ABSTRACT
Background: Sepsis and sepsis shock are leading causes of mortality around the world. Early identification, proper management and close monitoring are associated with improved patient outcomes. Echocardiography allows proper monitoring of fluid-guided therapy and early identification of patients requiring vasopressors and inotropic drugs, although it requires special training and has some limitations. This study aims to compare echocardiography (echo) derived indices to cardiometry-derived indices in management of septic patients.
Methods: This prospective, randomized study was carried out on 90 patients with sepsis. The patients were divided into three groups: cardiometry group, which included those who underwent evaluation by the electrical cardiometry monitor; echo group, which included patients who underwent resting transthoracic echocardiography; and early goal-directed therapy (EGDT) group, which included those who received intravenous fluid. Thirty-day mortality was recorded, in addition to total infused fluid, total dose of vasopressor and inotrope, mechanical ventilation (MV) duration, and the intensive care unit (ICU) and hospital stay period.
Results: Mortality in echo and cardiometry-guided therapy was significantly lower than in the EGDT group. There was significantly higher norepinephrine and dobutamine doses, prolonged time to weaning of vasopressors, MV, longer ICU and hospital stay duration in echo and cardiometry groups compared to the EGDT group. On the other hand, all measurements were comparable in echo and cardiometry groups.
Conclusion: Evidence supports the usefulness of echocardiogram-guided treatment, similar to cardiometry hemodynamic management. However, cardiometry showed the advantage of being a simple and noninvasive technique that does not require a trained cardiologist.
Trial registration: Pan African clinical trial registry on 08/05/2019 with unique identification number: PACTR201911842779294.
1. Introduction
Sepsis is considered a systemic illness that is a secondary consequence of body invasion by microorganisms, associated with a storm of inflammatory mediators such as cytokines and interleukins. These markers are responsible for the systemic inflammatory response syndrome (SIRS) initiation [1].
Sepsis can be associated with the development of cardiac depression with decreased cardiac output (CO) and blood pressure. This state of shock stimulated a compensatory increase in the systemic vascular resistance (SVR), to maintain an adequate vital tissue perfusion. On the other hand, drawbacks associated with high SVR increase afterload, which in turn decreases cardiac output (CO) and leads to a narrow pulse pressure [Citation1,Citation2].
The first therapeutic modality in septic patients is fluid therapy [Citation3]. But in patients not responding to adequate fluid resuscitation, vasopressors or inotropes must be administered to maintain optimal hemodynamic targets [Citation4]. This created a need for perfect predictors of fluid response and identifying patients requiring either vasopressors or inotropes early enough to optimize resuscitation and organ perfusion.
Echocardiography is not routinely done to patients with sepsis and septic shock, although it was helpful in the early goal-directed therapy protocol, to improve cardiac dysfunction, intravascular volume resuscitation and hypovolemia [Citation5] and help predict outcomes in patients with sepsis and septic shock [Citation6]. Despite its accuracy, some limitations of echocardiography still interfere with its use, as it requires special training and depends on the observer’s previous experience.
The use of echocardiography was a standard approach in early detection of the need for vasopressors or inotropes in critically ill patients, despite its sophistication and that it requires special training for proper assessment and measurements [Citation4]. One of the recent non-invasive continuous CO monitoring equipment used is electrical cardiometry, which is based on its precedent electrical impedance [Citation7–10].
The authors suggest that depending on two-dimensional echocardiography or electrical cardiometry in the guidance of fluid therapy, the use of vasopressors and inotropes in the resuscitation of septic patients can decrease the 30-day mortality, hospital stay, and ICU stay.
In this study, echocardiography-derived indices, cardiometry-derived indices and standard EGDT guided therapy were compared for management of septic patients. The primary outcome was the 30-day mortality among patients with sepsis. Secondary outcomes included the hospital and intensive care unit (ICU) stay period.
2. Materials and methods
This prospective, randomized-controlled study was carried out in Tanta University Hospitals in the anaesthesia, surgical ICU, pain medicine department and cardiology department, from June 2019 to January 2020. It was approved by the regional ethical committees (research ethical committee on 30/4/2019 with unique approval number: 33,086/04/19) and registered in the pan African clinical trial registry on 08/05/2019 with unique identification number: PACTR201911842779294. Every patient received an explanation of the purpose of the study and was assigned a secret code number, given the photos of only the part of the body linked to the research, to ensure privacy and confidentiality of data. Written informed consent was collected from the patients.
Ninety patients were included in this trial, aged 18–65 years old, non-pregnant, and of both genders, diagnosed with sepsis and admitted to ICU. Sepsis was defined according to the standard Surviving Sepsis Campaign criteria using the Sequential (sepsis-related) Organ Failure Assessment (SOFA) score [Citation11] (SOFA score ≥ 2 indicated organ dysfunction). Patients with any of the following were excluded: acute coronary syndrome, major cardiac dysrhythmia, valvular or congenital heart disease, left systolic dysfunction as cardiomyopathic and another primary lung disease, those known to be hepatic or have renal dysfunction, severe anemia and any other disease which affected cardiac hemodynamics or cardiac function, patients unable to lie supine and those who were pregnant.
Enrolment of patients occurred within 8 h of meeting the criteria for sepsis. All patients were randomized using the computer-generated software of randomization and sealed closed envelopes to allocate them to one of three groups (30 patients each).
2.1. Group I (cardiometry group)
All patients in this group with sepsis underwent evaluation by the electrical cardiometry monitor (ICON Cardiotronic, Inc., La Jolla, CA92307; Osyka medical GmbH, Berlin, AND Germany, model C3, serial number 1,725,303). Patients were held in supine position and four sensors were attached to each: the first one approximately 5 cm above the base of the neck on the left side, the second immediately on the left side of the base of the neck, the third at the level of the xiphoid process crossing the left anterior axillary line and the fourth approximately 5 cm below the 3rd electrode at the level of the left anterior axillary line. They were then connected to the sensor cable and the patient data started to feed in [Citation12,Citation13].
ICON was used as an indicator of contractility (patients below the normal range were managed by titrated doses of dobutamine (5–20 µg/kg/h)). Stroke volume variation (SVV) was used as an indicator of fluid responsiveness (patients diagnosed as fluid responders were managed by bolus doses of fluids (4–6 ml/kg)). SVR was used as an indicator for the need of vasopressors (patients with decreased SVR were managed by titrated doses of norepinephrine (0.05–1 µg/kg/h)).
2.2. Group II (echo group)
All subjects in this group underwent resting transthoracic 2-dimensional echocardiography as a baseline, using Philips CX50-Extreme edition USA with S5-1 MHz multi-frequency echo probe, according to the standard protocol.
Stroke volume (SV) was measured by multiplying the aortic valve area in the velocity-time integral of aortic blood flow (VTIAo) using the formula SV (ml) = (LVOTa) x (VTIAo). The aortic valve area was calculated from the measurement of the left ventricular outflow tract (LVOTd) measured at the insertion of the aortic cusp from the left para sternal axis view. The aortic valve area (LVOTa) was then calculated as π x (LVOTd/2)2, as the diameter of the aortic orifice is assumed to remain constant.
Apical 5 chamber view (VTIAo) was calculated from the area under the envelop of pulsed wave Doppler signal obtained at the level of aortic annulus. The echo-Doppler SV and SVV were calculated from six consecutive beats. Initially, the patients were assessed for the presence of pre-existing chronic cardiac dysfunction that can affect the interpretation, such as significant valvular heart diseases, congenital heart diseases, cardiomyopathies and extensive left atrial dilation. Cardiac output (CO) was calculated as the product of the heart rate and the SV. Echo was used to measure cardiac output and subsequent cardiac index, to determine whether the patient responded to the used management protocol.
Myocardial systolic function was then assessed by 2D Simpsons method and classified into either good or impaired systolic function according to the ejection fraction (EF) (EF < 40% indicated impaired systolic function). Patients with impaired systolic function were managed by titrating doses of inotropes (dobutamine 5–20 mcg/kg/min) and then reassessed by echo parameters, while in those with good left ventricular systolic function, fluid responsiveness was assessed by SV and SVV through measured velocity time integral (VTI). The fluid responders were managed by a fluid bolus (4–6 ml/kg) and then reassessed, while non-responders were managed by titration of vasopressors (norepinephrine 0.05–1 µg/kg/hr) and then reassessed again. Echocardiographic parameters were repeated after each change in the management of fluid and administration of vasopressors or inotropes.
2.3. Group III (early goal-directed therapy (EGDT) group)
Patients in this group received an IV fluid bolus (4–6 ml/kg and up to 30 ml/kg) till the target central venous pressure (CVP) (8–12 mmHg) was achieved. They then received norepinephrine 0.05–1 µg/kg/h to target a mean arterial pressure (MAP) of > 65 mmHg and dobutamine infusions (5–20 µg/kg/h) to achieve lactate normalization.
According to the surviving sepsis campaign (SSC) guidelines, before fluid administration and each hour for 6 hours, Acute Physiological and Chronic Health Evaluation (APACHE II) scoring system [Citation14] was used to calculate the score for each patient. Lactate serum level was assessed at admission and after 3 hours of fluid therapy to assess tissue hypoperfusion despite resuscitation [Citation15].
Discharge was based on the following criteria [Citation16]: conscious, with stable hemodynamic parameters with no intravenous inotropic/vasopressor support, stable respiratory status and oxygen requirements not more than 60%, weaned from MV for at least 24 hours, with a patent airway and normal cough reflex.
3. Measurements
For all groups, the following was recorded: 30-day mortality among patients with sepsis (primary outcome), the hospital stay period (calculated from ICU discharge till discharge from the hospital) and intensive care unit (ICU) stay (secondary outcomes), total infused fluid from ICU arrival till the CVP goal is achieved, total dose of vasopressor and inotrope taken during ICU stay period, duration of mechanical ventilation (MV), time to weaning of vasopressors, APACHI II score, serum lactate, heart rate (HR) and mean arterial pressure and CVP during ICU stay period. In echo and cardiometry groups, stock volume (SV), stock volume index (SVI), CO, cardiac index (CI), oxygen saturation (SpO2) and stock volume variation (SVV) were recorded. Also, a comparison was made within the same group between parameters before and after therapy.
4. Statistical analysis
The primary outcome of the study was the 30-day mortality. Based on the results of a previous study (17), the 30-day mortality was found to be 33.3% in sepsis patients receiving early goal-directed therapy. The sample size was thus calculated to be 28 patients in each group needed to detect a significant reduction of 20% in 28-day mortality at α error of 0.05 and power of study of 80%. Thirty cases were enrolled per group to overcome possible dropouts. Numerical variables were described as mean ± SD. Data were fed to the computer and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Categorical variables were described as percentages. The Kolmogorov–Smirnov test was used to verify the normal distribution of variables. Comparison between categorical variables between groups was assessed using Chi-square test, while quantitative variables were assessed using the student t-test for normally distributed variables. ANOVA was used to compare between more than two groups, while Post Hoc test was used in pairwise comparison. Comparison between different periods was carried out by paired t-test. Significance of the obtained results was judged at the 5% level.
5. Results
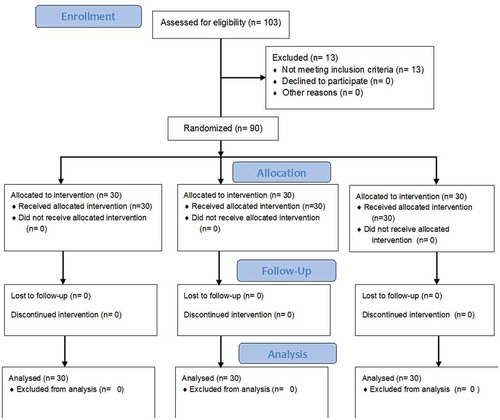
103 patients were recruited in this study. 13 were excluded (4 in group I, 4 in group II and 5 in group III): 7 patients were excluded due to major cardiac dysrhythmia, 3 patients had severe anemia, one patient was unable to lie in supine position and 2 patients had renal dysfunction. Only 90 patients with severe sepsis/septic shock were analyzed, 30 patients in each group ().
All groups in the study were comparable regarding baseline characteristics (age, sex and the causes of sepsis, as P = 0.497, 0.956, and 0.980, respectively) ().
Table 1. Comparison between the three studied groups according to age, sex and cause of sepsis
At 30 days, the mortality rate in EGDT group was 56.7% (17 patients), which was significantly higher than 23.3% (7 patients) in the echo group and 30.0% (9 patients) in the cardiometry group, P = 0.037 and 0.008, respectively. On the other hand, mortality rate in both echo and cardiometry groups were not significantly different (P = 0.559) ().
Table 2. Comparison between the three studied groups according to mortality
Norepinephrine and dobutamine doses were significantly higher in echo and cardiometry groups compared to the EGDT group. In addition, there was significant prolonged time to weaning of vasopressors, total MV days, ICU stay duration and duration of hospital stay in echo and cardiometry groups compared to the EGDT group. On the other hand, norepinephrine and dobutamine doses, time to weaning of vasopressors, total MV days, ICU stay duration and duration of hospital stay were comparable in echo and cardiometry groups ().
Table 3. Comparison between the three studied groups according to hospital stay, ICU stay, vasopressor duration, mechanical ventilation duration, dobutamine dose and norepinephrine dose
At baseline, APACHI II score, lactate, HR, MAP, CVP and SPO2 were comparable in the three groups. After therapy, there was significant improvement in each group. In addition, there was a significantly higher APCHI II score, lactate, HR and CVP and a significantly lower MAP and SPO2 in EGDT group than echo and cardiometry groups. On the other hand, APACHI II score, lactate, HR, MAP and SPO2 value after treatment were not significantly different between echo and cardiometry groups ().
Table 4. Comparison between the three studied groups according to APACHI II score, lactate, HR, MAP, CVP & SPO2 either between the three groups or within same group between before and after treatment
6. Discussion
Till this day, there is no uniform diagnostic approach to reliably determine which patients will or will not increase organ perfusion in response to aggressive fluids [Citation17] and which patients with impaired left ventricular systolic function required inotropes to support adequate organ perfusion [Citation18]. Although the static measures to predict fluid responders were widely studied, it has a poor predictive value when compared with dynamic measures [Citation19].
With dramatically improved technology and establishment of training fellowship programs, non-cardiologists will be able to properly perform bedside echocardiography [Citation20]. On the other hand, electrical velocimetry has been validated to monitor cardiac output non-invasively [Citation7]. Surprisingly, there is no previous literature comparing between echocardiography and cardiometry versus conventional fluid therapy in sepsis treatment. In the present work, the authors evaluated a group of patients whose treatment was guided by echo and by cardiometry and compared the results with a group of patients who received EGDT.
The study results regarding the mortality in both echo and cardiometry-guided therapy (23.3% and 30.0%, respectively) were significantly lower than EGDT group (56.7%). Moreover, there was significant increase of norepinephrine and dobutamine doses and significant prolonged time to weaning of vasopressors, total MV days, ICU stay and hospital stay in echo and cardiometry groups compared to EGDT group. The reason for this could be CVP guidance (targeting CVP between 8 and 12 mm Hg), which made the fluid therapy relatively higher in the EGDT group. Echo and cardiometry groups were comparable in mortality, norepinephrine and dobutamine doses, time to weaning of vasopressors, total MV days, ICU stay and hospital stay, either before or after treatment.
The study results were close to the reported incidence of mortality (20–30%, and up to 50%) in previous literature [Citation6,Citation21–23]. However, they were higher than those reported by Chertoff et al. [Citation24], who found 29.69% mortality rate in early goal-directed therapy group of patients.
In addition, in a study by Kanji et al., 28-day survival rate favoured patients receiving echo-guided therapy (66% versus 56%, respectively) over the standard management group (P = 0.04). Also, Kanji et al. reported significantly higher utilization of dobutamine in the echo group than the standard management (22% and 12%, respectively) (P = 0.01) [Citation25].
According to Pierrakos et al., MAP is not a reliable indicator of cardiac index (CI) changes after fluid challenge is performed in patients with septic shock. This result supported the current study observation about limitations of early goal-directed fluid therapy [Citation26].
Rivers et al. also reported that administration of sufficient intravenous fluids to achieve a CVP of 8–12 mm Hg was unable to predict significant changes in cardiac output or organ perfusion [Citation27].
Similarly, Zhang et al. found that treatment based on stroke volume variation and cardiac index assessed by Vigileo-FloTrac system was associated with increased PaO2/FiO2-ratio, a significant decrease in the required fluid volume for resuscitation and shorter intubation time in patients requiring thoracic surgery [Citation28].
Consistent with our results, Timsit et al. reported that trans-thoracic echo (TTE) was associated with a higher dose of norepinephrine, early vasopressors weaning, higher dobutamine and less mortality compared to the non-TTE group [Citation29].
On the other hand, ventilation-free days were not significantly different between echo group and non-echo groups, according to a study by Feng et al. [Citation30]
Different results were also reported by Lanspa et al., who found an insignificant difference between echo-guided resuscitation and EGDT, regarding mortality, lactate clearance and ICU stay. Their explanation was that this could be a result of delayed echo assessment after initial resuscitation [Citation31].
Overall, results of many studies indicated that echo, as an ideal monitoring technique in critically ill patients, was associated with better outcomes and significantly lower total fluid administration than EGDT. This study was limited by the potential bias of its unblinded design, as it was not possible to conduct it in a blinded manner for comparing goal-directed group with either echo or cardiometry-guided therapy in the same ICU. Other limitations rose from the small sample size, short duration of action and limited follow-up of studied patients. The use of echo was also limited by low echogenicity, was not suitable for continuous monitoring of cardiac output or pulmonary artery pressure, required adequate training which is not available at all centers and a limited value when used for single monitoring for repeated bedside assessment of hemodynamic variables. Finally, morbid obesity and atrial fibrillation still represent challenges in echocardiographic assessments and follow up due to inconsistency of cardiac output on LVOT VTI or limited window. Patients with these conditions were thus excluded from this study.
We believe that further randomized trials at different centers on a larger sample size, together with prolonged follow up, will be needed to compare between echocardiography-guided resuscitation and cardiometry in septic shock.
This was the first study to compare the outcome between EGDT and the use of echocardiographic and cardiometry-guided therapy in management of sepsis and septic shock. Results of the current work seem to be promising as in severe sepsis and septic shock, echo or cardiometry-guided management is superior and is associated with additional benefits over EGDT. Both echo and cardiometry are non-invasive tools and were associated with significant lower mortality and favorable outcomes. The present work recommends a routine use of cardiometry as a non-invasive bedside tool for the assessment and management of critically ill patients with severe sepsis and septic shock.
Disclosure statement
This paper was self-funded and did not receive financial support. The authors report no conflict of interest.
Additional information
Funding
References
- Singh Y, Katheria AC, Vora F. Advances in diagnosis and management of hemodynamic instability in neonatal shock. Front Pediatr. 2018;6(2):2–12.
- Fathi EM, Narchi H, Chedid F. Noninvasive hemodynamic monitoring of septic shock in children. World J Methodol. 2018;8(1):1–8.
- Soliman R. Prediction of fluid status and survival by electrical cardiometry in septic patients with acute circulatory failure. Egypt J Crit Care Med. 2017;5(2):65–68.
- Pollard S, Edwin SB, Alaniz C. Vasopressor and inotropic management of patients with septic shock. P & T: a Peer-Rev J Formulary Manag. 2015;40(7):438–450.
- Merx M, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802.
- Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005;365(9453):63–78.
- Rajput RS, Das S, Chauhan S, et al. Comparison of cardiac output measurement by noninvasive method with electrical cardiometry and invasive method with thermodilution technique in patients undergoing coronary artery bypass grafting. World J Cardiovasc Surg. 2014;4(7):1–8.
- Zoremba N, Bickenbach J, Krauss B, et al. Comparison of electrical velocimetry and thermodilution techniques for the measurement of cardiac output. Acta Anaesthesiol Scand. 2007;51(10):1314–1319.
- Schmidt C, Theilmeier G, Van Aken H, et al. Comparison of electrical velocimetry and transoesophageal Doppler echocardiography for measuring stroke volume and cardiac output. Brit J Anaesth. 2005;95(5):603–610.
- Narula J, Kiran U, Chauhan S, et al. Electrical cardiometry in patients undergoing cardiac catheterisation. Int J Perioperative Ul Appl Technol. 2013;2(3):102–107.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810.
- Bernstein DP, Lemmens H. Stroke volume equation for impedance cardiography. Med Bio Eng Comput. 2005;43(4):443–450.
- Bernstein DP. Bernstein-Osypka stroke volume equation for impedance cardiography: citation correction. Intens Care Med. 2007;33(5):923.
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829.
- Zhang L, Zhu G, Han L, et al. Early goal-directed therapy in the management of severe sepsis or septic shock in adults: a meta-analysis of randomized controlled trials. BMC Med. 2015;13(1):71–83.
- Nates JL, Nunnally M, Kleinpell R, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med. 2016;44(8):1553–1602.
- Lanspa MJ, Burk RE, Wilson EL, Hirshberg EL, Grissom CK, Brown SM. Echocardiogram-guided resuscitation versus early goal-directed therapy in the treatment of septic shock: a randomized, controlled, feasibility trial. 6(1):1–8, 2018 doi:10.1186/s40560-018-0319-3
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness?*: A systematic review of the literature and the tale of seven mares. Chest. 134(1):172–178, 2008
- De Backer, Daniel, Patrick Biston, Jacques Devriendt, Christian Madl, Didier Chochrad, Cesar Aldecoa, Alexandre Brasseur, Pierre Defrance, Philippe Gottignies, and Jean-Louis Vincent. Comparison of dopamine and norepinephrine in the treatment of shock. New Engl J. Med 362(9):779–789, 2010
- Monnet X, Teboul J-L. Assessment of volume responsiveness during mechanical ventilation: recent advances. Crit Care. 17(2):217–224, 2013
- Fletcher S, Grounds R. III. Critical care echocardiography: cleared for take up. Oxford University Press. BJA 109(4): 490–492, 2012 doi:10.1093/bja/aes323
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. 29(7):1303–1310, 2001 doi:10.1097/00003246-200107000-00002
- Boyd JH, Forbes J, Nakada T-a, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 39(2):259–265, 2011
- Vincent, Jean-Louis, Yasser Sakr, Charles L Sprung, V Marco Ranieri, Konrad Reinhart, Herwig Gerlach, Rui Moreno, Jean Carlet, Jean-Roger Le Gall, and Didier Payen. Sepsis in European intensive care units: results of the SOAP study. Intens Care Med. 34(2):344–353, 2006
- Chertoff J, Chisum M, Simmons L, King B, Walker M, Lascano J. Prognostic utility of plasma lactate measured between 24 and 48 h after initiation of early goal-directed therapy in the management of sepsis, severe sepsis, and septic shock. J Intensive Care. 4(1):13–21, 2016 doi:10.1186/s40560-016-0142-7
- Kanji HD, McCallum J, Sirounis D, MacRedmond R, Moss R, Boyd JH. Limited echocardiography–guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care. 29(5):700–705, 2014 doi:10.1016/j.jcrc.2014.04.008
- Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent J-L. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intens Care Med. 38(3):422–428, 2012
- Rivers, Emanuel, Bryant Nguyen, Suzanne Havstad, Julie Ressler, Alexandria Muzzin, Bernhard Knoblich, Edward Peterson, and Michael Tomlanovich. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 345(19):1368–1377, 2001
- Zhang J, Chen CQ, Lei XZ, Feng ZY, Zhu SM. Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: a pilot study. Clinics. 68(7):1065–1070, 2013
- Timsit, Jean-François, Mark Rupp, Emilio Bouza, Vineet Chopra, Tarja Kärpänen, Kevin Laupland, Thiago Lisboa, Leonard Mermel, Olivier Mimoz, and Jean-Jacques Parienti. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intens Care Med. 44(6):742–759, 2018
- Feng, Mengling, Jakob I McSparron, Dang Trung Kien, David J Stone, David H Roberts, Richard M Schwartzstein, Antoine Vieillard-Baron, and Leo Anthony Celi. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intens Care Med. 44(6):884-892, 2018.