ABSTRACT
Purpose
To evaluate the efficacy of renal resistance indices (RRIs) and neutrophil gelatinase-associated lipocalin (NGAL) for early prediction of occurrence and severity of acute kidney injury in patients suffered from severe preeclampsia.
Settings and Design
This study was a prospective and observational study.
Methods
A total of 80 patients suffered from severe preeclampsia admitted to Obstetric ICU after cesarean delivery, the renal color duplex was carried out on all patients participated in the study on the 1st day of admission, intrarenal resistance indices were measured, serum NGAL was determined for all patients enrolled in the study and according to the existence of AKI as defined by Acute Kidney Injury Network (AKIN), patients were further classified into two groups AKI and non-AKI group.
Results
RRIs (RI, PI, and Ved) were higher in the AKI group with a significant difference than the non-AKI group, with a cut-off value of (0.665, 1.14, and 14.36) respectively. Resistive index of 0.665, pulsatile index of 1.14 and End diastolic flow velocity of 14.36 have a sensitivity and specificity of (92.9% and 84.6%), (92.86%, and 84.62%), and (89.29%, and 84.62%), respectively, according to serum NGAL, it was higher in AKI group than the non-AKI group, the optimal cut-off value of serum NGAL in AKI group at or was 27.03 ng/ml with sensitivity and specificity of (82.1% and 92.3%).
Conclusion
This study concluded that patients with severe preeclampsia, renal resistance indices (RRIs), and NGAL can predict early the occurrence and severity of acute kidney injury.
Abbreviations: RRIs: Renal resistance indices; NGAL: Neutrophil gelatinase-associated lipocalin; AKIN: Acute kidney injury network; AKI: Acute kidney injury; RI: Resistive index; PI: Pulsatile index; Ved: End diastolic flow velocity; PE: Preeclampsia; GFR: Glomerular filtration rate; ATN: Acute tubular necrosis; RRT: Renal replacement therapy; BNP: Brain natriuretic peptide; IL: Interleukins; ICU: Intensive care unit; ELISA: Enzyme-linked immunosorbent assay; AUC: Area under the curve; ROC: Receiver operating characteristic; US: Ultrasound; CKD: Chronic kidney disease
1. Introduction
Hypertension is considered one of the serious medical complications of pregnancy and significantly affects the perinatal morbidity and mortality. Preeclampsia (PE) is a severe complication affecting 5%–7% of all pregnant females [Citation1]. It is considered a widespread multiple organ disorder of unknown cause [Citation2]. The different mechanisms of preeclampsia are still unknown, but are believed to result from an insufficient function of the placenta. The main pathological lesion in PE is the renal lesion named glomerular endotheliosis, which has been regarded as pathognomic for that condition [Citation2].
Both kidneys play a very essential role in the adaptive physiology of normal pregnancy and in the pathophysiology of preeclampsia and some changes in the renal function which are found to be common in term pregnancy and PE. But, the most essential question to every doctor is how to diagnose the renal dysfunction early at its reversible stage to prevent or at least to decrease the maternal morbidity and mortality [Citation3].
The expression “acute kidney injury (AKI)” was established recently to replace the old term of acute kidney failure or acute renal failure. Preeclampsia leads to the occurrence of generalized vasoconstriction and a decrease in the renal glomerular filtration rate (GFR). This leads to renal hypoperfusion and acute tubular necrosis (ATN). Early recognition of ATN is vital to manage the patient appropriately [Citation4,Citation5]. After the establishment of acute renal dysfunction in those patients, the morbidity and mortality become worse and increase the risk of chronic renal failure, which causes patients to require permanent renal replacement therapy (RRT). Therefore, identifying patients who may develop acute kidney injury is very important and can be lifesaving in many circumstances [Citation6].
The appearance of new novel biomarkers of renal injury has opened a new era for early recognition and prediction of AKI, including urine and plasma NGAL, cystatin C, kidney injury molecule 1, brain natriuretic peptide (BNP), IL6 and IL18, liver fatty acid-binding protein, and homovanillic acid sulfate (HVA SO4) [Citation7–9]. NGAL is a small 25-kDa glycoprotein excreted by activated neutrophils. It was recognized as a matrix protein of specialized granules of human neutrophils [Citation10].
Recently, the advent in the ultrasound field enables the anesthesiologists to use the ultrasonography in many practices in ICU and operative room. Renal resistance indices (RRIs) as defined by Doppler ultrasonography in the renal arteries are used for assessing the renal perfusion arising from acute and chronic disorders of renal parenchyma [Citation11,Citation12]. Renal resistance indices (RRIs) are considered an important parameter that may help in recognition of the early stage of renal dysfunction, renal vascular damages, and acute/chronic renal changes in renal vascular compliance [Citation13].
This study evaluated the renal resistance indices (RRIs) and NGAL as regard their ability to predict early the occurrence and severity of acute kidney injury in pregnant females suffered from severe preeclampsia.
2. Patients and methods
After approval of the Alexandria Faculty of Medicine Ethics Committee and a documented informed consent obtaining from all patients, the present study was done on 80 patients suffered from severe preeclampsia admitted to Obstetric ICU after delivery; this prospective and observational study was conducted in El-Shatby Maternity Hospital, College of Medicine, Alexandria University, Egypt, between June 2019 and May 2020. This study was registered at the Pan African Clinical Trial Registry (PACTR202001671840885).
All pregnant females included in this study suffered from severe preeclampsia which defined as:
Blood pressure: 160 mmHg or higher systolic or 110 mmHg or higher diastolic on two occasions at least 6 hours apart in a woman on bed rest.
Proteinuria: equal to 5 g or more in a 24-hour urine or 3+ or more on urine dipstick testing of two different urine samples collected at least 4 hours apart.
Other features: visual or cerebral disturbances, epigastric pain, impaired hepatic function, thrombocytopenia, and intrauterine growth retardation.
Patients with previous renal insufficiency, history of dialysis requirement, diabetes mellitus, essential hypertension, and peripheral vascular disease, use of nephrotoxic drugs before or during the study period, and patients with elevated serum urea and creatinine at the time of admission were all excluded from the study.
Renal color duplex was carried out on all patients included in this study on the 1st day of admission to the ICU. Bedside duplex ultrasound was carried out on the renal intralobular arteries of both kidneys with a GE Logiq P5 ultrasound equipped with a 5-MHz convex transducer [Citation14]. We estimated 3 different RRIs (Resistive index (RI), Pulsatility index (PI), and the End diastolic flow velocity (Ved) of the renal intralobular arteries).
The Resistive Index (RI) is a dimensionless value that determines the resistance of the vascular wall. It is determined by the subtraction between peak systolic velocities (PSV) and end-diastolic velocity (EDV) divided by the PSV. The Pulsatility Index (PI) is used when the final diastolic flow tends to zero or in cases of negative blood flow, considering all the velocity of a cardiac cycle. It is derived by the subtraction between PSV and EDV divided by the mean velocity (MV). End diastolic flow velocity (Ved) is an index measured in spectral Doppler ultrasound. The EDV corresponds to the point marked at the end of the cardiac cycle (just prior to the systolic peak).
Also, serum NGAL was determined for all patients enrolled in the study on the 1st day of admission to ICU, NGAL was measured in all specimens by (ELISA) technique according to the standard protocol, and values were expressed as ng/ml.
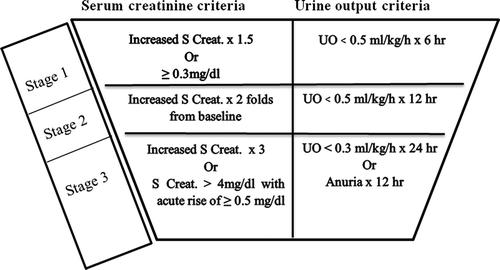
Using Acute Kidney Injury Network criteria () [Citation15], patients were further assessed every 24 hours for three consecutive days and classified into two groups: AKI and non-AKI group.
3. Statistical analysis
The sample size was statistically approved by the Community Medicine Department; Faculty of Medicine by using the G power 3.1.9.2 program for sample size calculation and according to data obtained from previous studies [Citation16,Citation17]. A sample size of 80 patients was estimated to achieve a 95% power, level of significance 5%, and effect size 0.5.
Analyzing of data was done using IBM SPSS software package version 20.0. Number and percent were used to describe the qualitative data while quantitative data were described by range (maximum and minimum), mean, standard deviation, median, and percentages. p < 0.05 was considered statistically significant.
We used Student’s t‑test for normally quantitative variables, to compare between two studied groups and paired t‑test for normally quantitative variables, to compare between two periods in the same group. Categorical data were analyzed using the Chi‑square (χ2) test. Mann-Whitney test used to compare between two independent different groups while the dependent group is ordinal or continuous, but normally not distributed.
4. Results
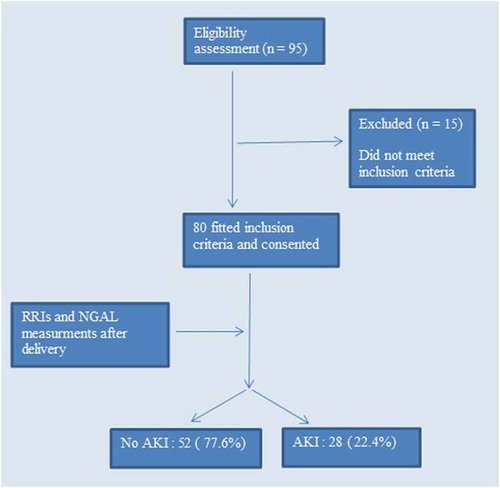
Ninety-fife patients were screened for eligibility to participate in this study and 80 patients subsequently consented and were enrolled in the study, with no patient drop-outs (). Age, body mass index, and arterial blood pressure (systolic and diastolic) were higher in the AKI group than non-AKI group, but without any significant differences.
5. Renal resistance indices measurement (RRIs): ()
1. Resistive index (RI):
Table 1. Comparison between the two studied groups according to RRIs
In the AKI group, it ranged from 0.68 to 0.84 with a mean value of 0.77 ± 0.05 and ranged from 0.52 to 0.71 with a mean value of 0.61 ± 0.04 in the non-AKI group, with a significant difference between the 2 groups (p = <0.001).
2. Pulsatile index (PI):
In the AKI group, it ranged from 1.01 to 1.39 with a mean value of 1.27 ± 0.09 and ranged from 0.83 to 1.21 with a mean value of 1.03 ± 0.09 in non-AKI group, with a significant difference between the two groups (p = <0.001).
3. End diastolic flow velocity (Ved) (cm/sec):
In the AKI group, it ranged from 14.24 to 18.88 with a mean value of 16.59 ± 1.49 and ranged from 9.84 to 15.44 with a mean value of 12.56 ± 1.58 in the non-AKI group, with a significant difference between the two groups (p = <0.001).
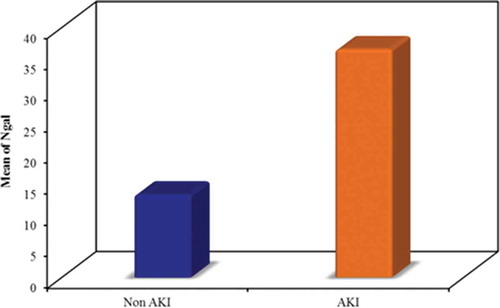
Serum NGAL level (ng/ml):
In the AKI group, it ranged from 19.24 to 55.21 ng/ml with a mean value of 36.53 ± 9.95 ng/ml and ranged from 4.54 to 25.24 ng/ml with a mean value of 13.28 ± 4.15 ng/ml in the non-AKI group, with a significant difference between the two groups (p = <0.001) ().
Classification/staging system for AKI:
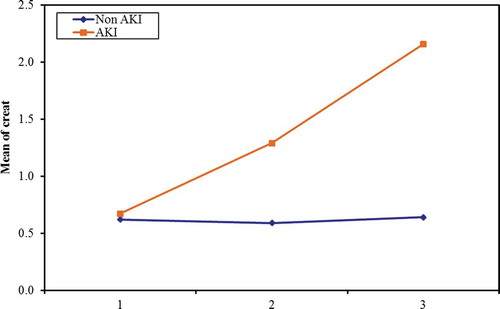
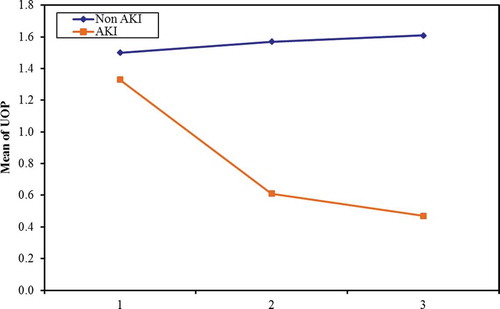
The changes in serum creatinine () and urine output () were measured every day starting from ICU admission.
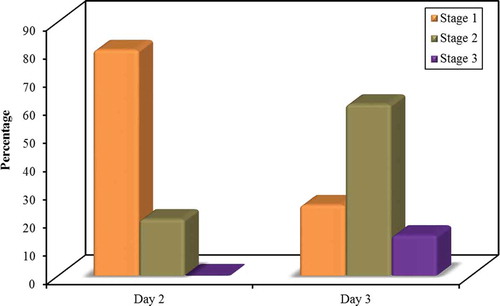
The incidence and stage of AKI were tabulated every 24 hours for the next 72 hours postoperative (). For the first day postoperative, according to AKIN staging, there was no incidence of AKI. For the second day of ICU admission; the 52 patients in the non-AKI group did not develop any degree of AKI according to AKIN criteria, but in the AKI group; 20 patients (25%) classified as stage 1 AKIN, 5 patients (20%) was stage 2 AKIN, and no patients (0%) were stage 3 AKIN. At the third day of ICU admission; 7 patients of the 28 (25%) classified as stage 1 AKIN, 17 patients (60.7%) were stage 2 AKIN, and 4 patients (14.3%) were stage 3 AKIN.
In the AKI group five patients (17.9%) needed dialysis while 23 (82.1%) did not need dialysis.
Diagnostic ability of renal resistive indices and serum NGAL:
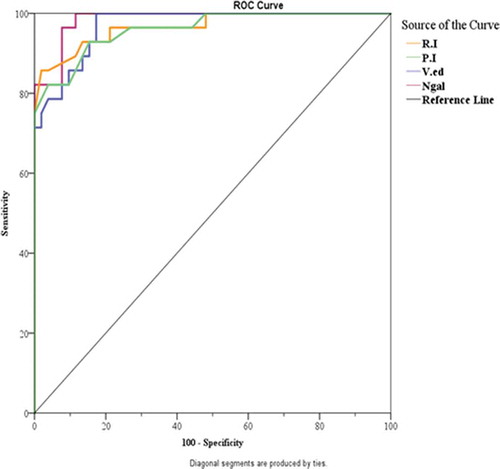
The performance of the studied renal resistive indices and serum NGAL in diagnosing AKI in patients with severe preeclampsia was tested for by the area under the curve and receiver operating characteristics (AUC and ROC) curve (; ).
Table 2. Diagnostic ability (sensitivity, specificity) for RI, PI, V.ed, and NGAL to predicate AKI patient
6. Discussion
This observational study revealed that renal resistance indices (RRIs) and NGAL have a very important role in the early prediction of the occurrence of acute kidney injury in patients who suffered from severe preeclampsia. The occurrence of acute kidney injury in pregnancy is not uncommon; its incidence ranged from 6% to 55% cases and considered to be a common cause of acute kidney dysfunction, contributing 20%–40% of all cases presented with AKI [Citation18].
Several clinical trials have established the role of many novel renal biomarkers in recognizing the occurrence of acute tubular injury [Citation19]. NGAL is one of these markers which are expressed early after kidney damage, when this early injury is still limitable or reversible. This marker is considered noninvasive and reliably measurable on available standardized clinical platforms [Citation20]. Ultrasound imaging of the renal arteries can be instrumental in the early detection of AKI. Indeed, blood flow to both kidneys is compromised at an early stage during acute tubular necrosis as a result of protracted renal vasoconstriction [Citation21]. According to these findings, renal resistance indices (RRIs) can be considered as vital parameters that may be beneficial in early detecting of renal dysfunction, renal vascular damages, acute and chronic renal changes in renal vascular compliance, and resistances [Citation22].
The present study was done on 80 female patients who suffered from severe preeclampsia admitted to Obstetric ICU after cesarean delivery. Using Acute Kidney Injury Network criteria 28 (22.4%) patients developed acute kidney injury and 52 (77.6%) patients did not develop AKI.
The results of the current study support the findings reached by Bahser et al. who studied whether the vasoconstrictive tonus in preeclampsia can be determined by elevations of renal resistance indices (RRIs), this study was performed between 2005 and 2007 at the Department of Gynecology at the Heinrich Heine University, Duesseldorf, Germany. Bahser et al. concluded that renal resistive indices are a very important classifier of preeclampsia, giving the potentiality to predict nephropathy [Citation17].
In agreement with the current study, Marty et al. evaluated the accuracy of RI for early prediction of acute kidney injury after knee and hip surgeries. The choice of AKIN classification for the diagnosis of acute kidney injury as it is more sensitive than other classifications such as the RIFLE classification. Renal RI was measured preoperatively and in the postanesthesia care unit, 16 patients presented with AKI in the postoperative period, the incidence of postoperative AKI was 32%, no patient required dialysis [Citation23]. Resistive index (RI) was increased in this group in both the preoperative (0.72 vs. 0.66) and postoperative periods (0.75 vs. 0.67), the most accepted cut-off value was a post-operative RI of 0.705 (sensitivity 94%, specificity 71%). Preoperative RI may reflect preexisting renal disorder and could help to determine patients at risk of AKI, postoperative RI reflects not the preexisting condition only but also changes which may follow the operation [Citation23].
Also, Marty et al. found that age is not the main determinant of postoperative AKI although it is known to affect the resistive index, as it changes vascular properties. He concluded that the postoperative resistive index is the best variable for early prediction of AKI after major orthopedic surgery [Citation23].
Additionally, Schnell et al. performed a prospective, double-center study to assess the efficacy of the ultrasound-based renal arterial resistive index and Cystatin values in recognizing the presence of AKI within 72 h of admission to the ICU. Schnell et al. concluded that Doppler-based renal RI which is measured at admission to the ICU is useful in predicting AKI on day 3, which may be useful to distinguish between prerenal azotemia and persistent acute renal injury [Citation24].
In contrast with the presenting study, Nakai et al., examined the flow waveform in renal interlobar arteries of 39 nonpregnant women, 77 healthy pregnant women at 16–40 weeks of gestation, and 15 women with PIH at 28–39 weeks of gestation by using color and pulsed Doppler US, he did not find a significant difference between women with PE and a healthy group as regards the RI of the interlobar artery measurements. The cause may be due to the relatively small sample size of women with PE included [Citation25].
On the other hand, Simonazzi et al. compared serum and urinary NGAL levels in women with preeclampsia and those with uncomplicated pregnancies to identify the severity of the disease. Between November 2009 and June 2010, a cross-sectional case–control study of 40 pregnancies was performed, 18 women affected by preeclampsia, and 22 women with uncomplicated pregnancy were enrolled at the Department of Obstetrics and Prenatal Medicine (University of Bologna) in collaboration with the Nephrology, Dialysis and Transplantation Unit, Orsola Malpighi, General Hospital, Bologna, Italy [Citation26].
Simonazzi et al. identified a positive trend for increasing NGAL values according to the severity of preeclampsia, but they could not detect any statistically significant difference by NGAL among cases of mild and severe forms of PE. These preliminary data indicated that NGAL may correlate with renal involvement in the severe form of preeclampsia. The main limitation of this study was a small sample size. This could reasonably explain why there was only a trend between serum NGAL and severity of preeclampsia, without demonstrating a significant correlation [Citation26].
Similarly, Yegenaga et al. compared NGAL with cystatin C as an indicator of the risk of future acute kidney injury. He concluded that NGAL concentrations on the second day of intensive care unit admission could be used to predict the development of acute renal injury in the following three to 7 days in the ICU; however, the cystatin C concentration did not have predictive value [Citation27].
Constantin et al. as well found that the plasma NGAL concentration in ICU patients with a RIFLE score of 0 during the first 48 hours after admission is predictive of the occurrence of AKI during the following week in the ICU (sensitivity: 93% and specificity: 97%) [Citation28].
In agreement with the current study, Gharishvandi et al. investigated the value of plasma NGAL (pNGAL) as an early renal marker of kidney dysfunction in hypertensive patients. The sensitivity was 96% and specificity was 100% for pNGAL (≥32.2 ng/ml), the most accurate cut-off value of pNGAL for detection of GFR < 78 ml/minute was 32.2 ng/ml, pNGAL cut-off value in this was much lower than previous studies, most likely because patients in their study were at early stages of chronic kidney disease. But against our study, he suggested that serial rather than the isolated single measurement of NGAL will provide the most useful data in patients affected by risk factors of CKD [Citation16].
7. Conclusion
The results of this study showed that in patients with severe preeclampsia, renal resistance indices (RRIs), and NGAL can predict early the occurrence and severity of acute kidney injury.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Tranquilli A, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104.
- Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 2016;27(2):71.
- Paauw ND, Luijken K, Franx A, et al. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci. 2016;130:239–246.
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73.
- Gilani SI, Anderson UD, Jayachandran M, et al. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol. 2017;28:3363–3372.
- Mehrabadi A, Dahhou M, Joseph K, et al. Investigation of a rise in obstetric acute renal failure in the United States, 1999–2011. Obstet Gynecol. 2016;127:899–906.
- Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51:335–351.
- Mahmoodpoor A, Hamishehkar H, Fattahi V, et al. Urinary versus plasma neutrophil gelatinase-associated lipocalin (NGAL) as a predictor of mortality for acute kidney injury in intensive care unit patients. J Clin Anesth. 2018;44:12–17.
- WFP IV, Maisel A, Kim J, et al. Neutrophil gelatinase associated lipocalin in acute kidney injury. Postgraduate Med. 2013;125:82–93.
- Fauzi A, Salasia SIO, Titisari N. Neutrophil gelatinase-associated lipocalin (NGAL) as a potential biomarker for early detection of acute renal failure. Comp Clin Pathol. 2016;25:387–392.
- Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37:68–76.
- Doi Y, Iwashima Y, Yoshihara F, et al. Renal resistive index and cardiovascular and renal outcomes in essential hypertensionnovelty and significance. Hypertension. 2012;60:770–777.
- Ninet S, Schnell D, Dewitte A, et al. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and meta-analysis. J Crit Care. 2015;30:629–635.
- Bahser N, Godehardt E, Hess AP, et al. Examination of intrarenal resistance indices indicate the involvement of renal pathology as a significant diagnostic classifier of preeclampsia. Am J Hypertens. 2013;27:742–749.
- Pachucki M, Ghosh E, Palanisamy K, et al. Acute kidney injury progression during the first five days of an ICU stay. Crit Care Med. 2018;46:660.
- Gharishvandi F, Kazerouni F, Ghanei E, et al. Comparative assessment of neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C as early biomarkers for early detection of renal failure in patients with hypertension. Iran Biomed J. 2015;19(2):76.
- Bahser N, Godehardt E, Hess AP, et al. Examination of intrarenal resistance indices indicate the involvement of renal pathology as a significant diagnostic classifier of preeclampsia. Am J Hypertens. 2014;27(5):742–749.
- Khanal N, Ahmed E, Akhtar F. Epidemiology, causes and outcome of obstetric acute kidney injury. Novel insights on chronic kidney disease, acute kidney injury and polycystic kidney disease Rijeka. InTech. 2012;7:67–81.
- Hartung EA. Biomarkers and surrogate endpoints in kidney disease. Pediatr Nephrol. 2016;31:381–391.
- Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase‐associated lipocalin: pathophysiology and clinical applications. Acta Physiol. 2013;207:663–672.
- Mohan S, Campenot E, Chiles MC, et al. Association between reperfusion renal allograft biopsy findings and transplant outcomes. J Am Soc Nephrol. 2017;28:3109–3117.
- O’Neill WC. Renal resistive index: a case of mistaken identity. Hypertension. 2014;64:915–917.
- Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery: a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37–43.
- Schnell D, Deruddre S, Harrois A, et al. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock. 2012;38(6):592–597.
- Nakai A, Asakura H, Oya A, et al. Pulsed doppler US findings of renal interlobar arteries in pregnancy-induced hypertension. Radiology. 1999;213(2):423–428.
- Simonazzi G, Capelli I, Curti A, et al. Serum and urinary neutrophil gelatinase-associated lipocalin monitoring in normal pregnancy versus pregnancies complicated by pre-eclampsia. in vivo. 2015;29(1):117–121.
- Yegenaga I, Kamis F, Baydemir C, et al. Neutrophil gelatinase-associated lipocalin is a better biomarker than cystatin C for the prediction of imminent acute kidney injury in critically ill patients. Ann Clin Biochem. 2018;55(2):190–197.
- Constantin J-M, Futier E, Perbet S, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25(1):176.e1-. e6.