ABSTRACT
Background
Auxiliary drugs augment the effect of local anesthetic in intravenous regional anesthesia (IVRA). The aim of our study was to estimate the median effective dose (ED50) of Dexmedetomidine in elective upper limb Lidocaine 0.5% IVRA.
Method
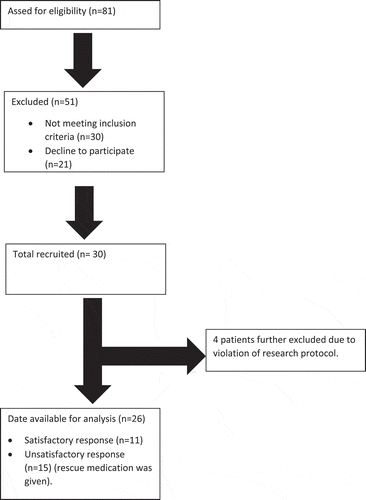
A prospective, double-blind, sequential study using modified Dixon’s up and down method. Thirty patients scheduled for minor upper limb surgeries were recruited in the Study, however, we ended up analyzing data of 26 patients due to protocol violation. The first patient in this sequential trial was randomly selected and received a mixed solution of 0.5% lidocaine and 0.4 µg/kg of dexmedetomidine, with 0.1 µg/kg dose interval. If IVRA outcome was satisfactory, the dose went down for the next patient by 0.1 µg/kg. Vice versa, if the outcome was unsatisfactory, the dexmedetomidine dose was stepped up by 0.1 µg/kg. Sensory and motor block onset, recovery times, hemodynamic variables, surgical and tourniquet VAS scores and time to first analgesic request were recorded.
Results
The series of patient responses to IVRA was satisfactory in 11 patients (42.3%) and unsatisfactory in 15 patients (57.6%) due to either surgical pain in 6 patients or tourniquet pain in the remaining 9 patients. In patients with satisfactory response to IVRA, intraoperative and postoperative mean arterial pressure and heart rate, compared with baseline (just prior to inflating the proximal cuff) measurement, showed no statistically significant differences (P = 0.754 and 0.324, respectively). Intergroup comparison of Patients with unsatisfactory response to IVRA time to first analgesic request showed no statistical difference (P > 0.05).
Conclusion
the ED 50 of Dexmedetomidine (95% confidence interval, CI) as adjuvant for satisfactory 0.5% lidocaine IVRA was 0.7 µg/kg (0.6–0.8).
1. Introduction
Since its innovation in 1908 by Dr August Bier of Germany, Intravenous regional anesthesia (IVRA) has stood the test of time as simple, safe and effective technique of regional anesthesia.[Citation1] The technique entails insertion of intravenous catheter into a surgical limb before isolating it from the general circulation by tourniquet then inject local anesthetic (LA) into this isolated limb. [Citation2] IVRA block is preferably used with short surgical procedures or maneuvers of the upper or lower extremity. Nevertheless, the technique has gained popularity for procedures of the upper extremity because of tourniquet issues like tourniquet pain, and other safety concerns that arise more often with lower extremity IVRA, essentially leakage of the of LA [Citation3]. Unfortunately, using LA solely for IVRA has not achieved optimum intraoperative analgesia or overcome the frequently reported tourniquet pain[Citation4]. A long list of adjuvants, to mention few: morphine, muscle relaxant and clonidine, have been used to augment LA action and ameliorate tourniquet pain. [Citation5] Dexmedetomidine (DEX) a potent α 2 agonist that have unique analgesic, anxiolytic and sedative profile has been called upon as attractive adjunct in general as well as regional anesthesia with enticing results. Several studies have pursued DEX median effective dose (ED50) in different settings, e.g. co-induction agent with Propofol, in laryngeal mask insertion and with local anesthetic to enhance the quality of spinal anesthesia. [Citation6] However, DEX effective median dose ED50 with IVRA for upper limb has not been investigated elsewhere, so far. The up and down sequential method of Dixon and Mood is a common sequential design method used in anesthesia research to detect the ED50. It is a sequence of experiments performed under the assumption that “satisfactory response” is followed by decrease in test dose for the next patient in the series, and “non-satisfactory response” is followed by increase in test dose for the next patient in the series. [Citation7] The purpose of this study is to estimate the median effective dose of Dexmedetomidine (DEX) with Lidocaine 0.5% IVRA in upper limb elective surgeries, using modified Dixon`s up and down method.
2. Patients and methods
Approval of the study proposal was granted from the ethics committee of Qena University Hospital, where the research took place, during the period from August 2019 to February 2020. Clinical trials registration number: NCT04304157. Written informed consent was obtained from each patient. Thirty patients aged between 18 and 60 years with American Society of Anesthesiologists physical status I or II undergoing minor upper limb orthopedic surgical procedures in the hand, wrist and forearms, were recruited in the study. Surgeries included: metacarpal fracture, tendon injury, and carpal tunnel release Exclusion criteria included patients with allergy to test drugs, vascular disease, contraindication to tourniquet applications, difficulty to manipulate the surgical limb, surgery expected to last more than an hour, and patient refusal.
In the operating theatre, a baseline recording of blood pressure, oxygen saturation, three leads ECG monitoring was initiated and continued through the entire procedure and post-operatively. An intravenous catheter was placed in the non-surgical limb for administration of fluids and rescue medications. Then, a double pneumatic cuff was applied to the surgical arm, and an intravenous catheter was inserted in the dorsum of the surgical hand before draining off the arm of blood, i.e. exsanguinate the limb by simply elevate the surgical limb above the level of the head of the supine patient for 3 minutes followed by applying Esmarch rubber band starting from the tip of the fingers towards the base of the arm. With the surgical arm elevated, we inflated the proximal pneumatic cuff to 100 mmHg above the patient’s systolic blood pressure, followed by removing the Esmarch rubber band. The absence of pulse oximetry tracing and non-palpable radial pulse confirmed that the vascular circulation to the limb was shut off and the limb was isolated from the general circulation. With the proximal cuff inflated, 10 ml lidocaine 2% plus predetermined dose of dexmedetomidine diluted with saline to total volume of 40 ml (to obtain Lidocaine 0.5%) was slowly infused over 3 min into the intravenous cannula of the surgical limb, then we removed the cannula and waited for the anesthetic to work.
The anesthetic mixture was prepared by a technician or anesthetist who were not aware of the study protocol and given by the attending anesthetist who collected the study parameters but remained blind to the content of the anesthetic mixture.
3. Monitoring sensory motor functions during and after the surgery
Intraoperatively, Sensation was assessed along the distribution of median, ulnar, radial and lateral antebrachial cutaneous nerves every minute, using pin prick. Onset of sensory loss was defined as time between completed infusion of the anesthetic mixture till loss of sensation to pin prick circumferentially along the limb. Motor block was tested also every minute by simply asking the patient to move his limb, and fingers. Onset of motor block was recognized as the time between complete infusion of the anesthetic mixture till complete paralysis of the limb. On reaching complete sensory and motor loss, the distal pneumatic cuff was inflated to 100 mm Hg above systolic pressure followed by deflation of the proximal tourniquet. The limb is ready for surgery to start.
For evaluation of both tourniquet and surgery pain we used visual analogue scale (VAS) 0–10 cm (0 being no pain and 10 unbearable pain). To monitor tourniquet pain, VAS scoring started at the inflation of the proximal tourniquet and every 5 mins till deflation. It is worth mentioning that tourniquet should be inflated for at least 30 minutes even if the surgical procedure was quick enough to end before half an hour. Likewise, for surgical comfort VAS scoring started at the surgical incision and every 5 minutes till the end of surgical procedure.
4. Primary outcome
Patients` response to IVRA was our primary goal. Indeed, it was classified into either satisfactory or unsatisfactory. We defined satisfactory outcome as VAS less than 4 and no need for supplementary iv analgesia or general anesthesia during the period extends from the onset of the sensory block till deflation of the tourniquet. While unsatisfactory outcome was defined as VAS ≥ 4 and the need for supplementary IV analgesia or general anesthesia during the period extending from the onset of the sensory block till deflation of the tourniquet. Intravenous Fentanyl 100 µg was given as rescue medication. The 50% effective dose (ED 50) of Dexmedetomidine was determined by calculating the average of mid-point doses of all independent pairs of patients subjected to crossover, i.e., failure to success.
We used simple randomization as follow: Patients names were kept in concealed envelopes and were randomly selected for assignment to different dexmedetomidine Doses in a sequential manner. The first patient in this sequential trial was randomly selected and tested at 0.4 µg/kg of dexmedetomidine, with 0.1 µg/kg dose interval. If IVRA outcome was satisfactory, the dose went down for the next patient by 0.1 µg/kg. Vice versa, if the outcome was unsatisfactory, the dexmedetomidine dose was stepped up by 0.1 µg/kg for the following patient. Although, we recruited 30 patients as sample size, only 26 patients were randomized as we eliminated 4, due to protocol violation.
5. Secondary outcomes
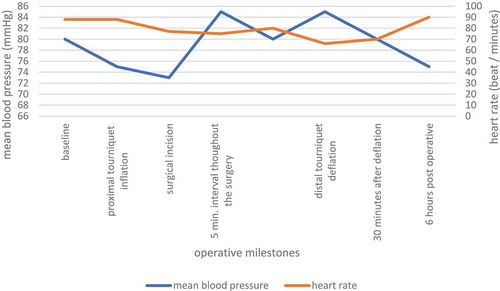
Hemodynamics in the form of mean arterial pressure (MAP) and heart rate (HR) were measured at baseline (just prior to inflating the proximal pneumatic cuff), after inflating the proximal cuff, intra-operatively at 5-minute intervals throughout the surgery until tourniquet deflation. Then, at 5-minute periods from deflation of the tourniquet for 30 minutes, followed by measuring every hour for 6 hours. Hypotension was defined as 30% of decrease from the baseline value (Hypotension was managed with ephedrine 3–6 mg bolus), and bradycardia as heart rate ≤ 45 beats/min, to which intravenous Atropine in the dose of 0.5 mg was at hand and, repeated if bradycardia persisted.
After conclusion of the surgery, tourniquet deflation was performed by the cyclic deflation technique. Time to recover sensation and complete motor power were assessed every 2 minutes, and time to first analgesic requirement was noted. Patients also were observed for any systemic side effects or complications due to systemic drug absorption. Postoperatively, patients VAS pain scores were assessed at 30 minutes, 2, 4 and 6 hours.
After tourniquet deflation and if patients VAS pain score ≥ 4 (time to first analgesic request) they were given intramuscular 75 mg diclofenac and the total diclofenac consumption in the first 6 hours postoperative was recorded. We have to point out that patients, care giver, data collector were blind to the study protocol.
6. Statistical analysis
Data were analyzed using computer software (SPSS version 13.0 interface). Results were expressed as mean ± standard or number of patients. Hemodynamic data were processed using analysis of variance (ANOVA) test and by post-hoc test. P value of <0.05 was considered statistically significant. Sample size was selected based on previous published data demonstrated that basically, in modified Dixon’s up and down method, 20–40 patients would provide stable estimates of the median dose for most realistic scenarios. [Citation8] For the purpose of this study, we enrolled 30 patients in our study; however, due to protocol violations, we ended up analyzing measurements of 26 patients.
7. Results
The main steps of our sequential analysis research is depicted in figure, 1.In our study, 26 patients completed the sequence, IVRA was satisfactory in 11 patients (42.3%) and unsatisfactory in 15 patients (57.6%) (). There was no statistically significant difference as regard age, sex, weight, ASA physical status, and duration of surgery or tourniquet time, .
Table 1. Patients’ profile.
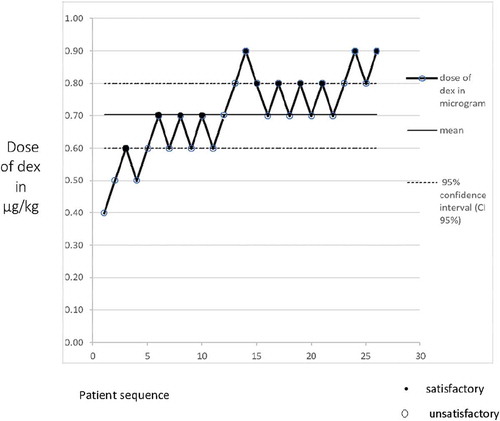
The series of satisfactory/unsatisfactory response of each patient to IVRA with 0.5% Lidocaine and dexmedetomidine using Dixon`s up and down technique is shown in . As depicted in the diagram, the ED 50 of Dexmedetomidine (95% confidence interval, CI) as adjuvant for satisfactory 0.5% lidocaine IVRA was 0.7 µg/kg (0.6–0.8).
Figure 2. The outcome of 26 patients on 0.5% Lidocaine IVRA and dexmedetomidine (dex) dose. The median effective dose (ED50) of dexmedetomidine for satisfactory IVRA response was (0.7 (0.6–0.8), with 95% confidence interval.
In patients with satisfactory response to IVRA, Hemodynamics values are shown in . When Mean arterial pressure and heart rate values obtained throughout the entire surgical process up to 30 min post-tourniquet deflation, were compared with baseline measurement, no statistically significant differences could be detected (= 0.754 and 0.324, respectively). depicts patients with unsatisfactory response to IVRA time to first analgesic request. Intergroup comparison of the time to the first need to analgesics showed no statistical difference (P > 0.05). In patients with unsatisfactory response to IVRA, time to first analgesic request for each patient in the series was statistically insignificant, compared to that of the first patient, table 3.
Table 2. Sensory and motor measurements, onset of tourniquet pain and first analgesic request, and overall intra-operative and postoperative rescue analgesic consumption.
Table 3. Patients with unsatisfactory response to IVRA time to first analgesic request per dose of dexmedetomidine.
8. Discussion
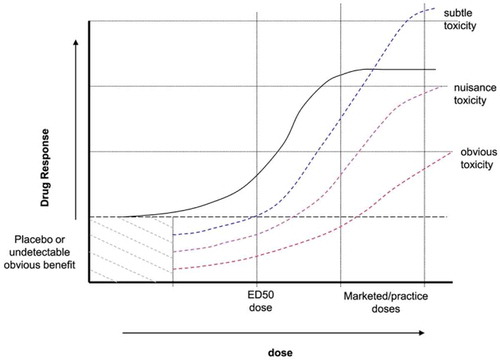
Our study showed that the median effective dose (ED 50) of Dexmedetomidine in IVRA in upper limb for short surgical procedure of not more than hour duration with 0.5% Lidocaine, was 0.7 µg/kg (95% confidence interval (CI) 0.6–0.8 µg/kg), using Dixon’s up and down sequential analysis. The initial starting dose in our sequential analysis was chosen based on the minimum dose of Dexmedetomidine anticipated to produce satisfactory response. Our starting dose selection was derived from our prior published research, in which we used 1 µg/kg of Dex with lidocaine 0.5% in IVRA of upper limb [Citation9] and other studies, used dose range 0.3–0.5 µg/kg of Dexmedetomidine as adjuvant for upper extremities IVRA. [Citation10,Citation11] To determine sample size for our series, 30 patients, we have gone through a short survey of the current literature that used Dixon`s sequential up and down method to determine median effective dose ED50. The original sample size postulated by Dixon in 1948, was 40 patients. Later, there was a tendency for smaller sample size, N ≤ 10. [Citation12] Actually, the core purpose of using Dixon`s sequential method is sample size reduction. Nowadays, some researchers use fixed sample size of 30, [Citation13,Citation14] while others go down to as few as 6 pair, N ≤ 6. [Citation15–Citation17] Moreover, Pace et al. stated that in a sequential design for median estimation, sample size ought to be between 20 and 40 patients. [Citation18] Indeed, this discrepancy in sample size is due to researchers` preferences and local institutional guidelines rather than theoretical concept. [Citation12] ED50 is the dose that elicits 50% response in half of the population received the medication. It is calculated by plotting a line on dose axis of the dose-response curve where 50% of the required response is observed, . Indeed, it is usually reported by manufacturer along with recommended therapeutic dosage. Indeed, ED50 is usually overlooked by clinicians once the drug is advertised and marketed with recommended dosage guidelines.
ED50 proves to be valuable variable in guiding clinicians to get maximum efficacy with least adverse effects. Dose-response curve shows that doses above ED50, show minimal increase in efficacy while side effects perpetually escalate, . In fact, selecting the lowest effective dose of a drug is warranted, especially when the medication is used for prevention of certain outcome, [Citation19] like in the case of our study, in which Dex is used to prevent surgical/tourniquet pain hence improves the quality of IVRA. Moreover, our study showed that the ED 50 of Dex was 0.7 µg/kg, there were patients in our sequential analysis who received the prior mentioned dose but showed unsatisfactory response to IVRA, . This came as no surprise because by definition, ED 50 is the dose that could elicit quantal (all or none) response in half of the people received the drug. That is to say, ED50 is usually used, as in our study, as rational prediction of the effect of a drug, but does not by all means, represent the recommended dose that anesthetists should adhere to. This simply because there is variation in ED50 among population attributed to different body size, pharmacodynamics and pharmacokinetics. This variation leads to a situation where the effective dose could be in many patients lower than the recommended one in clinical trials. [Citation20,Citation21] Theoretically any local anesthetic could be used for IVRA, Lidocaine is the drug of choice for decades due to its unique fast on/fast off pharmacokinetics which means quick onset and offset. [Citation22] Nevertheless, two troublesome aspects of Lidocaine IVRA cause considerable concerns; first tourniquet pain, a poorly localized dull pain at the tourniquet site, that could develop while the surgery is carried on and the quality of intraoperative anesthesia which could be short from optimum. [Citation23] Dex is a potent α 2 agonist that addresses these two points in many studies of regional anesthesia. Peripherally, which is our point of interest in our study, Dex is thought to exert its analgesic action through post-synaptic activation of G-coupled proteins and hence enhance potassium channels conduction. It also, works by inhibition of synaptic norepinephrine release. [Citation24–Citation26] Dex shows, when it is administered systemically, biphasic action on arterial blood pressure, a brief rise followed by a prolonged drop and a transient bradycardia. [Citation27] When used with IVRA, as the limb is isolated from the general circulation, we did not find statistically significant difference between MAP and HR measurements during surgery and baseline measurements. This is an added value, in our opinion, to the safety profile of Dex in IVRA.
There are several limitations in our study. First, our subjects of research are healthy individuals with ASA one or two, the ED 50 of Dex might change if our mean age population were older or taking medication or have co-morbidity. Second, the essence of Dixon up and down sequential method is to use small sample size as low as N ≤ 6, and although our sample size of 26 patients is universally accepted, smaller sample size would have saved resources and time. Finally, we did not include ED 95 in our protocol, which is more important to anesthetists than ED 50. However, Dixon up and down sequential analysis was not originally designed for ED 95. We may conjecture that the ED 50 of a solitary dose of Dexmedetomidine for satisfactory intravenous regional anesthesia of the upper limb is 0.7 µg/kg when Lidocaine 0.5% is used as local anesthetic blocking agent.
Disclosure statement
The authors declare that there is not conflict of interest.
Additional information
Funding
References
- Bier A. On local anesthesia with special reference to vein anesthesia. Edinburgh Med J. 1910;5:103–123.
- Morrison J. Intravenous local anesthesia. Br J Surg. 1930–1931;18:641–647.
- Holmes CMcK: intravenous regional analgesia. Lancet. 1963;1:245–247.
- Crews JC, Hilgenhurst G, Leavitt B, et al. Tourniquet pain: the response to the maintenance of tourniquet inflation on the upper extremity of volunteers. Regional Anesth. 1991 Nov-Dec;16(6):314–317.
- Choyce A, Peng P. A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anesth. 2002;49:32–45.
- Memis D, Turan A, Karamanlioglu B, et al. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98:835–840.
- Dixon WJ. The up-and-down method for small samples. J Am Stat Assoc. 1965 Dec;60(312):967–978.
- Stylianou M. Sequential analysis of Durham and Flournoy’s biased coin design for Phase I clinical trials. Washington, DC: American University, Department of Mathematics and Statistics, College of Arts and Sciences; 2000. p. i–xiii, 1–178.
- Mohamed AA, Aseeda SM, Salman OH. Lidocaine plus dexmedetomidine or nitroglycerine for intravenous regional anesthesia. JESMP. 2014 Apr;32:55–60.
- Subramanya V, Kapinigowda ST, Math AT, et al. Dexmedetomidine as an adjuvant for intravenous regional anesthesia in upper limb surgeries. Anesth Essays Res. 2017 Jul-Sep;11(3):661–664.
- Sardesai SP, Patil KN, Sarkar A. Comparison of clonidine and dexmedetomidine as adjuncts to intravenous regional anesthesia. Indian J Anaesth. 2015;59:733–738.
- Oron AP. Stationery and convergence properties of up and down methods. Department of statistics, university of Washington, Seattle. Teaching report No.506. Sept 27, 2006.
- Capogna G, Parpaglioni R, Lyons G, et al. Minimum analgesic dose of epidural fentanyl for first-stage labor analgesia. Anesthesiology. 2001;94:740–744.
- Camorcia M, Capogna G, Lyons G, et al. Epidural test dose with levobupivacaine and ropivacaine: determination of ED50 motor block after spinal administration. Br J Anaesth. 2004;92(6):850–853.
- Drover D, Litalien C, Wellis V, et al. Determination of the pharmacodynamic interaction of propofol and remifentanil during esophagogastroduodenoscopy in children. Anesthesiology. 2004;100:1382–1386.
- Lichtman A. The up-and-down method substantially reduces the number of animals required to determine antinociceptive ED50 values. J Pharma Toxic Meth. 1998;40(2):81–85.
- Sunderam R, Patra R, Julli M, et al. Use of the up-and-down acute toxicity test procedure to generate LC50 data for fish. Bull Environ Contam Toxic. 2004;72(5):873–880.
- Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007 Jul;107(1):144–152. Review.
- Dimmitt S, Stampfer H, Jennifer H. When less is more – efficacy with less toxicity at the ED50. Br J Clin Pharmacol. 2017 Jul;83(7):1365–1368.
- Peck C, Urquhart J. Postmarketing drug dosage changes of 499 FDA‐approved new molecular entities, 1980‐1999. Pharmacoepidemiol Drug Saf. 2002;11:439–446.
- Boxenbaum H, DiLea C. First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol. 1995;35:957–966.
- Benlabed M, Hamza J, Jullien P, et al. Alkanization of 0.5% lidocaine for intravenous regional anesthesia. Reg Anesth. 1990;15:59–60.
- Uluss A, Gurses E, Oztruk I. Serin S: compartive evaluatioh of two different volumes of lidocaine in intravenous regional anesthesia. Med Sci Monit. 2013;19:978–983.
- Salonen M, Reid K, Maze M. Synergistic interaction between alpha 2-adrenergic agonists and benzodiazepines in rats. Anesthesiology. 1992;76:1004–1011.
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610.
- Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268.
- Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142.