ABSTRACT
Objectives: Evaluation of urinary neutrophil gelatinase-associated lipocalin (UNGAL) and kidney injury molecule 1 (UKIM-1) as early predictors of development of acute kidney injury (AKI) in patients admitted to surgical ICU.
Patients & Methods: 188 ICU patients were clinically evaluated and gave blood samples for estimation of at admission (T0) serum creatinine (SCr) and urine samples for estimation of urinary creatinine (UCr), UNGAL and UKIM-1 and their values were adjusted to UCr level. At 48-hr after admission SCr levels were re-estimated and AKI was diagnosed and staged according to the Acute Kidney Injury Network criteria. Study outcomes included the ability of estimated markers for prediction of possible AKI development.
Results: At 48-hr after ICU admission, 73 patients (38.8%) developed AKI; 42, 21 and 10 mild, moderate and severe AKI, respectively. At T0, UNGAL and UKIM1, and their UCr ratios showed progressive increase with increased AKI grade. Calculated ∆SCr was positively correlated with clinical data and estimated levels of urinary markers. Regression analysis defined elevated BMI, TISS-28 score, UNGAL and UKIM-1 as early significant predictors for AKI. ROC curve analysis defined UNGAL and UKIM-1 and their ratios to UCr as the significant predictors for AKI development.
Conclusion: Development of AKI in ICU patients was related to severity of surgical trauma. High UNGAL and UKIM-1 at time of ICU admission can predict upcoming AKI and its AUC after adjustment to UCr levels had superior predictive value for AKI. A high uKIM-1/UCr ratio can be considered the most significant early predictor for AKI.
1. Introduction
Acute kidney injury (AKI) is the most common cause of organ failure in multiple organ dysfunction syndrome [Citation1] and despite advances in medical technologies and interventions, the incidence of AKI continues to rise [Citation2]. AKI is a life-threatening illness, which is associated with a high in-hospital mortality rate ranging between 20% and 50% according to the underlying conditions [Citation3].
Acute kidney injury is a frequent event among critically ill patients and is associated with increased morbidity and mortality [Citation4] especially for septic patients [Citation5], after major abdominal surgery [Citation6], open thoracic aortic surgery [Citation7], cardiac surgeries especially in patients with congenital heart disease [Citation8].
Neutrophil gelatinase-associated lipocalin (NGAL) is a member of the lipocalin protein family which is characterized by similar structure, but has diverse functions [Citation9]. Human NGAL is a 25-kDa secretory glycoprotein consists of 178-amino acid and exists in three molecular forms, including a 25-kDa monomer, a 45-kDa homodimer, and a 135-kDa disulfide-linked heterodimer bound to latent matrix metalloproteinase-9 [Citation10]. NGAL has dual effects as a siderophore: iron-binding protein and as a growth factor, and is markedly induced in kidney tubule cells, massively upregulated after renal tubular injury, so it may participate in limiting kidney damage [Citation11]. NGAL was detected in plasma and urine in animal models of ischemic, septic and nephrotoxic AKI [Citation12] and can differentially suggest the site of kidney injury where detection of the monomeric and dimeric forms of uNGAL suggest upper and lower urinary tract origins of disease, respectively [Citation13].
Kidney injury molecule-1(KIM-1) is a type I membrane protein consisting of extracellular and cytoplasmic portions [Citation14]. KIM1 is an epithelial phosphatidylserine receptor expressed transiently after acute kidney injury and chronically in fibrotic renal disease, promotes kidney fibrosis [Citation15]. KIM1 is the most highly upregulated protein in the renal proximal tubule after injury and its ectodomain which is heavily glycosylated and stable is released into the lumen of the tubule and appears in the urine after injury [Citation16]. KIM-1 acts as a double-edged sword that is implicated in both injury and healing of the kidney [Citation17] as it is markedly upregulated in injured and regenerating renal proximal tubular epithelial cells following ischemic or toxic insults [Citation18].
2. Hypothesis
Early prediction of AKI can help to improve patients’ outcome through early institution of the appropriate intervention [Citation5], thus the current study hypothesized that urine analysis for certain markers may provide an early knowledge about the possibility of oncoming kidney affection secondary to organ and tissue trauma affecting patients admitted to surgical ICU.
3. Objectives
The current study tried to evaluate the value of urinary markers as early predictors of possible development of AKI in patients admitted to surgical ICU.
4. Setting
Anesthesia & ICU Department, Security Forces Hospital, Riyadh, KSA
5. Design
Prospective observational three-year study
6. Patients & methods
All patients who were admitted to surgical ICU since Nov 2016 were eligible for evaluation for inclusion and exclusion criteria. Patients with manifest kidney disease, who had renal surgery, maintained on renal replacement therapy or had diabetic nephropathy were excluded from the study. Also, patients who had liver disease, endocrinopathies, morbid obesity that was defined as body mass index (BMI) >35 kg/m2 [Citation19] were also excluded from the study.
All patients were clinically evaluated for demographic and clinical data. Severity of injury and number of surgical interventions were evaluated using the simplified Therapeutic Intervention Scoring System (TISS-28) which evaluates the extent of injury of the body as divided into six major areas and each surgical intervention is assigned 1 to 4 points and the points are summed to obtain an overall score [Citation20]. The extent of impact of associated diseases on patients’ physiological and body organs’ functions was evaluated using the Acute Physiology and Chronic Health Evaluation (APACHE II) [Citation21] and sequential organ failure assessment (SOFA) [Citation22]. Higher scores indicate more severe illness and for the TISS-28 score, each therapeutic intervention is assigned 1 to 4 points, and the points are summed daily to obtain the overall score and higher score indicates a higher number of therapeutic interventions.
7. Diagnosis of AKI
Development of AKI within the first 48 hours after ICU admission and its staging was defined according to the Acute Kidney Injury Network criteria. Each stage was defined by the extent of change in serum creatinine level (∆SCr) as follows: Mild if Scr was increased by ≥0.3 mg/dL or ∆SCr was ≥1.5-2-fold from baseline, Moderate if ∆SCr was >2-3-fold from baseline and Severe if ∆SCr was increased by >threefold from baseline [Citation23].
8. Laboratory investigations
Blood samples were drawn under complete aseptic conditions at time of ICU admission (T0) and 48 hours thereafter (T48). Blood samples were put in clean dry tube and allowed to clot to separate serum for estimation of serum creatinine (SCr) using an enzyme linked immunosorbent assay (ELISA) kit (catalog no. ab65340; Abcam, Cambridge, USA) [Citation24].
Urine sample
Sampling: Catheter tubing with sampling port was inserted in the catheter orifice, urine was assured to flow in the tube, a clamp was applied about 6 cm below the level of the sampling port to allow urine to collect above the clamp so that a sample can be obtained. Sampling port was swabbed with alcohol-impregnated swab and allowed to dry, tubing was holding below the level of the sampling port, a syringe tip was inserted into the sampling port and 10-ml of urine was aspirated and then the clamp was released to allow urine to drain freely.
Processing & preservation: Urine was put into a sterile tube, centrifuged at 2000 g for 20 minutes, and the supernatant was frozen at – 80°C till being assayed within 4 months using ELISA kits according to the manufacturer’s instructions and were read using a 96 well microplate ELISA reader (Dynatech MR 7000)
Investigations
Spot Urinary creatinine (UCr) was estimated using the same procedure applied for SCr estimation [Citation24].
Urinary human neutrophil gelatinase-associated lipocalin (U-NGAL) was estimated using an ELISA kit (catalog no. ab215541; Abcam, Cambridge, USA) by sandwich enzyme-linked immunosorbent assay technology according to manufacture instructions. The limit of NGAL detection with this research assay is 46.9–3000 pg/ml [Citation25,Citation26].
Urinary human kidney injury molecule 1 (U-KIM1) was estimated using an ELISA kit (catalog no. ab235081; Abcam, Cambridge, USA) by sandwich enzyme-linked immunosorbent assay technology according to manufacture instructions. The limit of KIM1 detection with this research assay is 7.81–500 pg/ml [Citation27].
9. Study outcomes
The primary outcome is the ability of estimated urinary markers and their ratio in relation to urinary creatinine to be used as early predictors for patients susceptible to develop AKI. Early predictor was defined as a marker that changed early well before serum creatinine rise so as to allow prevention or treatment at very early stage of AKI [Citation28,Citation29] (Han et al., 2008; Liangos et al., 2009). The formulas used to calculate urinary KIM-1 and NGAL to urinary creatinine ratios were uKIM-1/Cre = KIM-1/Creatinine (pg/g) and uNGAL/Cre = NGAL/Creatinine (ng/g) [Citation30] (Żyłka et al., 2018).
The secondary outcomes include:
The number of patients who developed ∆SCr diagnostic of AKI and the extent of AKI severity.
The results of statistical analyses for the best predictor for upcoming AKI.
10. Statistical analysis
Obtained data were presented as mean±SD, numbers and percentages. Results were analyzed using paired t-test, One-way ANOVA Test and Chi-square test (X2 test). Possible relationships were investigated using Pearson’s correlation analysis. Regression analysis (Stepwise method) was used for stratification of studied parameters as specific predictors. Sensitivity & specificity of studied parameters as predictors for patients’ outcome were evaluated using the receiver operating characteristic (ROC) curve analysis judged by the area under the curve (AUC) that was compared versus null hypothesis that AUC = 0.5. Statistical analysis was conducted using the IBM SPSS (Version 23, 2015; IBM, South Wacker Drive, Chicago, USA) for Windows statistical package. P-value < 0.05 was considered statistically significant.
11. Results
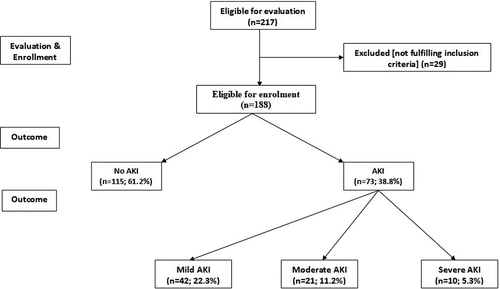
Two hundred and seventeen patients were admitted to surgical ICU for varied indications, 29 patients were excluded from the study for not fulfilling inclusion criteria, while 188 patients were included in the study. At time of admission, estimated SCr showed non-significant difference between patients who developed AKI (AKI group) and those who were free of AKI at 48-hr after admission (No AKI group). At 48-hr after ICU admission, all patients had elevated SCr with significantly higher T48-SCr levels in comparison to T0-SCr levels and 73 patients (38.8%) developed T48-SCr levels diagnostic for AKI. Forty-two patients (22.3%) had mild, 21 patients (11.2%) had moderate and 10 patients (5.3%) had severe AKI, while 115 patients (61.2%) had T48-SCr levels that were not diagnostic for AKI (, ).
Table 1. Patients’ distribution according to the development and severity of AKI as judged by change in serum creatinine
Demographic and clinical data of studied patients categorized according to development and severity of AKI showed non-significant differences apart from TISS-28 scores that were significantly lower in patients with no AKI in comparison to patients with AKI as shown in .
Table 2. At admission data of patients categorized according to development and severity of AKI
Mean levels of urinary creatinine (UCr) showed gradual decrease through AKI grades with significant difference versus no AKI. On contrary, urinary NGAL and KIM1 levels estimated at time of ICU admission showed progressive increase with increased AKI grade with significantly higher levels in AKI patients in comparison to no AKI. Adjustment of uNGAL and uKIM1 in relation to UCr levels assured the progressive increase with the increased AKI severity and showed significant differences in comparison to levels estimated in patients of No AKI group ().
Table 3. At admission laboratory findings of studied patients categorized according to development and severity of AKI
There was positive significant correlation between ∆SCr and patients’ age, BMI, APACHE II, SOFA and TISS-28 scores and with estimated levels of urinary markers as well as their adjusted ratio to UCr level while ∆SCr showed positive non-significant correlation with male gender. Moreover, estimated levels of urinary markers showed positive significant correlation with each other and with TISS-28 scores. Estimated UNGAL levels showed positive significant correlation with APACHE scores, while estimated UKIM-1 levels were positively correlated with SOFA scores ().
Table 4. Pearson’s correlation between development and severity of AKI as judged by ∆SCr and patients’ demographic, clinical and lab data
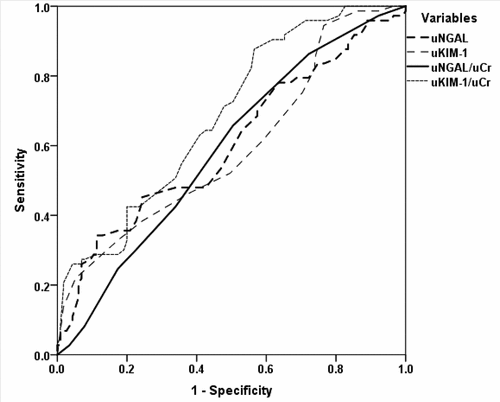
Regression analysis defined elevated BMI (β = 0.111, p = 0.001), TISS-28 score (β = 0.099, p = 0.003), urinary NGAL (β = 0.615, p < 0.001) and KIM-1 (β = 0.499, p < 0.001) levels estimated at time of admission as the early significant predictors for upcoming AKI. ROC curve analysis defined urinary levels of NGAL and KIM-1 and their ratios to UCr as the significant predictors for development of AKI (, ).
Table 5. Area under the ROC curves for urinary NGAL and KIM-1 and their ratio to UCr for early prediction of AKI development within 48-hr after ICU admission
12. Discussion
The current study detected a 38.8% incidence of AKI among patients admitted to surgical ICU, irrespective of the indication of ICU admission. The reported rate coincided with that reported by early studies which evaluated the incidence of AKI in surgical ICU and reported incidence rates of 44% among general population of patients admitted to surgical ICU [Citation31], 14% in patients with sepsis, shock, major surgery, and trauma [Citation32] and 13.4% among pediatrics admitted to trauma surgical ICU [Citation33]. Moreover, the reported figure also coincided with recent studies that detected AKI incidence of 19.4% at 48-hr after abdominal surgery [Citation6], 34.8% after isolated coronary revascularization [Citation34], 44% in ICU septic patients [Citation35] and 46.9% after robot-assisted laparoscopic radical prostatectomy [Citation36]. However, these discrepant figures for incidence could be attributed to the differences in the studied population.
Serum creatinine levels estimated at 48-hr after ICU admission were significantly higher in all patients’ samples in comparison to levels estimated at time of admission; a finding illustrative of the surgery &/or anesthesia-induced kidney insult, irrespective of the progress to AKI or not. In support of this finding, the incidence of AKI showed positive significant correlation with TISS-28 score which illustrates the multiplicity and severity of surgical interferences and with APACHE II score which manifest the impact of disease and surgery on patients’ physiology. In line with these findings, Zorrilla-Vaca et al. [Citation37] detected that ASA III–IV and open surgical approach are independent risk factors for AKI, and Xu et al. [Citation38] found radical not partial nephrectomy was an independent risk factor for AKI. Moreover, AKI incidence showed positive significant correlation with patients’ age and BMI. Similarly, multiple recent studies detected that age, male gender, BMI and body surface area are independent risk factors for postoperative AKI [Citation37,Citation39–41].
The extent of increased 48-SCr level (∆SCr) was significantly higher in AKI than in No AKI patients, but it has no predictive ability for upcoming AKI. Similarly, UCr level showed progressive decrease with AKI severity and can be used as severity measure but also has no predictive value. On contrary, urinary levels of NGAL and KIM1 estimated at time of ICU admission showed significant difference between AKI and No AKI patients with positive significant correlations with AKI severity as manifested by increased ∆SCr and with increased APACHE and TISS-28 scores. ROC curve analysis of patients’ variables defined both UNGAL and UKM1 as early predictors to discriminate patients who are liable to develop AKI prior to the increase of serum creatinine, and on adjustment in relation to UCr levels, ROC curve defined UKIM1/UCr ratio as the significant predictor for upcoming AKI with more significant difference in AUC versus the null hypothesis than AUC for UNGAL/UCr.
In line with these findings, NGAL Meta-analysis Investigator Group [Citation42] concluded that urinary and plasma NGAL concentrations can identify patients at high risk for AKI in clinical research and practice. Also, Törnblom et al. [Citation35] using ROC curve analysis found the corresponding AUCs for uNGAL were 0.690, 0.728, 0.769, and 0.600 for discrimination of AKI, severe AKI, renal replacement therapy and mortality of patients admitted to ICU with sepsis. Moreover, Rashidi et al. [Citation43] found NGAL was analytically superior to traditional AKI biomarkers such as creatinine and urine output for early prediction of AKI in sepsis and trauma patients and Fatani et al. [Citation44] found urinary KIM-1 levels were significantly associated with sepsis severity and AKI. Furthermore, Darawshi et al. [Citation45] found that increased urinary and serum NGAL in ICU patients who developed AKI while on inhibitors of sodium-glucose co-transporter-2 indicates acute tubular injury that principally affecting distal tubular segments. Similarly,
Unfortunately, few studies had evaluated the diagnostic ability of serum or urinary KIM1 in ICU setting, however, the obtained results go in hand with studies that evaluated KIM1 as biomarker for kidney affection in other settings as with Quang et al. [Citation46] who found urinary KIM-1 and NGAL are independent markers for early diagnosis of diabetic nephropathy with higher AUC for KIM1 as sensitive early marker and Khalil et al. [Citation47] who found high preoperative KIM1 level in morbidly obese patients undergoing sleeve gastrectomy correlate with serum creatinine and can discriminate patients with microalbuminuria and decreased postoperative KIM1 levels indicated kidney recovery.
13. Conclusion
Development of AKI in patients admitted to surgical ICU is a frequent complication with an incidence of 38.8% that was related to patients’ demographic data and severity of surgical trauma. High urinary levels of NGAL and KIM-1 at time of ICU admission can predict upcoming AKI and its AUC after adjustment to urinary creatinine levels had superior predictive value for AKI than other variables. High UKIM-1/UCr ratio can be considered the most significant early predictor for AKI.
14. Limitation
The study was limited by being a single-center study and by the use of only two markers for evaluation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wang H, Kang X, Shi Y, et al. SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail. 2020 Nov;42(1):638–645. .
- Harris P, Umberger R. Long-term renal outcomes in adults with sepsis-induced acute kidney injury: a systematic review. Dimens Crit Care Nurs. 2020;39(5):259–268.
- Oh D. A long journey for acute kidney injury biomarkers. Ren Fail. 2020;42(1):154–165.
- Husain-Syed F, Rosner M, Ronco C. Distant organ dysfunction in acute kidney injury. Acta Physiol (Oxf). 2020 Feb;228(2):e13357. .
- Deng F, Peng M, Li J, et al. Nomogram to predict the risk of septic acute kidney injury in the first 24 h of admission: an analysis of intensive care unit data. Ren Fail. 2020;42(1):428–436.
- Inácio R, Gameiro J, Amaro S, et al. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis. J Bras Nefrol. 2020 Aug 10;S0101–28002020005026202. DOI:10.1590/2175-8239-JBN-2019-0244.
- Ma X, Li J, Yun Y, et al. Risk factors analysis of acute kidney injury following open thoracic aortic surgery in the patients with or without acute aortic syndrome: a retrospective study. J Cardiothorac Surg. 2020 Aug 7;15(1):213. .
- Li D, Niu Z, Huang Q, et al. A meta-analysis of the incidence rate of postoperative acute kidney injury in patients with congenital heart disease. BMC Nephrol. 2020 Aug 17;21(1):350. .
- Lippi G, Meschi T, Nouvenne A, et al. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem. 2014;64:179–219.
- Bauvois B, Susin S. Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: saint or sinner? Cancers (Basel). 2018;10(9):336.
- Schmidt-Ott KM, Mori K, Li J, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007 Feb;18(2):407–413. .
- Kaucsár T, Godó M, Révész C, et al. Urine/ plasma neutrophil gelatinase associated lipocalin ratio is a sensitive and specific marker of subclinical acute kidney injury in mice. PLoS One. 2016;11(1):e0148043.
- Wu P, Hsu W, Tsai P, et al. Identification of urine neutrophil gelatinase-associated lipocalin molecular forms and their association with different urinary diseases in cats. BMC Vet Res. 2019 Aug 27;15(1):306. .
- Yin C, Wang N. Kidney injury molecule-1 in kidney disease. Ren Fail. 2016;38(10):1567–1573.
- Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013 Sep;123(9):4023–4035. .
- Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293–299.
- Lim A, Tang S, Lai K, et al. Kidney injury molecule-1: more than just an injury marker of tubular epithelial cells? J Cell Physiol. 2013 May;228(5):917–924. .
- Zhang Z, Cai CX. Kidney injury molecule-1 (KIM-1) mediates renal epithelial cell repair via ERK MAPK signaling pathway. Mol Cell Biochem. 2016;416(1–2):109–116.
- WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: World Health Organization; 1995.
- Miranda RR, de Rijk A, Schaufeli W. Simplified therapeutic intervention scoring system: the TISS-28 items — results from a multicenter study. Crit Care Med. 1996;24:64–73.
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
- Heinegard D, Tiderstrom G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973;43:305.
- Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863.
- Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095.
- Bonventre JV. Kidney injury molecule-1 (KIM1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24(11):3265–3268.
- Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869.
- Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–431.
- Żyłka A, Dumnicka P, Kuśnierz-Cabala B, et al. Markers of glomerular and tubular damage in the early stage of kidney disease in type 2 diabetic patients. Mediators Inflamm. 2018 Aug 9;2018:7659243. .
- Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–451.
- Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25.
- Talving P, Karamanos E, Skiada D, et al. Demetriades D: relationship of creatine kinase elevation and acute kidney injury in pediatric trauma patients. J Trauma Acute Care Surg. 2013;74(3):912–916.
- Nagpal A, Cowan A, Li L, et al. Starch or saline after cardiac surgery: a double-blinded randomized controlled trial. Can J Kidney Health Dis. 2020 Jul 28;7:2054358120940434. .
- Törnblom S, Nisula S, Petäjä L, et al. FINNAKI study group: urine NGAL as a biomarker for septic AKI: a critical appraisal of clinical utility-data from the observational FINNAKI study. Ann Intensive Care. 2020 Apr 28;10(1):51. .
- Sato H, Narita S, Saito M, et al. Acute kidney injury and its impact on renal prognosis after robot-assisted laparoscopic radical prostatectomy. Int J Med Robot. 2020 May 3;e2117. DOI:10.1002/rcs.2117.
- Zorrilla-Vaca A, Mena G, Ripolles-Melchor J, et al. Risk factors for acute kidney injury in an enhanced recovery pathway for colorectal surgery. Surg Today. 2020 Aug 12. DOI:10.1007/s00595-020-02107-2.
- Xu L, Li C, Zhao L, et al. Acute kidney injury after nephrectomy: a new nomogram to predict postoperative renal function. BMC Nephrol. 2020 May 14;21(1):181. .
- Li X, Zhang S, Xiao F. Influence of chronic kidney disease on early clinical outcomes after off-pump coronary artery bypass grafting. J Cardiothorac Surg. 2020 Jul 29;15(1):199. .
- Murphy C, Dunne T, Elliott J, et al. Acute kidney injury after esophageal cancer surgery: incidence, risk factors, and impact on oncologic outcomes. Ann Surg. 2020 Jul 24. DOI:10.1097/SLA.0000000000004146.
- Fu H, Chou N, Chen Y, et al. Risk factor for acute kidney injury in patients with chronic kidney disease receiving valve surgery with cardiopulmonary bypass. Asian J Surg. 2020 Jul 2;S1015-9584(20)30154–8. DOI:10.1016/j.asjsur.2020.05.024.
- Albert C, Zapf A, Haase M, et al. Neutrophil gelatinase-associated lipocalin measured on clinical laboratory platforms for the prediction of acute kidney injury and the associated need for dialysis therapy: a systematic review and meta-analysis. Am J Kidney Dis. 2020 Jul 14; S0272-6386(20)30780-0. DOI:10.1053/j.ajkd.2020.05.015.
- Rashidi H, Sen S, Palmieri T, et al. Early recognition of burn- and trauma-related acute kidney injury: a pilot comparison of machine learning techniques. Sci Rep. 2020 Jan 14;10(1):205. .
- Fatani S, Alkhatib K, Badr H, et al. Association of TNF-α-308 (G >A) (rs1800629) gene polymorphism with adverse outcomes of sepsis in critically ill patients. DNA Cell Biol. 2020 Jul 17. DOI:10.1089/dna.2020.5468.
- Darawshi S, Yaseen H, Gorelik Y, et al. Biomarker evidence for distal tubular damage but cortical sparing in hospitalized diabetic patients with acute kidney injury (AKI) while on SGLT2 inhibitors. Ren Fail. 2020;42(1):836–844.
- Quang TH, Nguyet M, Thao D, et al. Evaluation of urinary neutrophil gelatinase associated lipocalin and kidney injury molecule-1 as diagnostic markers for early nephropathy in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020 Jun 24;13:2199–2207. .
- Khalil R, Elghadban H, Abdelsalam M, et al. Kidney injury molecule-1: A potential marker of renal recovery after laparoscopic sleeve gastrectomy. Kidney Res Clin Pract. 2020 Jun 30;39(2):162–171.