ABSTRACT
Early identification of malnourished, critically ill patients helps initiate prompt treatment and improve patients’ outcomes. Most nutrition screening tools were not suitable for critically ill patients. This study was conducted to evaluate the modified nutrition risk in critically ill (mNUTRIC) score as a screening tool for nutrition risk in critically ill patients. Search was conducted in Medline, PubMed, and the Egyptian Knowledge Bank for cohort studies that were published in English until 1 March 2019. Eight studies with a total number of 4076 patients were included in this meta-analysis. Estimates and their 95% confidence intervals (CI) were calculated, then pooled for analysis. High mNUTRIC score (5 or above) in critically ill patients was related to increased risk of 28-day mortality (relative risk = 2.025; 95% CI = 1.488–2.758; p < 0.001; risk difference = 0.159; 95% CI = 0.120–0.198; p < 0.001), increased ICU length of stay (95% CI = 1.78–4.99 days; p < 0.001), and longer duration of mechanical ventilation (95% CI = 3.01–4.73 days; p < 0.001). Association of High mNUTRIC score with these parameters indicates that it might be used as a tool to predict poorer clinical outcomes in those patients.
1. Introduction
Malnutrition is a nutritional status that is caused by either shortage or excess of micro and/or macronutrients, which adversely affects the body size, function, composition, and clinical outcomes. By this definition, malnutrition is a term that encompasses both under and over nutrition. Undernutrition is usually the prevalent form of malnutrition encountered in critical care settings [Citation1].
Patients admitted to ICUs are at high risk of developing malnutrition, which is caused mainly by stress-induced catabolism and inadequate dietary intake. During the early phase of critical illnesses, catabolic hormones are secreted (e.g., glucagon, cortisol, and catecholamines), resulting in mobilization of amino acids and free fatty acids from muscles and adipose tissues for the generation of energy. Moreover, pro-inflammatory cytokines are released, contributing to the catabolic processes. Inflammation seems to play an important role in the pathogenesis of malnutrition in ICU patients [Citation2]. The second stage of critical illness is characterized by loss of body cell mass [Citation3]. In addition, ICU patients are likely to suffer from malnutrition before admission to ICU due to chronic illness or cancer [Citation2].
Malnutrition is associated with increased patient mortality and morbidity, including prolonged ICU stay, decreased immunity, increased rate of hospital-acquired infection, poor wound healing, and muscle wasting (leading to decreased ventilatory drive) [Citation4]. Therefore, malnutrition is considered among the main causes of increased health care costs [Citation3].
The prevalence of malnutrition was reported to range from 38% to 78% in acute critically ill patients [Citation5]. It is estimated that one in three patients at admission suffer from malnutrition in developing countries [Citation6].
Early identification of patients who are at high risk of malnutrition is essential to start appropriate and prompt treatment [Citation2], which may improve patients’ outcomes [Citation7]. Unfortunately, there is no unified standard protocol for screening of malnutrition, resulting in variations in practice across ICUs [Citation3]. Most nutritional screening tools are not suitable in ICU settings because of the difficulty to obtain some parameters, such as accurate history of dietary intake and weight loss [Citation3].
Heyland et al. [Citation8] developed NUTRIC score to quantify the risk of adverse outcomes in critically ill patients that may be improved by nutrition therapy. Patients who are at high nutritional risk are likely to benefit more than patients with low risk by therapeutic nutritional intervention [Citation9]. Recent studies suggest using the modified NUTRIC (mNUTRIC) score for screening and subjective global assessment of nutritional status in adjunction with other parameters, such as laboratory markers, sarcopenia index, and handgrip strength [Citation10].
This meta-analysis was carried out to evaluate mNUTRIC as a screening tool for nutrition risk in critically ill patients. The objectives of the meta-analysis included assessment of the association between the score and the 28-day mortality (primary outcome), the length of ICU stay, the duration of mechanical ventilation, the incidence of infection and its relationship with APACHE II and SOFA scores (secondary outcomes).
2. Methods
2.1. Ethical considerations
This meta-analysis was conducted in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [Citation11]. Ethical approval was not required because this study was a literature-based work.
2.2. Search strategy for identification of studies
The related studies were retrieved from the electronic databases of Medline, PubMed, and the Egyptian Knowledge Bank, using the search keywords of modified NUTRIC score, malnutrition, critically ill, and intensive care. Filters were used to include only cohort studies that were published in English until 1 March 2019.
2.3. Inclusion criteria
Articles were included if they fulfilled the following criteria: cohort in design; focused on assessing mNUTRIC score in critically ill patients admitted to ICUs who are above 20-years-old; relative risks were reported with their corresponding 95% CIs or original data were available to allow for computing them; and at least one of these outcomes was assessed: a) 28-day mortality, b) length of ICU stay, C) duration of mechanical ventilation and d) incidence of infection. A critically ill patient was defined as patient who has a life-threatening multisystem process that can result in significant morbidity and mortality, and in most cases is preceded by a period of physiological deterioration [Citation12].
2.4. Exclusion criteria
The following types of publications were excluded from this meta-analysis: duplicate reports, abstracts, case reports, review articles, editorials, and clinical guidelines. In addition, studies with unavailable full text or incomplete data were excluded.
2.5. Data extraction
A copy of each identified paper was obtained, and relevant data were extracted by two independent reviewers for a quantitative overview. The point estimates of the assessed outcomes along with their 95% CIs and the country where the study was carried out were also ascertained. Any disagreements between the two reviewers were resolved either by consensus or by consulting a third reviewer.
2.6. Examination of publication bias
Publication bias was assessed by examination of the funnel plots of the effect size measures, the Begg-Mazumdar rank correlation, and Egger regression test.
2.7. Statistical considerations
Statistical analysis was conducted using an R-based software (Openmeta). Studies included in the meta-analysis were tested for heterogeneity of the estimates using the Cochran Q chi-square test and I-square (I2) index. Statistically significant Cochran Q chi-square test (p < 0.1) denoted heterogeneity among the studies. An I-square (I2) index = 30% to 60% indicated moderate heterogeneity, from 50% to 90% indicated substantial heterogeneity, and from 75% to 100% denoted considerable heterogeneity.
Outcomes from included studies were combined using either fixed or random effect models. Reasons for heterogeneity for studies were explored. If heterogeneity across studies was moderate or low (I2 < 50%), the fixed effects model was utilized for pooling estimates using the Mantel-Haenszel fixed-effects method. The random effects model was utilized if I2 was 50% or above [Citation13], using the Der Simonian laird random-effects method. Comparison of outcomes was done by estimation of the risk ratios with their 95% CI and risk difference with their 95% CI. P-values < 0.05 were considered statistically significant.
3. Results
3.1. Literature search
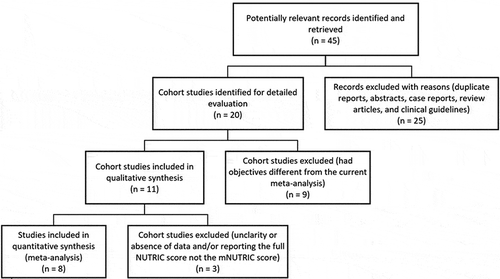
We identified forty-five studies that were potentially relevant to the present meta-analysis. We excluded 25 studies that were duplicate reports, case reports, review articles, abstracts or clinical guidelines. Assessment of the full-text of the remaining 20 studies led to removal of nine of them because their objectives differed from the objectives of the current meta-analysis. After reviewing the remaining eleven articles, 3 studies were excluded due to unclarity or absence of data about the assessed outcomes or the number of patients with high and low mNUTRIC score. One study [Citation8] among the three excluded articles reported the full NUTRIC score, not the mNUTRIC score. Therefore, eight studies were considered eligible for inclusion in the current meta-analysis [Citation14–21]. is the flow diagram that outlines the search process, the included and excluded articles, and the causes of exclusion.
3.2. Characteristics of the included studies
summarizes the baseline characteristics of the included studies. They were published between 2017 and 2019, contained a total of 4076 patients (mean sample size = 509.5; range: 75–1143 patients). Two studies were multi-center [Citation14,Citation20], while the other six were conducted in a single center [Citation15–19,Citation21].
Table 1. Characteristics of the included studies in the meta-analysis
shows the number of patients with high and low mNUTRIC score included in each study, their gender, and age. Three studies reported the lack of statistically significant difference between high and low mNUTRIC score regarding the patients’ gender [Citation16,Citation18,Citation20]. Four studies found that patients with high mNUTRIC score had a significantly higher mean/median age than those with low mNUTRIC score [Citation15,Citation16,Citation18,Citation20].
Table 2. Systematic review results for gender, age and number of patients with high and low mNUTRIC score
3.3. Meta-analysis of 28-day mortality
Five out of the eight included studies assessed the 28-day mortality using the risk ratio and risk difference [Citation15–17,Citation20,Citation21]. The total number of patients with high mNUTRIC score in these five studies was 1119 and death was encountered in 347, while the total number of patients with low mNUTRIC score was 857 and death occurred in 160 patients.
Analyses of mortality risk ratio are shown in and . The heterogeneity test for the five studies was statistically non-significant [I2 = 52.69%; Q (df = 4) = 8.4542; p = 0.0763]. There was no evidence of publication bias (Begg-Mazumdar: Kendall’s tau = 0.6; p = 0.2333; Egger bias = 1.875646; p = 0.1322). The funnel plot showed also no evidence of publication bias. The random effects model was used to calculate the pooled risk ratio and its 95% CI. A statistically significant difference was detected between the groups with high and low mNUTRIC score (Risk ratio = 2.025; 95% CI = 1.488–2.758; p < 0.001). Therefore, the risk of 28-day mortality in cases with high mNUTRIC score was 2.025 times the risk in cases with low mNUTRIC score.
Table 3. The pooled estimates for 28-day Mortality (risk ratio and risk difference)
Figure 2. Forest and funnel plots for mortality outcome in the included studies. A: Forest plot – risk ratio; B: Forest plot – risk difference; C: Forest plot – predictive performance; D: Funnel plot – risk ratio; E: Funnel plot – risk difference; F: Funnel plot – predictive performance
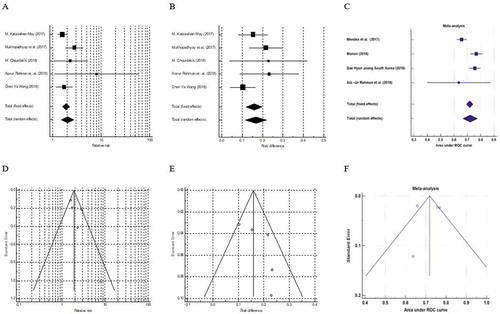
Analyses of mortality risk differences are also shown in and . The heterogeneity test for the four studies was statistically non-significant [I2 = 39.7%; Q (df = 4) = 6.6335; p = 0.1566]. There was no evidence of publication bias (Begg-Mazumdar: Kendall’s tau = 0.4; p = 0.4833; Egger bias = 2.078234; p = 0.2118). The funnel plot showed no evidence of publication bias. The fixed effects model revealed a statistically significant difference between patients with high and low mNUTRIC score (Risk difference = 0.159; 95% CI = 0.120–0.198; p < 0.001). Therefore, the risk of 28-day mortality in cases with high mNUTRIC score was 15.9% higher than in cases with low mNUTRIC score.
Four studies evaluated the predictive performance of mNUTRIC score [Citation14,Citation17–19]. The heterogeneity test for the four studies was statistically significant [I2 = 82.9%; Q (df = 3) = 17.567; p < 0.001]; therefore, the random effects model was chosen. The mNUTRIC score with an area under the curve of 0.722 (95% CI = 0.667–0.777) could fairly predict mortality. The pooled sensitivity, specificity, positive predictive value, and negative predictive value were 70.3%, 61.3%, 47%, and 78.9%, respectively ( and ).
Table 4. The predictive performance and pooled estimates of mNUTRIC score for 28-day Mortality
3.4. Meta-analysis of the length of ICU stay
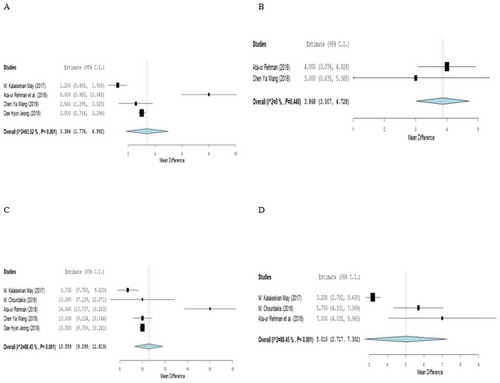
Four out of the eight included studies assessed the length of ICU stay [Citation15,Citation17,Citation18,Citation20], as shown in and . The heterogeneity test for the four studies was statistically significant [I2 = 93.52%; Q (df = 2) = 46.323; p < 0.001]. High mNUTRIC score was significantly associated with increased ICU length of stay among ICU patients when compared to patients with low mNUTRIC score by an estimate of the difference of 3.384 (95% CI = 1.776–4.992; p < 0.001).
Table 5. The pooled estimates for ICU length of stay and duration of mechanical ventilation
3.5. Meta-analysis of the duration of mechanical ventilation
Two out of the eight included studies assessed the duration of mechanical ventilation [Citation17,Citation20], as demonstrated in and . The heterogeneity test was statistically non-significant [I2 = 0%; Q (df = 1) = 0.596; p = 0.440]. Patients with high score had significantly longer duration of mechanical ventilation than those with low score (The estimate of the difference = 3.87; 95% CI = 3.01–4.73; p < 0.001).
3.6. Meta-analysis of the relationship between mNUTRIC and APACHE II scores
shows that five out of the eight included studies assessed the relationship between mNUTRIC score and APACHE II score [Citation15–18,Citation20]. The heterogeneity test was statistically significant [I2 = 88.5%, Q (df = 4) = 34.6; p < 0.001]. The estimate of the difference was 10.6 (95% CI = 9.3–11.8).
3.7. Meta-analysis of the relationship between mNUTRIC and SOFA scores
Three out of the eight included studies assessed the relationship between mNUTRIC score and SOFA score [Citation15–17], as demonstrated in . The heterogeneity test was statistically significant [I2 = 88.5%; Q (df = 4) = 17.3; p < 0.001]. The estimate of the difference was 5.0 (95% CI = 2.7–7.3).
3.8. mNUTRIC score sensitivity, specificity, positive predictive value, negative predictive value, and number of comorbidities
shows that mNUTRIC score had a total sensitivity, specificity, positive predictive value, and negative predictive value of 70.3%, 61.3%, 47%, and 78.9%, respectively. Also, it shows correlation of higher mNUTRIC score with comorbidities (p < 0.001).
Table 6. Systematic review results for mNUTRIC score sensitivity, specificity, positive predictive value, negative predictive value, and number of comorbidities
4. Discussion
This meta-analysis aimed to evaluate mNUTRIC score as a screening tool for nutrition risk in critically ill patients. The studied outcomes included the 28-day mortality (primary outcome), the length of ICU stay, the duration of mechanical ventilation, incidence of infection and its relationship with APACHE II and SOFA scores (secondary outcomes).
Early identification of malnourished, critically ill patients is essential to initiate prompt and appropriate treatment; hence, the patients’ outcomes may improve. The mNUTRIC score is a promising screening tool for malnourishment among the ICU patients. Most of nutrition screening tools before mNUTRIC score were not suitable for critically ill patients because malnutrition in ICU is linked with inflammation and hypermetabolic state, and the previous tools didn’t include these important causes for malnutrition. So, mNUTRIC score is considered the first validated specific nutritional screening tool in critically ill patients [Citation22].
However, the efficacy of mNUTRIC score is subject to some limitations. The score is mainly concerned with the administration of macronutrients, protein, and energy. The score may not detect patients who may benefit from pharmaconutrient supplementation (e.g., antioxidants). During the development of the NUTRIC score, nutritional history and practices were suboptimally taken into consideration [Citation8].
In the present meta-analysis, we reviewed the eight retrieved studies [Citation14–21] that assessed the performance of mNUTRIC score as a predictor of outcomes in critically ill patients. The mNUTRIC score is derived from NUTRIC score after exclusion of interleukin-6 level, which is not routinely assessed in clinical settings. The mNUTRIC score comprises five parameters: age, SOFA score, APACHE II score, number of co-morbidities, and days from hospital to ICU admission [Citation8]. The modified score has been validated. Multiple studies confirmed that mNUTRIC score correlated well with clinical outcomes in ICU patients [Citation14,Citation21–23].
High mNUTRIC score was associated with increased risk of 28-day mortality. The funnel plot showed no evidence of publication bias in the studies that used either risk ratio as the point of estimate or evaluated the risk difference or the performance of mNUTRIC score for prediction of mortality.
The mortality rate in Ata Ur-Rehman study was 26%, which is comparable to that of Kalaiselvan et al. [Citation15] who reported a mortality rate of 31.4%. However, Moretti et al. [Citation23] reported a higher mortality rate of >50% in mechanically ventilated patients with similar NUTRIC scores.
Higher mNUTRIC score was associated with increased length of stay (95% CI 1.175–4.712; p < 0.0001) by total random effect due to heterogeneity (I2 = 93.52%). As regards days on mechanical ventilation, estimate of the difference was about 3.87 (95% CI 3.007–4.728), p < 0.001.
Mendes et al. and Kalaiselvan et al. [Citation14,Citation15] reported that 48.6% and 42.5% of mechanically ventilated patients respectively had NUTRIC scores ≥ 5 regardless of the duration of mechanical ventilation.
We could not assess the incidence of infection in our study because none of the included eight studies assessed the incidence of infection.
Up to the best of the authors’ knowledge, this is the first meta-analysis to evaluate mNUTRIC as a screening tool for nutrition risk in critically ill patients. The study had some limitations that may affect the interpretation of the results. The sample size was relatively small as only eight studies were included in this meta-analysis, which may affect the heterogeneity across the studies and consequently the pooled analyses. Significant heterogeneity across some studies has already been observed when analysis was performed for predictive performance of mNUTRIC, length of hospital stay, APACHE II, and SOFA scores. We were unable to retrieve unpublished studies or studies published in languages other than English.
5. Conclusions
The current evidence points that mNUTRIC appears to be an effective tool for screening of malnutrition in critically ill patients who are at risk of developing adverse outcomes. The use of mNUTRIC score is recommended in the settings of critical illness. However, the small number of included studies warrants further research with larger sample sizes for confirmation of the score’s effectiveness and its association with adverse patients’ outcomes. Further studies with larger number of patients are required to prove the correlation of mNUTRIC with the incidence of infection.
Disclosure of interest
The authors report no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hamilton C, Boyce VJ. Addressing malnutrition in hospitalized adults. JPEN J Parenter Enteral Nutr. 2013;37(6):808–815.
- Tappenden KA, Quatrara B, Parkhurst ML, et al. Critical role of nutrition in improving quality of care: an interdisciplinary call to action to address adult hospital malnutrition. JPEN J Parenter Enteral Nutr. 2013;37(4):482–497.
- Lee ZY, DK H. Determination of nutrition risk and status in critically Ill patients: what are our considerations? Nutr Clin Pract. 2019;34(1):96–111.
- Kirkland LL. Extent and impact of malnutrition in critically Ill patients. In: Rajendram R, Preedy VR, Patel VB, editors. Diet and nutrition in critical care. New York, NY: Springer; 2015. p. 265–278.
- Lew CCH, Yandell R, Fraser RJL, et al. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review [Formula: see text]. JPEN J Parenter Enteral Nutr. 2017;41(5):744–758.
- Kirkland LL, Kashiwagi DT, Brantley S, et al. Nutrition in the hospitalized patient. J Hosp Med. 2013;8(1):52–58.
- Elia M, Zellipour L, Stratton RJ. To screen or not to screen for adult malnutrition? Clin Nutr. 2005;24(6):867–884.
- Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268.
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421.
- Brantley S, Mills M. Overview of enteral nutrition. ASPEN adult nutrition support core curriculum. 2nd ed. Silver Springs: American Society for Parenteral and Enteral Nutrition; 2012. p. 170–184.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- Robertson LC, Al-Haddad M. Recognizing the critically ill patient. Anaesth Intensive Care Med. 2013;14(1):11–14.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Mendes R, Policarpo S, Fortuna P, et al. Nutritional risk assessment and cultural validation of the modified NUTRIC score in critically ill patients-A multicenter prospective cohort study. J Crit Care. 2017;37:45–49.
- Kalaiselvan MS, Renuka MK, Arunkumar AS. Use of nutrition risk in critically ill (NUTRIC) score to assess nutritional risk in mechanically ventilated patients: a prospective observational study. Indian J Crit Care Med. 2017;21(5):253–256.
- Chourdakis M, Grammatikopoulou MG, Poulia KA, et al. Translation of the modified NUTRIC score and adaptation to the Greek ICU setting. Clin Nutr ESPEN. 2019;29:72–76.
- Ata Ur-Rehman HM, Ishtiaq W, Yousaf M, et al. Modified nutrition risk in critically Ill (mNUTRIC) score to assess nutritional risk in mechanically ventilated patients: a prospective observational study from the Pakistani population. Cureus. 2018;10:e3786.
- Jeong DH, Hong SB, Lim CM, et al. Comparison of accuracy of NUTRIC and modified NUTRIC scores in predicting 28-day mortality in patients with sepsis: a single center retrospective study. Nutrients. 2018;10(7):911. .
- de Vries MC, Koekkoek WK, Opdam MH, et al. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr. 2018;72(3):428–435.
- Wang CY, Fu PK, Huang CT, et al. Targeted energy intake is the important determinant of clinical outcomes in medical critically Ill patients with high nutrition risk. Nutrients. 2018;10(11):1731.
- Mukhopadhyay A, Henry J, Ong V, et al. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin Nutr. 2017;36(4):1143–1148. .
- Rahman A, Hasan RM, Agarwala R, et al. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–162.
- Moretti D, Bagilet DH, Buncuga M, et al. Study of two variants of nutritional risk score “NUTRIC” in ventilated critical patients. Nutr Hosp. 2014;29:166–172.