Brzan Simenc et al., who evaluated the use of ONSD measured within 24 hours from delivery in fluid assessment of thirty severe preeclampsia patients by investigating its relation with EVLW estimated by lung ultrasound and found that the median ONSD was 5.7 mm and median ECS value 19 with significant correlation between them and they concluded that optic nerve ultrasound is a helpful diagnostic tool for detection of preeclampsia patients with fluid overload [Citation21].
On the other hand, Ortner et al. who studied the abnormalities in point of care ultrasound including cerebral edema, pulmonary interstitial syndrome, and cardiac dysfunction prevalence in late onset severe preeclampsia and their associations with severe features. The authors reported no association between increased ONSD and any parameter in cardiac or pulmonary ultrasound or the albumin concentration [Citation22]. This could be attributed to performing different lung ultrasound method in their study as they rely on the 8-region method and diagnose the pulmonary interstitial syndrome depending on the B-line pattern which is defined by the presence of two or more positive regions per side, the lung region is considered positive if there are 3 or more B lines and the sum of B-lines counted in all regions yield the B-line score. While in our study we depend on the more detailed 28-rib interspaces technique to estimate the EVLW and ECS.
There is a well-established association of severe PE with the increased amount of EVLW and the high incidence of pulmonary edema both before and immediately after delivery [Citation3] and this increased EVLW amount decreases rapidly in the first postpartum days [Citation8,Citation18] such that at 24 hours after delivery we observed a significant decrease in the Echo Comet Score and ONSD mean values compared with the pre-delivery mean values. This is in agreement with Ambrozic et al., who reported higher Echo Comet Score values in severe PE patients before delivery and a rapid decrease within 24 hours following delivery [Citation18]. The changes of ECS values over time were observed in another study by Ambrozic et al., who demonstrated a significant higher ECS in severe preeclampsia patients both before and 1 day after delivery than in healthy controls and no difference at day 4 post-delivery and this significant decrease of ECS values in severe PE patients was between day 1 and 4 after delivery while there was no difference in ECS values before delivery versus 1 day after delivery [Citation8].
The development of cerebral and pulmonary edema in PE patients had many risk factors that could include colloid osmotic pressure with its main determinant the serum albumin level, increased vascular permeability and capillary leak, as well as the capillary hydrostatic pressure [Citation22]. In our study, we observed a significant low albumin level in severe PE patients with severe degree of pulmonary congestion (2.86 ± 0.24 gm/dl) versus those with mild and moderate degrees of pulmonary congestion (3.6 ± 0.18 gm/dl and 3.31 ± 0.25 gm/dl for mild and moderate degrees, respectively) with the mean serum albumin level of all enrolled PE patients in our cohort was 3.34 ± 0.36 mg/dl. This finding is further corroborated by the results reported by Gojnic et al. in their study found that serum albumin concentration was correlated to PE severity [Citation23] and Seong et al., who found that the serum albumin level of 3 gm/dl is the threshold below which pulmonary edema is more likely to develop [Citation24].
ONSD cutoff point seems to be affected by several factors such as the underlying pathology of intracranial hypertension and its duration [Citation23]. Dubost et al. found an increase in ONSD in 19% of preeclampsia patients with a median ONSD of 5.4 mm [Citation11]. The same 5.4 mm ONSD was found in late onset preeclampsia females included in Ortner et al. study [Citation22]. While Arzpeyma et al. in their case control study demonstrated that 4.55 mm is the best ONSD cutoff for diagnosing existing preeclampsia [Citation13]. Several previous studies document that ONSD more than 5.8 mm equals intracranial pressure above 20 mmHg [Citation25–27]. Brzan Simenc et al. reported 43% of severe preeclampsia females with the measured ONSD above 5.8 mm [Citation12] also; Arzpeyma et al. reported that 34.2% of their preeclampsia patients had ONSD ≥ 5.8 mm [Citation13].
Our results indicate that ONSD seems to be a marker of systemic edema and fluid overload in severe preeclampsia by its significant correlation with EVLW in these patients and we found that it could effectively predict the amount of excess EVLW and the degree of pulmonary congestion and so guide the fluid management by identifying patients who would benefit from fluid restriction or even the need for diuretics. ONSD cutoff between 5.6 and 6.4 mm could predict moderate pulmonary congestion while severe pulmonary congestion and pulmonary edema should be anticipated with ONSD > 6.4 mm.
One of the main strengths of this study is evaluating the ONSD correlation with EVLW both before and after delivery and also we opted to include only patients with severe preeclampsia as they are actually critically ill pregnant females with increased incidence of pulmonary edema.
On the other hand, there were several limitations to our study. First, this study was not designed to interfere in the clinical management and we did not evaluate the prognostic value of ONSD measurements in these patients. Yet, our findings suggest that ultrasound evaluation is a useful adjunct to clinical examination and of a great importance in the prediction of disease severity. Second, we did not include healthy pregnant controls as lung ultrasound in previously published literature found no B-patterns in healthy pregnant females >36 weeks gestation [Citation28]. Moreover, previous preeclampsia studies applying lung ultrasound found no B-lines pattern in control groups [Citation7,Citation8]. Also, ONSD has not been found to be increased in any healthy controls of previous studies [Citation11,Citation22]. Lastly, the assessment of ECS with 28 ribs interspaces technique which requires several measurements and is time consuming. Therefore, there is a need for further studies to evaluate the simplified eight regions lung ultrasound method.
5. Conclusion
In conclusion, the rapid, safe, and repeatable ultrasound measurement of ONSD is significantly correlated with EVLW estimated by lung ultrasound in severe preeclampsia patients and it effectively predicts the amount of excess EVLW and the degree of pulmonary congestion with cutoff >6.4 mm predicts severe pulmonary congestion. The investigators of this study believe that ONSD as an easily accessible noninvasive test could be used for monitoring and assessment of volume status and for prediction of complications in severe preeclampsia and that a large scale future study focusing in confirmation of this finding and examining the effectiveness of using ONSD for diagnosing and reducing maternal complications related to fluid management in severe preeclampsia.
Disclosure statement
There are no conflicts of interest.
References
- Hariharan N, Shoemaker A, Wagner S. Pathophysiology of hypertension in preeclampsia. Microvasc Res 2017; 109:34–7.
- Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in Preeclampsia: an overview. Circulation. 2014; 130:703–14.
- Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia 2012; 67: 646–59.
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia. Current understanding of its pathophysiology. Nat Rev Nephrol 2014; 10: 466–80.
- Pretorius T, van Rensburg G, Dyer RA, Biccard BM. The Influence of Fluid Management on Outcomes in Preeclampsia: A Systematic Review and Meta-Analysis. Int J Obstet Anesth. 2018; 34:85–95.
- Shyamsundar M, Attwood B, Keating L, Walden AP. Clinical review: the role of ultrasound in estimating extra-vascular lung water. Crit Care. 2013; 17:237.
- Zieleskiewicz L, Contargyris C, Brun C, Touret M, Vellin A, Antonini F, et al. Lung ultrasound predicts interstitial syndrome and hemodynamic profile in parturients with severe preeclampsia. Anesthesiology. 2014; 120:906–14.
- Ambrozic J, Brzan Simenc G, Prokselj K, Tul N, Cvijic M, Lucovnik M. Lung and cardiac ultrasound for hemodynamic monitoring of patients with severe pre-eclampsia. Ultrasound Obstet Gynecol 2017; 49: 104–9
- Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014; 40(9):1267–74
- Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000; 217: 371–6.
- Dubost C, Le Gouez A, Jouffroy V, Roger-Christoph S, Benhamou D, Mercier FJ, Geeraerts T. Optic nerve sheath diameter used as ultrasonographic assessment of the incidence of raised intracranial pressure in preeclampsia: a pilot study. Anesthesiology. 2012; 116(5):1066–71.
- Brzan Simenc G, Ambrozic J, Prokselj K, Tul N, Cvijic M, Mirkovic T, et al. Ocular ultrasonography for diagnosing increased intracranial pressure in patients with severe preeclampsia. Int J Obstet Anesth 2018; 36: 49– 55.
- Arzpeyma SF, Khorram PB Asgharnia M, Mohtasham-Amiri Z. Investigating the relationship between ultrasound measured optic nerve sheath diameter and preeclampsia AIMS Medical Science 2019; 6(3): 250– 9.
- Ferro F, Rocha E, Nobrega L, Amorim MM, Katz L. Transorbital ultrasonographic measurement of the optic nerve sheath diameter in preeclampsia. Obst Gyn 2016; 127: 87.
- Chen H, Wang XT, Ding X, Zhang HM, Zhao H, Chao YG, et al. Correlation between optic nerve sheath width and volume status in patients after cardiac surgery. Chinese Journal of Internal Medicine 2016, 55(10):779–83.
- American College of Obstetricians and Gynecologists) ACOG): Gestational Hypertension and Preeclampsia, Obstetrics & Gynecology 2020; 135(6): e237–e260.
- Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS): International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–91.
- Ambrozic J, Prokselj K, Lucovnik M. Lung ultrasound and echocardiography for peripartum haemodynamic monitoring of patients with severe preeclampsia. The Journal of Maternal-Fetal & Neonatal Medicine 2016; 29: (2):25.
- Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. European Heart Journal. 2016; 37: 2097–104
- Pordeus AB, Katz L, Soares MC, Maia SB, Amorim MMR. Acute pulmonary edema in an obstetric intensive care unit, A case series study, Medicine 2018; 97 (28): 1–5.
- Brzan Simenc G, Ambrozic J, Prokselj K, Tul N, Cvijic M, Mirkovic T, et al. Optic nerve ultrasound for fluid status assessment in patients with severe preeclampsia. Radiol Oncol 2018; 52(4): 377–82.
- Ortner CM, Doz P, Krishnamoorthy V, Neethling E, Flint M, Swanevelder JL, et al. Point-of-Care Ultrasound Abnormalities in Late-Onset Severe Preeclampsia: Prevalence and Association With Serum Albumin and Brain Natriuretic Peptide. Anesth Analg 2019; 128:1208–16.
- Gojnic M, Petkovic S, Papic M, Mostic T, Jeremic K, Vilendecic Z, et al. Plasma albumin level as an indicator of severity of preeclampsia. Clin Exp Obstet Gynecol 2004; 31: 209– 10.
- Seong WJ, Chong GO, Hong DG, Lee TH, Lee YS, Cho YL, et al. Clinical significance of serum albumin level in pregnancy-related hypertension. J Obstet Gynaecol Res. 2010; 36(6):1165–73.
- Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J Neurosurg 1997; 87: 34–40.
- Geeraerts T, Launey Y, Martin L, Pottecher J, Vigue´ B, Duranteau J, et al. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med 2007; 33: 1704–11.
- Bäuerle J, Nedelmann M. Sonographic assessment of the optic nerve sheath in idiopathic intracranial hypertension. J Neurol 2011; 258: 2014–9.
- Arbeid E, Demi A, Brogi E, Gori E, Giusto T, Soldati G, et al. Lung Ultrasound Pattern Is Normal during the Last Gestational Weeks: An Observational Pilot Study. Gynecol Obstet Invest. 2017; 82(4):398–403.