ABSTRACT
Backgroun: Though Emergence Delirium (ED) in children is a short duration, often self-limited, episodes, its prevention is essential. This study aims to evaluate the effect of single-dose dexmedetomidine on the incidence of ED in pediatrics who underwent strabismus surgery under sevoflurane-based anesthesia.
Methods: The study consisted of 70 ASA I–II children who were 3–7 years old and scheduled for strabismus correction surgeries under sevoflurane anesthesia. The clinical trial registry is PACTR201911530033705, registered on the 3rd of November, 2019. Patients were randomly allocated into two groups (dexmedetomidine and control groups). Patients were administered either dexmedetomidine (0.3 µg/kg intravenous in 10 ml normal saline) or 10 ml normal saline, ten minutes before the end of the procedure (before discontinuation of sevoflurane). Our primary aim was to measure the incidence of ED. Watcha and PAED scores were measured simultaneously with FLACC score on arrival to PACU every ten minutes until 30 minutes, then at discharge. Besides, recovery time from anesthesia, length of PACU stay, parent’s satisfaction, and adverse effects were also recorded.
Results: The incidence of ED was 17.6% and 57.6% in groups D and C, respectively. The Watcha and PAED scores were significantly higher in group C during PACU stay while FLACC score was significantly higher in group C on PACU arrival. Despite the significant delay of anesthetic recovery (p < 0.001), PACU stay was significantly shortened (p < 0.001) in group D. Parent’s satisfaction score was significantly better in group D (p = 0.002) without significant differences between both groups regarding adverse effects.
Conclusions: This study demonstrates that a single dose of dexmedetomidine is effective in prophylaxis against ED in children after sevoflurane-based anesthesia. Despite its association with delayed recovery, dexmedetomidine shortened PACU stay and could be used safely in children.
1. Introduction
Postoperative Emergence Delirium (ED) is a frequent and well-known challenge among pediatric cases with a reported incidence up to 80% that causes disappointment and depression for both parents and children and health care providers [Citation1].
Children during ED have the risks of injuring themselves or their caregivers, wound dehiscence, operative site bleeding, removing Intravenous (IV) lines or drains, and increasing pain. This behavior may require more nursing supervision, overloading nursing resources [Citation2].
Although ED episodes are of short duration, their prevention is essential. Children experiencing ED may exhibit new-onset maladaptive behavioral abnormalities in the postoperative period such as enuresis, night-time crying, and separation anxiety, up to 14 days post-surgery [Citation3].
Suggested contributors to ED are related to surgery, e.g., type of surgery, anesthesia (volatile anesthetics), patients’ characters (age and preexisting behavior), and the surrounding environment (parental anxiety). Several prophylactic strategies against ED include behavior management, choice of anesthetic drugs and techniques, perioperative analgesia, and choice of adjuvant medications [Citation4].
Prophylactic drugs include the adjuvant use of propofol, midazolam, fentanyl, ketamine, and α2 adrenoreceptor agonists such as dexmedetomidine. The use of these drugs with their adverse effects should be weighed against the fact that ED is a self-limited disorder [Citation5,Citation6].
The purpose of this trial is to evaluate the effect of single-dose dexmedetomidine on the incidence of ED in preschool pediatric cases that underwent strabismus surgery under sevoflurane-based general anesthesia.
We hypothesized that dexmedetomidine may decrease the incidence of ED in preschool pediatrics who underwent sevoflurane-based anesthesia; it could be safe with no major adverse effects in children.
2. Materials and methods
This prospective double-blinded randomized study was carried out in Tanta University Hospitals from November 2019 to April 2020 after the approval of the institutional ethical committee, clinical trial registry No. (PACTR201911530033705). Written informed consent was acquired from the parents of the participant children.
The study consisted of 70 children of 3–7 years old of American Society of Anesthesiologists’ (ASA) physical status I and II and scheduled for strabismus correction surgeries under sevoflurane anesthesia.
The exclusion criteria were parents’ refusal, children with preexisting abnormal behavior, psychiatric disorders, developmental delay or central nervous system diseases (epilepsy), children who are anxious to a degree that necessitates preoperative sedation, and parents not cooperating with health care providers (abnormal behavior or trouble makers).
The sample size calculation was conducted by G*Power 3.1.9.2 (Universitat Kiel, Germany). It was based on the following parameters: 80% power, 5% confidence limit, and the incidence of ED (our primary outcome) was 26% with dexmedetomidine and 60.8% without dexmedetomidine after sevoflurane-based General Anesthesia (GA) according to a previous study [Citation7]. So, at least 33 patients were needed in each group and two cases were added to overcome the dropout.
Patients were randomly allocated into two groups by a computer-generated random numbers system concealed in sealed opaque envelopes. One anesthetist conducted general anesthesia and another collected the data (without informing the group assignment). Also, parents, patients, and nurses in PACU were blinded to the group assignment.
To control environmental influences, children were asked to bring their favorite toys or books on the day before the surgery. The preoperative evaluation and intraoperative anesthesia were performed by the same anesthetist to be familiar to the children. Also, there were toys and television movies in the preoperative waiting area. On the day of surgery, children stayed in the waiting area with their parents and we shortened the length of their preoperative stay while limiting the number of medical staff who were in contact with the children.
Pediatric cases were anesthetized in an area immediately near to the operating room (OR) in the presence of their parents. Inhalational induction by sevoflurane (5–8%) in oxygen until loss of consciousness was given in all pediatrics, then IV lines were inserted and patients were transferred to OR.
On arrival to OR, standard monitoring (pulse oximetry, noninvasive blood pressure, electrocardiogram, end-tidal gas analysis, and temperature probe (Cardiocaps/5; DatexOhmeda, Helsinki, Finland)) were applied to all participants.
All pediatrics received the same general anesthetic management: inhaling sevoflurane at 3% with a face mask–assisted ventilation. IV fentanyl (1 µg/kg) and atracurium (0.5 mg/kg) were given after the trachea was intubated with initial mechanical ventilation. Ventilation was adjusted to deliver a minute volume of 70–80 ml/kg to maintain normocapnia with a flow rate of 1–2 l/min.
After anesthesia induction, cases received 0.5 mg/kg IV ketorolac for controlling postoperative pain and 0.1 mg/kg IV dexamethasone for controlling postoperative nausea and vomiting.
Maintenance of anesthesia was by sevoflurane in O2 and air (50: 50) and atracurium (0.1 mg/kg) incremental doses as needed.
According to group assignment, dexmedetomidine 0.3 µg/kg (group D) and normal saline (group C) were given ten minutes before the end of the procedure before the discontinuation of sevoflurane.
At the surgery end, paracetamol IV(15 mg/kg) and ondansetron IV (0.15 mg/kg) were administered, neuromuscular block was reversed, and smooth tracheal extubation occurred while the patients were awake.
All spontaneous breathing cases were transferred to the Post-Anesthesia Care Unit (PACU). Every child was received by one of their parents who stayed with them until discharge (preferably the mother). Without any stimulation, the children were allowed up to 30 minutes postoperation to return to full consciousness.
At the PACU, we measured the incidence of ED (primary outcome). We used the Watcha scale [Citation6] (0 = Asleep, 1 = Calm, 2 = Crying but consolable, 3 = Crying but inconsolable, 4 = Agitated and thrashing around) to detect delirium and the pediatric anesthesia emergence delirium (PAED) scale [Citation8] (1- The child makes eye contact with the caregiver, 2- The child’s actions are purposeful, 3- The child is aware of their surroundings, 4- The child is restless, 5- The child is inconsolable) to assess its degree. Patients were evaluated on PACU arrival every ten minutes until 30 minutes elapsed. Then, they were discharged. We considered patients to be suffering from ED when Watcha score was less than two or PAED score less than or equal to ten. If so, patients received 0.03 mg/kg of IV midazolam.
In the PAED scale, items 1, 2, and 3 are scored as follows: 4 = not at all, 3 = just a little, 2 = quite a bit, 1 = very much, 0 = extremely. Items 4 and 5 are scored as follows: 0 = not at all, 1 = just a little, 2 = quite a bit, 3 = very much, 4 = extremely. The degree of ED increased in a directly proportional pattern with the total score.
Our secondary variables were assessment of pain by face, legs, activity, crying, being consolable or not (FLACC) scale [Citation9] on PACU arrival every ten minutes until a total time of 30 minutes lapse. Then, the patients were discharged. Each of the five categories is scored within 0–2 with a total score of 0–10. Patients received IV fentanyl 0.5 µg/kg when the score was less than three.
Also, the quality of PACU stay of the pediatric cases involved in the trial was assessed by parents by (Parents’ Satisfaction Scale): 1 = excellent, 2 = good, 3 = poor, and 4 = bad.)
We recorded recovery time which is defined as the interval from discontinuation of anesthesia and reversal of muscle relaxant until spontaneous eye opening; the length of the PACU stay was recorded. Patients were discharged from the PACU when they were hemodynamically stable, awake, free of pain or agitation, began oral intake, and had no active adverse events. Also, children were not discharged for 45 additional minutes if fentanyl and/or midazolam were administered.
Finally, any adverse postoperative events until discharge from PACU, e.g., bradycardia, hypotension, nausea, and vomiting, laryngospasm, oxygen desaturation, respiratory depression) were recorded.
3. Statistical analysis
Analysis of the data was done by SPSS 25 (IBM Inc., Chicago, IL, USA). The normality of data was verified by the Shapiro-Wilks test and histograms. Quantitative parametric variables were described as mean and Standard Deviation (SD) and were compared by an unpaired Student T-test. Quantitative nonparametric variables were described as median and Interquartile Range (IQR) and were compared by the Mann-Whitney test. Categorical variables were described as frequency and percentage and were analyzed by the Chi-square test. Spearman correlation was done to estimate the degree of correlation between nonparametric variables. P values less than 0.05 were considered statistically significant.
4. Results
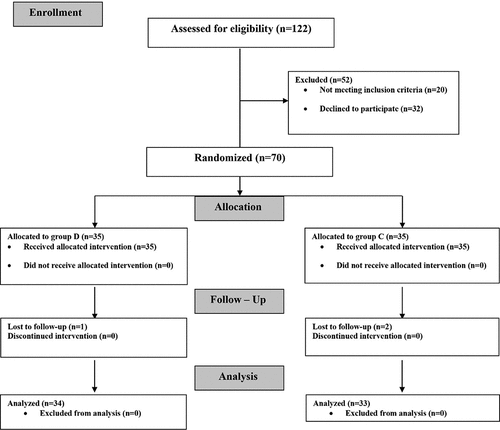
Over six months, 122 patients were assessed for eligibility. 52 patients were excluded (20 patients did not meet the criteria of inclusion and 32 patients refused to participate in the study). Three patients were lost during the follow-up. Lastly, 67 patients were analyzed (34 patients in group D and 33 patients in group C) as shown in the CONSORT flow diagram, .
shows the patients’ characteristics (age, sex, weight, ASA physical status, and history of previous operations) and the duration of surgery and anesthesia. All these parameters were comparable between both groups.
Table 1. Patients demographics (age, weight, sex, American Society of Anesthesiologists (ASA) physical status classification, history of previous operations), duration of anesthesia and surgery, Parent’s satisfaction score, an d adverse effects
The incidence of ED was 17.6% in group D and 57.6% in group C with a significant decrease in group D (p< 0.001). There were significant differences between the two groups in Watcha and PAED scores at all time points of measurement during PACU stay while there were significant differences of FLACC scores only on arrival to PACU (p= 0.005) as shown in .
Table 2. Comparison of Watcha score between the two groups
Table 3. Comparison of Pediatric agitation emergence delirium (PAED) score between the two groups
Table 4. Comparison of face, legs, activity, cry, consolability (FLACC) score between the two groups
There was no significant correlation between the history of previous operations and FLACC scores and both Watcha and PAED scores. Meanwhile, there was a negative correlation between the duration of anesthesia and both Watcha and PAED scores. There was a significant positive strong correlation between PAED and Watcha scores as shown in .
Table 5. The correlation between the face, legs, activity, cry, consolability (FLACC) score, duration of anesthesia, and history of previous operation and both Watcha score and pediatric anesthesia emergence delirium (PAED) score
Whilst there was delayed recovery from anesthesia in group D with a significant difference between the two groups (8.18 ± 1.77 minutes, 5.61 ± 1.54 minutes; p< 0.001), there was a significantly higher median [IQR] of the length of PACU stay in group C than group D (30 [20–40] minutes, 20 [20–40] minutes, respectively; p< 0.001).
Although there was a significant difference (p= 0.002) between the two groups regarding parents’ satisfaction score, there were more adverse effects in group D (three patients with bradycardia and two patients with nausea) than group C (three patients with nausea and four patients with vomiting) with no significant differences between the two groups ().
5. Discussion
ED remains a challenging situation that complicates the recovery of children from GA [Citation10]. Hence, this research is a modest contribution to the ongoing discussion about the effective prophylactic medication against ED in pediatric cases.
The successful prevention of postoperative ED reduces the workload faced by the post-anesthetic care providers, the ED related complications, as well as the stress of the guardians and disturbance of other children in the recovery [Citation11].
In many studies, different medications were used for the prevention of postoperative ED with variables success rates [Citation12]. Further, dexmedetomidine prophylaxis was studied, but its effective dosage and timing are still conflicting [Citation13].
Our trial was designed to assess the efficacy and safety of intraoperative use of single-dose dexmedetomidine in decreasing ED in the preschool pediatrics recovering from sevoflurane-based GA. There was a significant decrease in the incidence and severity of ED and a shorter PACU stay, associated with a significantly higher parent’s satisfaction. Moreover, dexmedetomidine was used safely in children with no significant adverse effects.
It was reported that ED is more frequent among preschool compared with school children [Citation14]. Additionally, ED incidence was significantly more associated with sevoflurane-based anesthesia [Citation15]. This was considered in this work, where preschool children who underwent sevoflurane-based GA were recruited. Furthermore, to obtain valid results, ED in this study was assessed by the PAED score. It is a reliable and valid rating scale that was developed to standardize the evaluation of ED for better reporting and comparison of the results of various studies [Citation16]. Moreover, recording of PAED score was performed by one investigator to overcome errors during the evaluation of its items. Also, the mean duration ED is 5–15 minutes and it is known to mainly happen in the 1st 30 minutes postoperatively [Citation17]. So, the studied patients were followed up at all times where ED occurrence is anticipated.
In our study, the intraoperative use of single-dose dexmedetomidine in strabismus surgery played a role in decreasing the total incidence of post-sevoflurane ED (17.6% vs. 57.6%). Additionally, there was a difference in the severity of ED in favor of dexmedetomidine use where D group showed a significant decrease in PAED score at all the studied time points. Dexmedetomidine is a centrally acting α2 adrenergic agonist. It has sedative, analgesic, and anxiolytic actions that justify the role in providing better sedation and prevention of ED [Citation18].
Dexmedetomidine infusion 0.2 μg·kg−1·h−1 in the perioperative period lowered the incidence of ED to 26% vs. 60.8% in the placebo group [Citation12]. While, intraoperative single IV dexmedetomidine dose decreased the incidence of ED from 37% in the control group that received saline to 17% and 10% in the groups that received 0.15 μg·kg−1 and 0.3 μg·kg−1, respectively, in pediatric cases that recovered from sevoflurane-based anesthesia and caudal block for genital and superficial lower abdominal surgeries [Citation19]. Also, dexmedetomidine administration given five minutes before the end of adenotonsillectomy surgeries decreased the agitation and prolonged the times of extubation and emergence [Citation20]. Preoperative intranasal dexmedetomidine was suggested for the prevention of ED in pediatric patients recovering from GA; however, the scientific evidence on its effectiveness in comparison with oral midazolam is still deficient [Citation21].
A network metaanalysis concluded a better protective effect of dexmedetomidine (the lowest odds ratio of 0.18) in comparison with other anesthetic adjuvants such as fentanyl, sufentanil, ketamine, clonidine, propofol, remifentanil, and midazolam (the greatest odds ratio of 0.46) into sevoflurane anesthesia [Citation22]. Currently, a systematic review and metaanalysis support the superiority of dexmedetomidine over placebo, midazolam, or opioid in significantly decreasing the incidence of ED in pediatrics after anesthesia for various types of surgeries [Citation23]. The same evidence was also confirmed in a network analysis of pediatric anesthesia for adenotonsillectomy operation [Citation24].
In our trial, dexmedetomidine was given as a single IV dose (0.3 μg/kg). Various doses of dexmedetomidine were shown to assist in the prevention of EA after sevoflurane-based anesthesia in pediatrics [Citation25]. Supporting evidence suggested by Zhang Y-Z et al [Citation26] that a dose of 0.3 μg/kg dexmedetomidine prevented 95% of sevoflurane-associated ED during pediatric tonsillectomy and adenoidectomy.
Postoperative pain can cause agitation, and it is considered a confounder in all trials investigating ED [Citation27]. In this study, we chose the strabismus correction surgery where the pain is mild and easily controllable. The pain was controlled in both groups via administration of IV ketorolac after the induction of anesthesia and supplementation with IV paracetamol. These drugs were sufficient to provide analgesia at surgeries end [Citation28]. Assessment of pain by FLACC score showed insignificant differences between the studied groups except on arrival to the PACU. Moreover, there was an insignificant correlation between the FLACC score and both Watcha and PAED scores. Therefore, in our cases, postoperative pain is not the cause of developing ED mostly.
In addition to pain, duration, and type of surgeries, the newer inhalational anesthetic drugs were accompanied by ED development [Citation29]. In the present trial, these factors were similar in the two groups, and the trial involved only one surgical procedure with standardized anesthetic management.
The recovery time from anesthesia was significantly longer in patients administered dexmedetomidine. This agrees with previous studies that showed a negative correlation between the time to awakening and ED scores [Citation28].
Dexmedetomidine administration had also a positive impact on the parents. There was a significant difference in the parent’s satisfaction scores between both groups. Similar findings were reported when dexmedetomidine infusion was used before operations on children undergoing hernia repair [Citation30]. Currently, usage of dexmedetomidine in the perioperative period has shown to significantly improve outcomes in the postoperative period especially when used in the enhanced recovery after surgery protocols were accompanied with less time of discharge from PACU and better patient’s satisfaction [Citation31].
Dexmedetomidine causes bradycardia and hypotension which is dose dependent. Using a dose of 0.3–0.5 µg/kg bolus was reported to be safe with no hemodynamic effects [Citation19,Citation20,Citation32]. Similarly, adverse effects were similar in the two groups without significant hemodynamic effects of dexmedetomidine in our study.
The limitation of this study was the lack of long-term follow-up of children with delirium. Further evaluation of different doses and timing of dexmedetomidine with other adjuvants and different types of surgeries are recommended for better clinical practice.
6. Conclusions
Using single-dose dexmedetomidine before the termination of sevoflurane-based anesthesia lowered the incidence and severity of ED and the time of discharge in preschool pediatric cases that underwent strabismus surgery. Additionally, its use was safe with no reported significant adverse effects.
Data availability statement:
Raw data were generated at Tanta University Hospitals. Derived data supporting the findings of this study are available from the corresponding author M R E upon reasonable request.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Bong C, Lim E, Allen J, et al. A comparison of single‐dose dexmedetomidine or propofol on the incidence of emergence delirium in children undergoing general anaesthesia for magnetic resonance imaging. Anaesthesia. 2015;70(4):393–399.
- Mountain BW, Smithson L, Cramolini M, et al. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. Aana J. 2011;79(3):219–224. PMID: 21751690.
- Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesthesia Analg. 2004;99(6):1648–1654.
- Mason K. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. BJA. 2017;118(3):335–343.
- Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane-and desflurane-related emergence agitation in children: a meta-analysis of published studies. BJA. 2010;104(2):216–223.
- Reduque LL, Verghese ST. Paediatric emergence delirium. Continuing Educ Anaesthesia, Crit Care Pain. 2013;13(2):39–41.
- Shukry M, Clyde MC, Kalarickal PL, et al. Does dexmedetomidine prevent emergence delirium in children after sevoflurane‐based general anesthesia? Pediatr Anesthesia. 2005;15(12):1098–1104.
- Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiol J Am Soc Anesthesiologists. 2004;100(5):1138–1145.
- Nilsson S, Finnström B, Kokinsky E. The FLACC behavioral scale for procedural pain assessment in children aged 5–16 years. Pediatr Anesthesia. 2008;18(8):767–774.
- Yudkowitz FS, Davis RL. Pediatric Post-anesthesia Care Unit Challenges Update. Curr Anesthesiol Rep. 2019;9(2):92–9. DOI:10.1007/s40140-019-00317-0
- Moore AD, Anghelescu DL. Emergence Delirium in Pediatric Anesthesia. Paediatr Drugs. 2017;19(1):11–20.
- Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatric anaesthesia.. 2005;15(12):1098–104. DOI:10.1111/j.1460-9592.2005.01660.x
- Santana L, Mills K. Retrospective study of intranasal dexmedetomidine as a prophylactic against emergence delirium in pediatric patients undergoing ear tube surgery. Int J Pediatr Otorhinolaryngol. 2017;100:39–43.
- Aono J, Ueda W, Mamiya K, et al. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997;87(6):1298–1300.
- Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109(2):225–232.
- Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–45. DOI:10.1097/00000542-200405000-00015
- Singh R, Kharbanda M, Sood N, et al. Comparative evaluation of incidence of emergence agitation and post-operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anaesthesia. Indian J Anaesth. 2012;56(2):156–161.
- Kim NY, Kim SY, Yoon HJ, et al. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J. 2014;55(1):209–215.
- Ibacache ME, Muñoz HR, Brandes V, et al. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesthesia Analg. 2004;98(1):60–63.
- Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15(9):762–766.
- FitzSimons J, Bonanno LS, Pierce S, et al. Effectiveness of preoperative intranasal dexmedetomidine, compared with oral midazolam, for the prevention of emergence delirium in the pediatric patient undergoing general anesthesia: a systematic review. JBI Database System Rev Implement Rep. 2017;15(7):1934–1951.
- Wang X, Deng Q, Liu B, et al. Preventing Emergence Agitation Using Ancillary Drugs with Sevoflurane for Pediatric Anesthesia: A Network Meta-Analysis. Mol Neurobiol. 2017;54(9):7312–7326.
- Rao Y, Zeng R, Jiang X, et al. The Effect of Dexmedetomidine on Emergence Agitation or Delirium in Children After Anesthesia-A Systematic Review and Meta-Analysis of Clinical Studies. Front Pediatr. 2020;8:329.
- Jiao H, Wang H, Jiang Z, et al. Comparative efficacy of ancillary drugs in sevoflurane-related emergence agitation after paediatric adenotonsillectomy: A Bayesian network meta-analysis. J Clin Pharm Ther. 2020;45:1039–1049.
- Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2014;58(6):642–50. DOI:10.1111/aas.12292
- Zhang Y-Z, Wang X, Wu J-M, et al. Optimal Dexmedetomidine Dose to Prevent Emergence Agitation Under Sevoflurane and Remifentanil Anesthesia During Pediatric Tonsillectomy and Adenoidectomy. Front Pharmacol. 2019;10:1091.
- Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesthesia Analg. 2007;104(1):84–91.
- Mehrotra S. Postoperative anaesthetic concerns in children: postoperative pain, emergence delirium and postoperative nausea and vomiting. Indian J Anaesth. 2019;63(9):763–770.
- Zhang C, Hu J, Liu X, et al. Effects of intravenous dexmedetomidine on emergence agitation in children under sevoflurane anesthesia: a meta-analysis of randomized controlled trials. Plos One. 2014;9(6):e99718.
- Du Z, Zhang X-Y, Qu S-Q, et al. The comparison of dexmedetomidine and midazolam premedication on postoperative anxie//g;ty in children for hernia repair surgery: a randomized controlled trial. Paediatr Anaesth. 2019;29(8):843–849.
- Kaye AD, Chernobylsky DJ, Thakur P, et al. Dexmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. 2020;24(5):21.
- Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: A comparison of dexmedetomidine and propofol. Saudi J Anaesth. 2013;7(3):296–300.