?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: The inflammatory response due to cardiopulmonary bypass (CBP) activates different inflammatory pathways that affect multiple organs. Dexmedetomidine is proved to affect inflammatory marker production.
Objectives: Evaluation of the effect of Dexmedetomidine on the inflammatory response associated with pediatric open-heart surgery using interleukin-6 (IL-6) and interferon-gamma (INF-ɤ) levels.
Methods: 61children aged between one to 8 years undergoing elective repair of non-cyanotic congenital heart disease with CPB were randomly assigned into two groups. The control group (31 patients) received normal saline, whereas the Dex group (30 patients) received an initial bolus of Dexmedetomidine 0.5 µg/kg followed immediately by infusion of 0.5 µg/kg/hr continued till the end of CPB. The level of IL-6 and INF-ɤ was measured. Hemodynamic, ICU, and hospital data were recorded.
Results: IL-6 and INF -ɤ levels were increased significantly with time in control group, with no increase in their levels in the Dex group. They were significantly lower in the Dex group compared to the control group in samples taken during bypass, 6 h and 24 h after the operation (the end of surgical procedure). There was a significant difference between the groups regarding inotropic score and mechanical ventilation. There was no significant difference between the groups regarding complications, duration of ICU or hospital stay.
Conclusion: The use of Dexmedetomidine in pediatric cardiac surgery for non-cyanotic heart disease had significantly attenuated the inflammatory response. It was useful in decreasing the level of inflammatory mediators, inotropic support, and duration of mechanical ventilation, but not the ICU or hospital stay.
Trial registration: https://clinicaltrials.gov. (Identifier: NCT03163238).
1. Introduction
Inflammatory response due to cardiopulmonary bypass (CBP) activates different pathways, resulting in cytokine production, cellular edema, endothelial injury, and organ ischemia [Citation1] that affect multiple organs like lung, heart, brain, and kidneys [Citation2–5]. This inflammatory response is exaggerated in pediatrics because of multiple factors such as; Major hemodilution due to small body surface area compared to the circuit area, which exposes them to more blood transfusion and abrupt temperature changes; need for higher perfusion rates due to higher metabolic demand increasing shear stress; and the immaturity of organs which increases the risk and severity of injury[Citation6]. Several methods have been employed to decrease this inflammatory response. The effect of prophylactic corticosteroid infusion during CPB on inflammatory response and on postoperative course is controversial [Citation7,Citation8]. Other pharmacologic agents are also used in practice and at the experimental level. However, evidence of the effectiveness of these therapies requires further evaluation[Citation9]. these agents include phosphodiesterase inhibitors[Citation10], dopexamine[Citation11], aprotinin[Citation12], free radical scavengers, and antioxidants (such as allopurinol, N-acetyl-cysteine, mannitol), ketamine[Citation13], angiotensin-converting enzyme inhibitors[Citation14], H2 antagonists[Citation15], and specific C5a monoclonal antibodies[Citation16].
Dexmedetomidine is α-2 adrenergic receptors agonist that affects the production of cytokines and tumor necrosis factor-α (TNF-α) by macrophages[Citation17]. The potent inhibitory effect of dexmedetomidine on inflammatory reactions, including edema, accumulation of inflammatory cells, and production of TNF-α and cyclooxygenase-2 (COX-2) has been described[Citation18].
We hypothesized that dexmedetomidine decreases the inflammatory response associated with pediatric open-heart surgery for non-cyanotic congenital heart diseases. To test this hypothesis, we measured interleukin-6 (IL-6) and interferon-gamma (INF-ɤ) levels as markers of inflammatory response in Children undergoing elective repair of congenital heart disease with CPB who received dexmedetomidine compared with saline placebo.
2. Patients and methods
This prospective, randomized, double-blind, parallel assignment clinical trial was carried out between December 2017 and May 2019. The study was carried out at Orman Heart Hospital, Assiut University.
2.1. Ethical considerations
It was approved by the Medical Ethics Committee, Faculty of Medicine, Assiut University (reference number IRB000087632). The trial was registered prior to patient enrollment at Clinical Trials. gov (NCT03163238). A written informed consent was obtained after detailed discussion with the parents or the legal guardians of the study participants.
2.2. Patients
Children between 1 and 8 years undergoing elective repair of congenital heart disease with CPB were included. Exclusion criteria included patients with cyanotic congenital heart diseases, re-intervention surgery, preoperative intake of corticosteroids, deep hypothermia, preoperative low cardiac output, those with non-palpable peripheral pulses before surgery (e.g., accompanying coarctation of the aorta), and those with inflammatory diseases like rheumatic heart disease or diabetes mellitus.
2.3. Randomization and blindness
A computer-based program was used to generate a sequence of random numbers, and patients were assigned into two groups to receive dexmedetomidine (bolus and maintenance infusion), (DEX group) and placebo controls (Control group) following simple randomization processes. The group assignments were kept in well-sealed opaque envelopes and opened after enrollment of the patients to the study.
For each group two syringes were prepared, one of them is 10 ml (for bolus dose) and the other is 50 ml (for continuous infusion). The syringes of both groups are matched with each other in volume and color of the content. The 10 ml syringe contained only 10 ml of 0.9% normal saline (NS) for control group or Dexmedetomidine (0.5 mcg/kg) diluted with 10 ml 0.9% normal saline for DEX group. The second syringe (50 ml) contained only 50 ml NS for control group or Dexmedetomidine diluted in 50 ml NS for DEX group Concentration of Dexmedetomidine was diluted according to the body weight to fix the rate of infusion (1 ml/kg/hr) to achieve a concentration of 0.5 mcg/kg/h for DEX group. All patients and their guardian, outcome collectors, and data analysts were blinded to the study groups. The anesthetist, physician, and others in the operative team, such as technicians involved in drug preparation, were aware of patient’s grouping.
2.4. Study protocol
2.4.1. Anesthetic technique
All patients of both groups received the same technique of anesthesia and CPB. An interview questionnaire regarding preoperative patient’s characteristic (age, sex, body weight, and height) and clinical data (diagnosis, echo findings, surgery planned, and preoperative investigations) were recorded. Patients were premedicated with oral midazolam (0.5 mg/kg) 30 minutes before induction of anesthesia. Sealed envelope of randomization was opened by anesthetist. Patients’ heart rate (HR), rhythm, blood pressure, temperature, oxygen saturation, end tidal capnography, and degree of neuromuscular block were monitored. Induction of anesthesia was performed by sevoflurane plus fentanyl (5 mcg/kg) and cisatracurium (0.1 mg/kg) and was maintained with sevoflurane, fentanyl (1–2 mcg/kg/hr), and cisatracurium (0.05 mg/kg per dose).
After insertion of a peripheral line and giving induction anesthetics, patients received the bolus syringe (10 ml) for over 10 minutes, followed immediately by a continuous infusion (1 ml/kg/h) of the infusion syringe (50 ml) according to group randomization. This was continued throughout the operation till the end of CPB.
Arterial line for invasive blood pressure monitoring and central venous line were inserted after induction.
2.4.2. Cardiopulmonary bypass technique
The routine strategy was performed by a non-pulsatile heart-lung machine (Stockert Centrifugal Pump S5 Console Roller pump 150- part No: 10–80-00, Ser No: 10E03400, Germany). Priming of the CPB circuit was done with mannitol, sodium bicarbonate, and packed red cells given to obtain a hematocrit of 22–26%. Heparin 400 IU/kg was given to the patient and once activated clotting time reached ≥450 seconds, CPB was initiated. The aorta was clamped and cold blood cardioplegia (15–20 ml/kg) was administered into the aortic root. The body (core) temperature was cooled to 30–32°C. The cardioplegia solution was repeated every 20 minutes. pH-stat blood gas strategy was used; partial pressure of carbon dioxide in arterial blood (PaCO2) was maintained between 35 and 45 mmHg. During CPB, mean systemic arterial pressure (MAP) was preserved between 30–60 mmHg, and anesthesia was conserved by sevoflurane 0.8–1.5%, fentanyl (1–2 mcg/kg/hr.) and cisatracurium (0.05 mg/kg per dose). Weaning from CPB was done at 37°C after completion of surgery. Ventilation was started, and patient hemodynamics and arterial blood gases were stabilized. Heparin was reversed by 1 mg protamine for every 100 IU heparin. The patients were transported to post-operative pediatric cardiac intensive care unit (PICU) post-surgery.
After arrival to the PICU, all patients were mechanically ventilated till criteria of weaning from mechanical ventilation and extubation were fulfilled weaning from mechanical ventilation was done as early as possible. Patients received I.V. paracetamol and non-steroidal anti-inflammatory drugs for analgesia. Patients received no further sedation.
2.4.3. Assessment parameters
2.4.3.1. Primary outcome
The levels of IL-6 and INF-ɤ were measured in arterial blood samples withdrawn preoperatively (baseline), after full flow CBP, 6 h, and 24 h post-surgery in the pediatric cardiac intensive care unit (PICU). IL-6 level and INF-ɤ levels were measured using serum samples via ELISA kits (manufactured by SinoGeneClon Biotech Co) with sandwich ELISA detection method.
2.4.3.2. Secondary outcome
Hemodynamic variables (HR, MAP) were recorded during surgery and ICU admission. The inotropic score was measured using the following equation:
Complications as arrythmias e.g., bradycardia (decrease in HR > 30%), heart block, pulmonary edema (defined as the occurrence of bilateral chest crepitations, hypoxemia, and the presence of frothy bloody secretions from the endotracheal tube), etc., were treated and recorded intra-operatively and post-operatively. Duration of mechanical ventilation, length of ICU and hospital stay, and morbidity were recorded.
2.5. Statistical methods
2.5.1. Power calculation
Based on data from previous study [Citation19], sample size was calculated using G*power software 3.1.9.2. to be able to detect a significant difference of 48 picogram/ml of interleukin 6 level between both groups, with standard deviation of 48 picogram/ml (effect size = 1), power of the study of 95%, alpha error of 5% with allocation ratio of 1:1 in a two-tailed study, we need to include total sample size of 27 patients in each group. Five patients were added to each group to compensate for dropouts.
2.5.2. Statistical tests
Shapiro–Wilk test was used to test the normality of continuous data. Parametric data were presented as mean ±SD while nonparametric data were presented by median (IQR). Categorical data were presented as numbers (percentages).
Difference between the two studied groups was analyzed by t-test for parametric data, Mann–Whitney U test for nonparametric data and ƙ2 test for categorical data. Statistical significance was taken for p value < 0.05. All statistical analyses were performed using IBM SPSS statistics version 22 (SPSS Inc., Chicago, IL, USA).
3. Results
In this study, 31 patients in control group and 30 patients in DEX group are analyzed []. There were no significant differences between both groups in age, sex, weight, and height (P > 0.05) []. The Pathological Diagnosis, ischemic time, bypass time, the duration of surgery, dose of analgesics (fentanyl), and sedatives (midazolam) were comparable in both groups with non-significant differences, (P > 0.05) [].
Table 1. Patients’ characteristics and operative data in the two study groups
3.1. Inflammatory markers
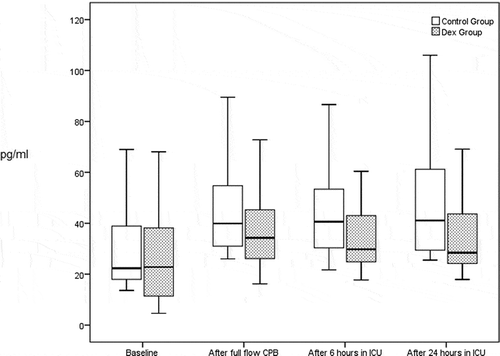
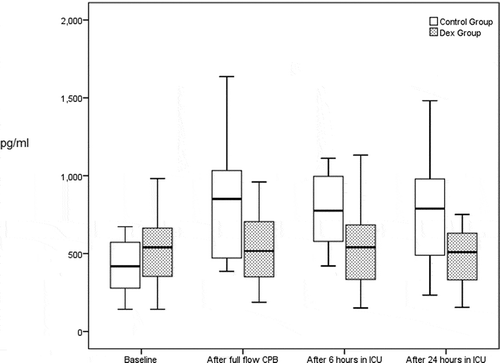
Levels of IL-6 and INF-ɤ increased significantly with time in control group, with no increase in their levels with time in DEX group. The levels of both inflammatory markers were significantly lower in DEX group compared to control group at all operative and postoperative times [ and ].
3.2. Inotrope score
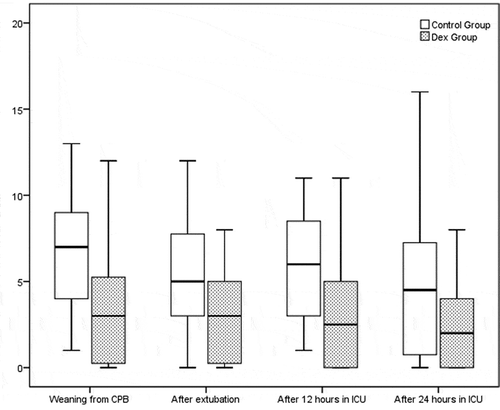
There was significant difference between the two groups after weaning from CBP (control group 7.37 ± 5.44, DEX group 3.81 ± 3.39, p value 0.009), after extubation (control group 6.21 ± 5.48, DEX group 3.57 ± 3.22 p-value 0.046), and 12 h (control group 6.53 ± 4.98, DEX group 3.17 ± 3.03, p value 0.007) after ICU admission [].
3.3. Hemodynamic variables
There was no significant difference between the two groups regarding MAP and HR except after extubation as they were significantly lower in DEX group.
There was no significant difference between the two groups regarding the incidence of perioperative complications []. The duration of mechanical ventilation was significantly shorter in the DEX group compared with the control group (p value = 0.023) []. No significant difference was recorded between the two studied groups in the length of ICU stay (p = 0.385) or hospital stay (p = 0.087) [].
Table 2. Clinical outcomes in both study groups
4. Discussion
Our primary outcome is to determine the levels of intraoperative and postoperative inflammatory markers IL-6 and INF -ɤ. The levels of both markers increased significantly with time in control group, with no increase in their levels in DEX group. The levels of IL-6 and INF -ɤ were significantly lower in DEX group compared to control group in samples taken during bypass, 6 h and 24 h post-surgery.
The anti-inflammatory effect of dexmedetomidine was studied in adult patients by Ueki M. et al in 2014. Patients in their study were scheduled for valve replacement and coronary artery bypass grafting (CABG) surgery. They investigated the levels of the nuclear protein plasma high-mobility group box 1 as an early mediator of inflammation and organ damage in ischemia-reperfusion injury, and plasma IL-6 levels. Dexmedetomidine started as 1 µg/kg for 10 min after aortic cross-clamping, and 0.5 µg/kg/h intra-operatively attenuated the rise in both mediators during and after CPB[Citation20].
In 2016, Bulow NMH. et al concluded that dexmedetomidine as a component of total intravenous anesthesia modified the inflammatory response in 23 adult patients scheduled for elective CABG surgery under mini-CPB. Dexmedetomidine was associated with a significantly reduced increase in plasma levels of IL-1, IL-6, TNF-α and INF-γ as samples were taken at baseline, 90 min and 5 h after starting mini-CPB and 24 h after the end of surgery. They infused a low dose of dexmedetomidine at 0.3 µg/kg/h during the surgery, which is lower than the average of the commonly used dose to avoid bradycardia as sufentanil and propofol were infused simultaneously[Citation21].
Recently, a pilot study was conducted by Zhou et al in 2019 that considered valve replacement surgeries to observe the effect of dexmedetomidine infusion started before CPB on myocardial injury, oxidative stress, and inflammatory response indicators in peripheral blood. Their data suggested that dexmedetomidine can reduce cardiac troponin I concentration (cTnI) 24 h after CPB and provide synergic benefits on inflammatory response[Citation22].
In pediatric cardiac surgery, dexmedetomidine was investigated for its anti-stress effect by Mukhtar et al in 2006. They demonstrated that intraoperative dexmedetomidine infusion significantly attenuated the hemodynamic and neuroendocrinal response to surgical trauma and CPB in 30 pediatric patients undergoing corrective surgery for congenital heart disease. The use of dexmedetomidine in pediatric cardiac surgery resulted in decreasing HR and MAP, with a concomitant reduction in cortisol, catecholamines, and blood glucose levels as markers of stress response. However, the effects on anti-inflammatory response were not evaluated[Citation23].
In our study, there was no significant difference in MAP and HR between the two groups, except after extubation as they were significantly lower in DEX group. This can be explained by the sedating and sympatholytic effect of dexmedetomidine. Dexmedetomidine was associated with a shorter period of mechanical ventilation (p value 0.023).
As regard the inotropic score, it was significantly lower in DEX group in the first 24 hrs. The decrease in inotropic score can be explained by the protective effects of dexmedetomidine on the myocardium. Alpha 2-adrenergic agonists have protective effects against myocardial ischemia by increasing the cAMP level and enhancing adenosine-induced coronary vasodilatation effect [Citation24].
Gupta, et al concluded that prolonged dexmedetomidine use in critically ill children with heart disease was associated with decreased inotropic support [Citation25]. It has been proposed that α2 agonists such as dexmedetomidine help to restore vascular reactivity to exogenously administered catecholamines in patients with catecholamine-refractory shock. α 2 agonists lower intrasynaptic catecholamine concentrations leading to reduction in α1 desensitization, leading to gradual resensitization of α1 receptors and a better response to exogenous catecholamines [Citation26,Citation27].
Clinical outcomes such as complications, duration of pediatric intensive care unit, or hospital stay showed no significant differences between both the groups.
A few studies focused on the effect of dexmedetomidine on the clinical outcomes in cardiac surgery. Ji F et al investigated the outcome in CABG and valvular replacement cardiac surgery using dexmedetomidine and found a decrease in post-operative complications, delirium, and mortality rate by up to one year[Citation28].
A meta-analysis was done in 2016 by Geng J et al. on the effects of perioperative dexmedetomidine on patients with cardiac surgery. They concluded the association of dexmedetomidine with decreased risk of ventricular tachycardia, atrial fibrillation, and delirium. Dexmedetomidine also shortened the duration of ICU stay and hospitalization. They also reported that dexmedetomidine administration was associated with increased risk of bradycardia and hypotension[Citation29].
5. Limitations
This study had some limitations. First, we used IL-6 and INF-ɤ as biomarkers for inflammatory response to surgery and CPB, however additional inflammatory markers should have been investigated. Secondly, the study was performed in non-cyanotic congenital cardiac surgery which has less inflammatory response. Finally, a wide range of age was investigated with possible effects on the results.
6. Conclusion
In pediatric cardiac surgery for non-cyanotic heart disease; dexmedetomidine administration can decrease the inflammatory response to surgery and CBP. It was useful in decreasing the level of inflammatory mediators, inotropic support, and duration of mechanical ventilation, but not the length of ICU or hospital stay. Future studies are recommended to focus on different infusion doses of dexmedetomidine in Pediatrics to reach the optimum dose with the best possible hemodynamics, to study the effect of dexmedetomidine after bypass – especially in patients that are extubated on it in the OR. Future studies will be required to investigate the clinical outcome.
Acknowledgments
Authors are grateful to the colleagues and staff of the PICU for their co-operation in data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hall RI. Cardiopulmonary bypass and the systemic inflammatory response: effects on drug action. J Cardiothorac Vasc Anesth. 2002;16:83–98.
- Wan S, J-L L, Vincent J-L. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692.
- Cuccurullo L, Accardo M, Agozzino L, et al. Ultrastructural pathology of pediatric myocardium in acute ischemia: bioptic study before and after treatment with cardioplegic solution. Ultrastruct Pathol. 2006;30:453–460.
- Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–1353.e3.
- Brix‐Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. 2001;45:671–679.
- Manrique AM, Vargas DP, Palmer D. et al. 2020, The effects of cardiopulmonary bypass following pediatric cardiac surgery. Munoz RA, Morell VO, Vetterly CG(Eds.), . Crit Care Child Hear Dis
- Pasquali SK, Li JS, He X, et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics. 2012;129:e385–91.
- Poyrazoğlu HH, Duman Z, Demir Ş, et al. Investigating the impacts of preoperative steroid treatment on tumor necrosis factor-alpha and duration of extubation time underwent ventricular septal defect surgery. Balkan Med J. 2016;33:158–163.
- Durandy Y. Minimizing systemic inflammation during cardiopulmonary bypass in the pediatric population. Artif Organs. 2014;38:11–18.
- Craciun EM, Altfelder F, Kuss N, et al. Anti-inflammatory effects of selected drugs on activated neonatal and adult neutrophils. Scand J Clin Lab Invest. 2013;73:407–413.
- Bach F, Grundmann U, Bauer M, et al. Modulation of the inflammatory response to cardiopulmonary bypass by dopexamine and epidural anesthesia. Acta Anaesthesiol Scand. 2002;46:1227–1235.
- Greilich PE, Brouse CF, Whitten CW, et al. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of ϵ-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003;126:1498–1503.
- Welters ID, Feurer M-K, Preiss V, et al. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br J Anaesth. 2011;106:172–179.
- Brull DJ, Sanders J, Rumley A, et al. Impact of angiotensin converting enzyme inhibition on post-coronary artery bypass interleukin 6 release. Heart. 2002;87:252–255.
- Kambam J, Meszaros R, Merrill W, et al. Prophylactic administration of histamine 1 and histamine 2 receptor blockers in the prevention of protamine-related haemodynamic effects. Can J Anaesth. 1990;37:420–422.
- Allan CK, Newburger JW, McGrath E, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244–1251.
- Straub RH, Herrmann M, Berkmiller G, et al. Neuronal regulation of interleukin 6 secretion in murine spleen: adrenergic and opioidergic control. J Neurochem. 1997;68:1633–1639.
- Sukegawa S, Inoue M, Higuchi H, et al. Proceedings of the American Society of Anesthesiologists Annual Meeting 2011, A1590
- Seghaye M-C, Duchateau J, Grabitz RG, et al. Influence of low-dose aprotinin on the inflammatory reaction due to cardiopulmonary bypass in children. Ann Thorac Surg. 1996;61:1205–1211.
- Ueki M, Kawasaki T, Habe K, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69:693–700.
- Bulow NMH, Colpo E, Pereira RP. et al. Dexmedetomidine decreases the inflammatory response to myocardial surgery under mini-cardiopulmonary bypass. . Brazilian J Med Biol Res
- Zhou H, Zhou D, Lu J, et al. Effects of Pre–Cardiopulmonary Bypass Administration of Dexmedetomidine on Cardiac Injuries and the Inflammatory Response in Valve Replacement Surgery With a Sevoflurane Postconditioning Protocol: A Pilot Study. J Cardiovasc Pharmacol. 2019;74:91.
- Mukhtar AM, Obayah EM, Hassona AM. The use of dexmedetomidine in pediatric cardiac surgery. LWW. 2006;103:52–6.
- Roekaerts PM, Prinzen FW, Willigers HM, et al. The effects of alpha2-adrenergic stimulation with mivazerol on myocardial blood flow and function during coronary artery stenosis in anesthetized dogs. Anesthesia Analg. 1996 Apr 1;82(4):702–711.
- Gupta P, Whiteside W, Arash Sabati TM, et al. Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease. Pediatr Crit Care Med. 2012;13(6):660–666.
- Pichot C, Géloën A, Ghignone M, et al. Alpha-2 agonists to reduce vasopressor requirements in septic shock? Med Hypotheses. 2010 Dec 1;75(6):652–656.
- Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928.
- Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–1584.
- Geng J, Qian J, Cheng H, et al. The influence of perioperative dexmedetomidine on patients undergoing cardiac surgery: a meta-analysis. PLoS One. 2016;11(4).