ABSTRACT
Background: Epidural analgesia has been established to be the best modality for painless labor. This study hypothesized to evaluate the effect of adding tramadol as an adjuvant to bupivacaine in epidural labor analgesia.
Methods: Sixty parturients were allocated into two equal groups. Group B received a total volume of 10 ml bupivacaine 0.25% in normal saline. Group BT received a total volume of 10 ml bupivacaine 0.25% plus tramadol 5 mg/ml in normal saline as a loading dose, followed by continuous infusion of bupivacaine 0.125% at rate 10 ml/h 15 min after a loading dose for both groups. The onset of analgesia, degree of motor block, sedative effect, hemodynamic parameters, visual analogue scale (VAS), number of top-up doses, fetal outcomes, any related side effects, and maternal satisfaction were recorded.
Results: Faster onset of analgesia was achieved in BT group compared to B group (7.9 ± 2.68 vs 15.3 ± 3.3, respectively: P < 0.001). BT group has a longer duration of analgesia (181.1 ± 44.3 min) than B group (89.6 ± 32.6 min) (P < 0.001). VAS was significantly decreased at 10, 15, 60, 120 and 180 min in BT group compared to B group (P < 0.001, < 0.001, 0.001, < 0.001, and 0.006, respectively). Motor block was comparable between both groups. Higher levels of sedation were reached in group BT.
Conclusion: Tramadol in epidural labor analgesia as an adjuvant to bupivacaine can provide a better quality of analgesia with faster onset, and prolonged duration with no relevant adverse effects
Pan African Clinical Trial registration: (PACTR201804003261749)
1. Introduction
Normal delivery birth experience is an exciting moment, however, the pain associated with it has been considered as one of the most excruciating pain. The fear of labor pain has an increased propensity to cesarean section[Citation1]. Labor pain is associated with many psychological and physiological changes, leading to increased level of catecholamine, hyperventilation with increased oxygen demand resulting in uteroplacental hypoperfusion and fetal hypoxia[Citation2].
Different modalities for painless labor has been implemented [Citation3]. Epidural labor analgesia is the golden method for painless labor with higher maternal satisfaction. Bupivacaine is the most common local anesthetics used for this purpose. To achieve a better quality of analgesia and a longer duration of action, several adjuncts have been added to local anesthetics [Citation3,Citation4].
Tramadol is a synthetic opioid that has multimodal anti-nociceptive effects. It has been used for postoperative pain in different routes, such as intramuscular, intravenous, and oral [Citation5]. In addition to its moderate µ–opioid receptor affinity, it inhibits norepinephrine reuptake at the level of α-2 adrenergic receptors and acts as a 5-hydroxytryptamine (5HT) reuptake inhibitor together with presynaptic stimulation of 5HT release at 5-HT3 receptors. These accentuate the descending inhibitory pathway in the spinal cord enhancing analgesia [Citation6,Citation7]. It is also suggested by many studies that tramadol has a local anesthetic properties, although weaker than lidocaine, by blocking nerve conduction either by interaction with calcium receptors [Citation8] or inhibition of K+ channels [Citation9]. Therefore, tramadol can provide effective analgesia when given epidurally as local anesthetic adjuvants with no relevant side effects [Citation10,Citation11].
This study was designed to study the effects of adding tramadol as an adjuvant to bupivacaine in epidural painless labor on the quality of analgesia, the motor block, and the sedation level.
2. Material and methods
This prospective randomized double-blind study was conducted at the Obstetrics and Gynecological department, after it was approved by Faculty of Medicine, Tanta University, Local Ethical Committee (approval number 32,141/02/18), and Clinical Trial registration (PACTR201804003261749). Sixty nulliparous parturients aged from 21 to 30 years, ASA physical status II, requesting epidural labor analgesia were recruited in the study after signing written informed consent. The parturients included in the study had a single fetus, vertex presentation, active labor (cervical dilatation 3–5 cm), and more than 37 weeks of gestation. The controlled study was carried out between March 2018 to March 2020. Multiparous, mal-presentation, high-risk pregnancies (preeclampsia, diabetes, or cardiac), multiple pregnancies, coagulopathy, allergy to any used medications, BMI > 35 kg/m2, and patient refusal were excluded from the study.
Randomization of the participants was achieved using a computer-engendered software program then were allocated into two groups in a 1:1 ratio by opening sealed envelopes. Group B (30 parturient) received a total volume of 10 ml bupivacaine 0.25% in normal saline, whereas Group BT (30 parturient) received a total volume of 10 ml bupivacaine 0.25% plus tramadol 5 mg/ml in a normal saline as a loading dose (5 ml increments every 5 min) followed by continuous infusion of bupivacaine 0.125% at a rate 10 ml/h for both groups 15 min after a loading dose. The infusion was stopped when the cervix was fully dilated. The mixed solutions in the syringes were prepared by an anesthesiologist not involved in the study then endorsed blindly to the anesthesiologist who was responsible for the placement of the epidural labor analgesia and recording the data. Parturient, Obstetrician, Neonatologist, and ward nurse were also blinded to group allocation.
All the parturients included in the study received 500 ml of Ringer’s solution intravenous as preload and connected to routine monitors (pulse oximetry, non-invasive blood pressure and electrocardiography). Also, they were trained how to quantify the pain using 10-cm linear visual analogue scale (VAS) (0 = no pain and 10 = worst imaginable pain).
The parturient was placed in a sitting position and all aseptic precautions with skin sterilization were performed. Identification of L 3–4 or L 4–5 intervertebral space followed by local infiltration of the skin and subcutaneous tissue with lidocaine 2%. Localization of epidural space was done with an 18-gauge Tuohy needle (Perifix®, Braun, Germany) using a loss of resistance to air technique. Epidural catheter (20-gauge multi-orifice) was threaded in the epidural space and fixed to the depth of 4–5 cm after confirmation of negative aspiration for cerebrospinal fluid or blood. The catheter was secured with plaster over the back of the parturient. Then, the parturient was positioned supine with a left lateral tilt to avoid aortocaval compression. A test dose of 3 ml lidocaine 2% plus 15 µg adrenaline was injected to confirm the absence of inadvertent intravascular or intrathecal placement of the catheter prior to injection of the study solution. To evade the risk of increased spread of study drugs into epidural space the injection was given in between uterine contraction.
The injection time of the study drug was recorded as T0. The onset of analgesia was estimated as the time to achieve VAS < 3. The duration of analgesia was evaluated as time from T0 to reach VAS > 3 and the occurrence of breakthrough pain. Additional top-up dose of 5 ml bupivacaine 0.125% was injected into the epidural catheter to manage breakthrough pain. The number of top-up doses was recorded. VAS was assessed before the placement of epidural then at 5, 10, 15, 30, 45, 60 min then every hour after injection of the study drugs until the delivery time.
The degree of motor block was assessed using a modified Bromage scale [Citation10] before the placement of epidural then at every 15 min in the first hour then every 30 min after injection of the study drugs until the delivery time. The infusion rate of bupivacaine was decreased when Bromage score was ≥ 2 until the score became ≤ 1. The level of sedation was assessed by sedation core [Citation10] at the same time interval.
Hemodynamic monitoring of blood pressure and heart rate was recorded before placement of the epidural and at 5, 15, 30, 60 min then every hour until delivery. Hypotension was defined as decreased mean arterial blood (MAP) pressure ≥ 30% from baseline and/or systolic blood pressure less < 100 mm/Hg and was treated by intravenous fluid boluses and/or 10 mg ephedrine and can be repeated if needed. Bradycardia was defined as a heart rate < 60 beats/min and was treated with 0.5 mg atropine. Hypotension, bradycardia, and any other adverse effects such as nausea and vomiting (treated with metoclopramide 10 mg) or pruritus (treated with diphenhydramine 50 mg) were recorded. Cervical dilatation at the beginning of the study, duration of the first and second stage of labor, and the mode of delivery (spontaneous vaginal delivery, assisted or cesarean section) were recorded.
Fetal heart rate was monitored continuously by cardiotocography, and any fetal heart rate abnormalities detected were managed initially by giving oxygen or intravenous fluid to the mother, stopping oxytocin, and ensuring a left uterine displacement. Taking into account the comparison of the tracing recorded 30 min before the placement of epidural and during epidural analgesia. Neonatal outcome was assessed by neonatologist using Apgar score at 1 and 5 min and umbilical venous blood PH.
Patients satisfaction about the quality of analgesia were assessed after 24 h of delivery with five point Likert’s scale (1 = poor; 2 = fair; 3 = good; 4 = very good; 5 = excellent).
The onset of analgesia was the primary outcomes. The secondary outcomes were the degree of motor block, the hemodynamic changes, and the sedation score.
3. Sample size
Calculation of sample size based upon the results of previous trial [Citation12] revealed that at least 21 patients were required in each group to detect a significant change of the onset of sensory block of 5 minutes at alpha value of 0.05 and 95% power of study. Thirty patients were included in each group to overcome the possibility of dropout cases.
4. Statistical analysis
Statistical analysis of data was done utilizing IBM SPSS 20.0 (Armonk, NY: IBM Corp) software program. Checking of normal distribution of data was done by Kolmogorov–Smirnov or Shapiro tests. Data were presented as mean ± standard deviation. Comparison of numerical data between the two groups was done utilizing Student’s independent t- test for data showing normal distribution or by Mann – Whitney U test, if otherwise. VAS score, motor block and sedation score was presented as median (interquartile range) and analyzed by Mann–Whitney test between studied groups. Categorical variables were presented as patients’ number and percentage (%) and were analyzed utilizing the Chi-square test or Fisher’s exact test when appropriate. P-values < 0.05 was considered significant.
5. Results
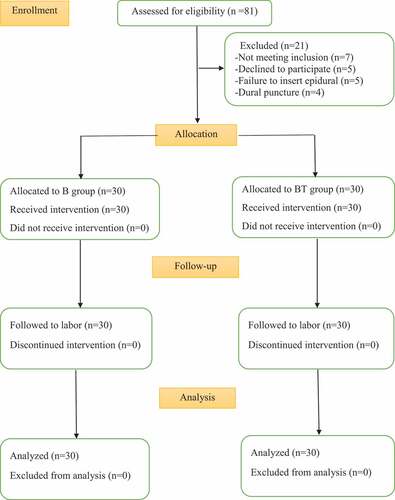
Out of eighty-one eligible parturients, sixty parturients were enrolled and allocated into two groups in this study (). Regarding demographic characteristics and labor progress data, there were comparable as shown in
.Table 1. Demographic characteristic and labor progress in the two studied groups
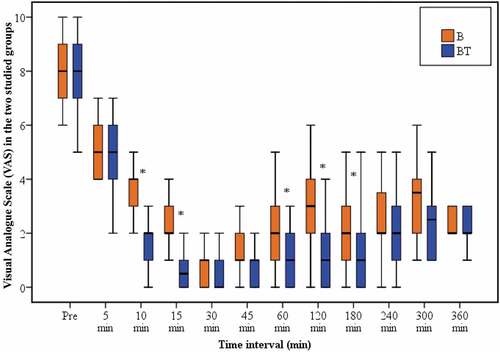
Group BT has a shorter onset of analgesia (7.9 ± 2.68 min) in comparison with group B (15.3 ± 3.3 min) and this was statistically significant (P < 0.001) (). The VAS was significantly lower in group BT at 10 and 15 min than group B (P < 0.001, < 0.001, respectively) (). The duration of analgesia was significantly longer in group BT () and this was associated with significantly lower VAS at 60, 120, and 180 min (P = 0.001, < 0.001, 0.006, respectively) as compared with group B (). VAS was comparable between the two groups at any subsequent reading. The number of top up doses and the number of parturients requested it presented in .
Table 2. Analgesic quality, maternal side effects, parturient satisfaction, and neonatal outcomes in the two studied groups
Sedation score of 2 or 3 was more frequent in group BT (56.7%) compared to group B (0%) and this was statistically significant at 30, 45, 60, and 120 min ().
Table 3. Motor block and sedation in the two studied groups
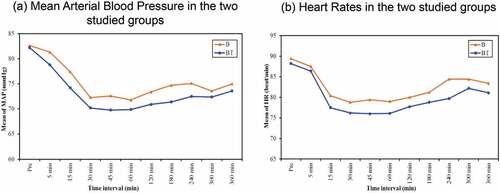
Only 6 (20%) parturients in group B and 8 (26.6%) parturients in group BT were developed motor block of grade 2. No one developed grade 3 motor block (). Hemodynamic changes () and side effects were comparable between the two groups ().
Figure 3. Hemodynamic parameters, (a) Mean Arterial Blood Pressure in the two studied groups, (b) Heart Rates in the two studied groups
The neonatal outcomes were statistically insignificant between both groups (). Maternal satisfaction was significantly better in group BT as compared with group B (P = 0.037) ().
6. Discussion
Labor pain management has a lot of challenges for both Obstetricians and Anesthetists. Several modalities have been used, however, epidural analgesia has been established to be the best modality for pain relief during labor. The drugs used in epidural analgesia should provide a better quality of analgesia with rapid onset and higher maternal satisfaction, on the other hand, reduce adverse effects for both mother and neonate [Citation13]. It is important to choose a suitable local anesthetic concentration and appropriate adjuvants.
This study was designed to show the analgesic properties, the motor block, and the sedative effect of administrating tramadol as an adjuvant to bupivacaine in epidural painless labor.
Our results showed that adding tramadol has been associated with rapid onset of analgesia (7.9 ± 2.68 min versus 15.3 ± 3.3 min) and longer duration of action (181.1 ± 44.3 min versus 89.6 ± 32.6 min) compared to bupivacaine alone.
Furthermore, the addition of tramadol had statistically significantly decreased VAS at 10, and 15 min compared to bupivacaine alone group which determines rapid onset of action with more patients recorded VAS < 3. Also, it decreased significantly at 60, 120, and 180 min which indicates a prolonged duration of action. Moreover, the first request for a top-up dose due to breakthrough pain was delayed with a significantly decreased number of parturient requested analgesia (30%) in the tramadol group as compared with bupivacaine alone group (76.6%).
Türkoğlu et al. [Citation14] compared patient-controlled epidural analgesia with the administration of levobupivacaine 0.125% either plus tramadol 100 mg or morphine 100 µg. They showed lower VAS in morphine and tramadol group after major abdominal surgery. A study conducted by Singh et al. [Citation11] showed that either tramadol 1 mg/kg or 2 mg/kg provides significantly prolonged postoperative analgesia and lower VAS when added to ropivacaine 2% in epidural for upper abdominal surgery compared to ropivacaine 2% alone. The duration of analgesia was longer in tramadol 2 mg/kg (584 ± 58 min) compared with tramadol 1 mg/kg (394 ± 46 min) or ropivacaine alone (283 ± 35 min).
Also, Fan et al. [Citation15] in their study comparing the efficacy of adding tramadol (5 mg/ml) or fentanyl (3 µg/ml) to ropivacaine 0.125% in epidural labor analgesia, the onset of analgesia and VAS were comparable with no significant difference between both groups. They concluded that tramadol seems to be as effective as fentanyl in providing analgesia with minimal side effects.
Moreover, in a study conducted by Jaitley et al. [Citation12] comparing epidural and IV tramadol, they reported that epidural tramadol group has an onset of analgesia with a mean (10.83 ± 4.346) min and duration of analgesia with a mean (3.77 ± 0.573) h. This was consistent with our study. Imani et al. [Citation10] concluded that epidural anesthesia with tramadol adding to lidocaine in a patient scheduled for cesarean section has a rapid onset of sensory and motor blockade and delayed request of analgesia. Previous studies [Citation16,Citation17] have evaluated the effectiveness of adding tramadol to bupivacaine in caudal epidural analgesia in children, and they reported a longer duration of analgesia and decreased the need for supplementary analgesia with lower VAS.
It is probably the slow absorption of tramadol through the dura or slow uptake from the epidural space into the systemic circulation is the mechanism of prolonged duration of action [Citation18,Citation19].
However, Chatrath et al. [Citation2] in their prospective-randomized study comparing the efficacy of levobupivacaine with tramadol or fentanyl for combined spinal-epidural analgesia in labor, concluded that fentanyl has a rapid onset of analgesia whereas tramadol has a longer duration of analgesia.
Ambulation during labor is imperative as well as good expulsive force to allow normal spontaneous vaginal delivery and appropriate fetal outcomes. Motor block due to labor epidural analgesia may be one of the adverse effects that is related directly to the concentration of local anesthetics [Citation20]. This might result in a prolonged second stage of labor and the possibility of increased instrument delivery and/or CS. In our study, the majority of patients reported Modified Bromage score (0, 1) in both groups, only 6 (20%) parturients in bupivacaine group and 8 (26.6%) parturients in tramadol group were developed motor block of grade 2. This can be explained by a higher concentration of bupivacaine (0.25%) that affects A α fiber responsible for motor block and also the epidural test dose of lidocaine that leads to undesirable loss of proprioceptive and motor functions. The incidence of assisted delivery was (10%) in bupivacaine group and (6.7%) in tramadol group while CS incidence was (6.7%) in bupivacaine group and (3.3%) in tramadol group with no significant difference between the two groups.
Our result was in agreement with Merson et al. [Citation21] who used different concentrations of bupivacaine and ropivacaine, they reported increased motor blockade of bupivacaine 0.25% without a significant increase in the rate of instrumental delivery or CS. Also, Rodríguez-Ramón et al. [Citation22] concluded the same results when comparing bupivacaine 0.25% and 0.125%. Moreover, Ahmed et al. [Citation23] reported lower limb motor weakness with bupivacaine 0.25% especially with the lumbar insertion of epidural catheter compared to lower thoracic.
After epidural administration of tramadol, it absorbed through epidural venous plexus and distributed to serum [Citation6]. This is maybe responsible for any systemic effects. Sedation score was significantly higher in tramadol group from 30 to 120 min reading as compared to bupivacaine group. That is maybe of benefit as it decreased maternal anxiety. Gupta et al. [Citation24] reported that 14 patients out of 30 patients had sedation in tramadol group when compared to butorphanol group in epidural analgesia. However, Gupta et al. [Citation25] in their study comparing the effect of adding either dexmedetomidine or tramadol to ropivacaine in caudal anesthesia in pediatric, they reported that the level of sedation in tramadol group was generally less than 2. Also, Results from Swathi et al. [Citation26] reported a lower sedation score. Both of these studies differ in that they use the Ramsay sedation scale.
Nausea and vomiting are unpleasant sensations, and the parturient are more susceptible. The incidence of nausea and vomiting was higher in tramadol group (20%) while only (3.3%) in bupivacaine group, and this is perhaps due to the action of tramadol on the 5-HT receptor. Results for studies conducted by Kundra et al. [Citation27], Türkoğlu et al. [Citation14] and Swathi et al. [Citation26] reported the same, however, Prakash et al. [Citation17] reported a lower incidence. Heart rate and blood pressure were comparable between groups with no significant difference, although, they were significantly lower regarding the baseline that can be explained by autonomic blockade of bupivacaine 0.25%
Regarding neonatal outcomes, the addition of tramadol to bupivacaine did not seem to affect Apgar score or umbilical PH. The results between both groups were comparable, and this was consistent with other studies [Citation10,Citation12,Citation15]. Tramadol has been used for labor analgesia in many studies either through intravenous [Citation28] or intramuscular [Citation29,Citation30] routes, and it provides satisfactory analgesia with safety for both mother and fetus without respiratory depressant effect.
Excellent maternal satisfaction was (63.3%) in tramadol group, however only (30%) in bupivacaine group. Jailety et al. [Citation12] concluded that maternal satisfaction was excellent in (36.67%) of the patients who received epidural tramadol and only in (10%) of the patients who received intravenous tramadol.
Limitation in our study, first we didn’t have a control group who received either no analgesia or I.V analgesia only to compare the effect of epidural on the duration of the first and second stages of labor. Second, we selected bupivacaine 0.25% to be injected in the epidural instead of bupivacaine 0.125% which is commonly used in ambulatory labor analgesia which increased the incidence of motor block. Third, we studied only one dose of tramadol (50 mg) [Citation10], so further studies needed to compare different doses to reach the optimum dose.
7. Conclusions
Tramadol in epidural labor analgesia as an adjuvant to bupivacaine can provide a better quality of analgesia with faster onset, and prolonged duration with no relevant adverse effects.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Yerby M. Pain in childbearing: key issues in management. 6th ed. London: Elsevier Health Sciences; 2000. p. 17–42.
- Chatrath V, Khetarpal R, Sharma S, et al. Fentanyl versus tramadol with levobupivacaine for combined spinal-epidural analgesia in labor. Saudi J Anaesth. 2015;9(3):263–267.
- Ali HM, Wahdan A. Using dexamethasone as an adjuvant to levobupivacaine in epidural anesthesia to change the pain intensity and duration in painless labor. Saudi J Anaesth. 2018;12(2):209–214.
- Hasanein R, El-sayed W, Khalil M. The value of epidural magnesium sulfate as an adjuvant to bupivacaine and fentanyl for labor analgesia. Egypt J Anaesth. 2013;29(3):219–224.
- Lewis KS, Han NH. Tramadol: a new centrally acting analgesic. Am J Health-Syst Pharm. 1997;54(6):643–652.
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923.
- Arcioni R, Della Rocca M, Romanò S, et al. Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT3 spinal receptor involvement in acute pain in humans. Anesth Analg. 2002;94(6):1553–1557.
- Mert T, Gunes Y, Guven M, et al. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439(1–3):77–81.
- Mert T, Gunes Y, Guven M, et al. Differential effects of lidocaine and tramadol on modified nerve impulse by 4-aminopyridine in rats. Pharmacology. 2003;69(2):68–73.
- Imani F, Entezary SR, Alebouyeh MR, et al. The maternal and neonatal effects of adding tramadol to 2% lidocaine in epidural anesthesia for cesarean section. Anesth Pain Med. 2011;1(1):25–29.
- Singh AP, Singh D, Singh Y, et al. Postoperative analgesic efficacy of epidural tramadol as adjutant to ropivacaine in adult upper abdominal surgeries. Anesth Essays Res. 2015;9(3):369–373.
- Jaitley A, Singh S, Srivastava U, et al. A comparison between epidural and IV tramadol for painless labor and effect on perinatal outcome. J Obstet Gynecol India. 2011;61(1):42–47.
- Wangping Z, Ming R. Optimal dose of epidural dexmedetomidine added to ropivacaine for epidural labor analgesia: a pilot study. Evid Based Complement Alternat Med. 2017;2017:7924148.
- Türkoğlu Z, Karacaer F, Biricik E, et al. Comparison of the effects of epidural Levobupivacaine with tramadol or morphine addition on postoperative analgesia following major abdominal surgery. Turk J Anaesthesiol Reanim. 2019;47(4):287–294.
- Fan Y, Ji M, Zang L, et al. Comparison of epidural tramadol-ropivacaine and fentanyl-ropivacaine for labor analgesia: a prospective randomized study. Ups J Med Sci. 2011;116(4):252–257.
- Demiraran Y, Kocaman B, Akman R. A comparison of the postoperative analgesic efficacy of single-dose epidural tramadol versus morphine in children. Br J Anaesth. 2005;95(4):510–513.
- Prakash S, Tyagi R, Gogia A, et al. Efficacy of three doses of tramadol with bupivacaine for caudal analgesia in paediatric inguinal herniotomy. Br J Anaesth. 2006;97(3):385–588.
- Murthy B, Pandya K, Booker P, et al. Pharmacokinetics of tramadol in children after iv or caudal epidural administration. Br J Anaesth. 2000;84(3):346–349.
- Kubota R, Komiyama T, Miwa Y, et al. Pharmacokinetics and postoperative analgesia of epidural tramadol: a prospective, pilot study. Curr Ther Res. 2008;69(1):49–55. .
- Albers LL, Anderson D, Cragin L, et al. The relationship of ambulation in labor to operative delivery. J Nurse Midwifery. 1997;42(1):4–8. .
- Merson N. A comparison of motor block between ropivacaine and bupivacaine for continuous labor epidural analgesia. Aana J. 2001;69(1):54–58.
- Rodríguez-Ramón R, Márquez-González H, Jiménez-Báez MV, et al. Analgesic efficacy of two concentrations of bupivacaine in women in labor: randomized, controlled, triple blind clinical trial. Rev Col Anest. 2015;43(3):179–185.
- Ahmed A, Baig T. Incidence of lower limb motor weakness in patients receiving postoperative epidural analgesia and factors associated with it: an observational study. Saudi J Anaesth. 2016;10(2):149–153.
- Gupta R, Kaur S, Singh S, et al. A comparison of epidural butorphanol and tramadol for postoperative analgesia using CSEA technique. J Anaesthesiol Clin Pharmacol. 2011;27(1):35–38.
- Gupta S, Sharma R. Comparison of analgesic efficacy of caudal dexmedetomidine versus caudal tramadol with ropivacaine in paediatric infraumbilical surgeries: A prospective, randomised, double-blinded clinical study. Indian J Anaesth. 2017;61(6):499–504.
- Swathi N, Ashwini N, Shukla MI. Comparative study of epidural bupivacaine with butorphanol and bupivacaine with tramadol for postoperative pain relief in abdominal surgeries. Anesth Essays Res. 2016;10(3):462–467.
- Kundra TS, Kuthiala G, Shrivastava A, et al. A comparative study on the efficacy of dexmedetomidine and tramadol on post-spinal anesthesia shivering. Saudi J Anaesth. 2017;11(1):2–8.
- Jianjing L, Yun Y. Patient controlled intravenous analgesia with tramadol for labor pain relief. Chin Med J. 2003;116(11):1752–1755.
- Jain S, Arya V, Gopalan S, et al. Analgesic efficacy of intramuscular opioids versus epidural analgesia in labor. Int J Gynecol Obstet. 2003;83(1):19–27.
- Kushtagi P, Surpaneni N. A thought for tramadol hydrochloride as labor analgesic. Anesth Essays Res. 2012;6(2):147–150.