ABSTRACT
Background: To investigate reliability of Bio-impedance Cardiometer parameters in comparison with basic non-invasive parameters for early detection of haemodynamic changes during percutaneous nephrolithotomy (PNL) under spinal anesthesia.
Methods: Forty patients were enrolled. Basal and 10-min interval parameters were recorded, included systolic blood pressure, mean arterial blood pressure, heart rate, O2 saturation and bio-impedance parameters. According to blood loss as measured by haemoglobin HB level, patients were divided into two groups; significant blood loss group > 20% from basal HB were compared with non-significant loss group.
Results: Significant blood loss included 14 (35%) patients while other group included 26 (65%) patients. No difference was detected in demographics, basic non-invasive and bio-impedance cardiometer parameters either at basal values or at minute-120. No difference was detected in recorded data per 10-min interval in both groups throughout procedure except for heart rate and systolic time ratio (STR). STR changes were noticed 20 minutes prior to HR changes (at 80 minutes and 100 min, respectively). Mean±SD of STR at minute-80 was higher in group of significant blood loss (0.44 ± 0.07 Vs 0.38 ± 0.05, P 0.018). HR started to be significantly higher at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05).
Conclusions: STR is a more reliable tool than MBP and HR for early detection of haemodynamic collapse in case of non-measurable bleeding during PNL. STR should be integrated as a routine monitoring tool during PNL.
1. Introduction
Early detection of any haemodynamic changes during surgery is a principal responsibility of anesthesiologist. In certain surgical maneuvers, such haemodynamic changes could be attributed to pharmacodynamics of given drugs, active blood loss, septicemia, changes in patient position or summation of all these risk factors. Percutaneous nephrolithotomy (PNL) is considered to be typical example of such case. Vast majority of PNL procedures are done under potential hazards of high-level spinal anesthesia and hidden blood loss. Intra-operative hypotension reaches up to 26.6% [Citation1], it has been established that intraoperative hypotension is an independent risk factor for acute kidney injury (AKI) after PNL [Citation1]. Technically, burden and magnitude of blood loss is difficult to be noticed by urologist during performance of this particular procedure. The delusional effect of irrigation fluid used for washing purpose sometimes masks the real estimation of blood loss. In this situation, surgeon would be in need to hear a statement “stop procedure at this point”. Moreover, PNL is classically done in prone position. Turning the patient from lithotomy to prone position leads to decrease in cardiac output due to reduced venous return and direct effects on atrial filling together with reduced left ventricular compliance secondary to intrathoracic pressure elevation. Inferior vena cava compression, exacerbated by abdominal compression, leading to decreased cardiac output, increased bleeding, venous stasis and the consequent potential thrombotic complications [Citation2]. Therefore, there is true need for accurate methods for early detection of haemodynamic changes during PNL.
Invasive blood pressure (IBP) remains the gold standard in critically ill patients [Citation3]. Swan Ganz catheter and Trans-pulmonary thermo-dilution devices [Citation4,Citation5] are recommended in patients with refractory or severe shock. However, its invasive nature, high cost and technical difficulty are major limitations for routine utilization during PNL [Citation6]. On the other hand, basic noninvasive monitoring tools including blood pressure (NIBP), heart rate (HR), and pulse oximetry O2 Saturation are non-reliable tools for early diagnosis of haemodynamic changes [Citation7] which endangers the patient safety.
Recently, continuous and real-time non-invasive measurements gradually replace the intermittent invasive haemodynamic monitoring techniques. Noninvasive monitoring devices are user-independent, reliable, continuous, cost-effective with fast response time enough for rapid detection of haemodynamic changes [Citation8]. Thoracic bioimpedance algorithm is one of the most recent non-invasive techniques for CO measurement. It is based on the theory that the thorax is a blood-filled cylinder and regarding Ohm’s law (resistance = voltage/current). Impedance of thoracic tissue is parallel to blood impedance which changes repeat themselves with every heart beat and are linked to cardiac activity [Citation9]. ICON Cardiometer is the only operator-independent, non-invasive haemodynamic monitors, FDA cleared for use in adults, pediatrics and neonates. Over 20 clinical publications have validated the accuracy and clinical utility of the monitors, including studies comparing EC monitor with thermodilution [Citation10] and transesophageal doppler echocardiography [Citation11] in adults and thermodilution [Citation12] transthoracic echocardiography [Citation13,Citation14] and direct Fick [Citation15] in pediatrics and neonates.
Up till now, there is no study has addressed reliability of advanced intraoperative noninvasive thoracic bioimpedance for early detection of haemodynamic changes during PNL in prone position under spinal anesthesia. We hypothesized that ICON Cardiometer parameters could detect haemodynamic collapse earlier than the routinely used basic haemodynamic monitoring variables response.
Therefore, this study was conducted aiming at comparison between the credibility of basic haemodynamic monitors and thoracic bioimpedance in reflecting the real haemodynamic state during PNL in prone position under spinal anesthesia.
2. Materials and Methods
A prospective observational study in tertiary referral center was conducted on 40 patients as single group to detect the changes over time for each variable of both non-invasive bioimpedance cardiometer and the basic haemodynamic variables, furthermore, the collected data was subdivided according to blood loss into two groups; Group 1 (significant group) included 14 (35%) patients with significant blood loss. Group 2 (non-significant group) included 26 (65%) patients with insignificant blood loss.
Study protocol has approval of IRB (Institutional Review Board) code no MFM-IR.18.03.103 on 5/5/2018 and clinical trial registry code NCT03828175. An informed consent was taken from all involved patients at night of surgery. Exclusion criteria involved patients less than 18 or more than 70 years, with ASA score more than II, “cardiac, hepatic, renal or respiratory disorders”, history of hypersensitivity to amide local anesthetics, general contraindications to spinal anesthesia, difficult communications and who refused to be involved in the current trial.
3. Pre-operative Preparation and induction of anesthesia
All patients were counseled about steps of anesthetic procedure. Standard monitors (ECG, NIBP, pulse oximeter) then were attached. Intrathecal anesthesia was conducted in the sitting position under complete aseptic condition, after subcutaneous infiltration 1 ml Lidocaine 1%, heavy bupivacaine 15 mg (3 ml) plus 10 µg dexmedetomidine intrathecal injection using 25 G needle. During PNL, patient’s position passes with three situations. Supine position, just after induction of spinal anesthesia and it usually lasts for 2 minutes to ensure haemodynamic stability of patient and ensure fixed anesthesia level at T4. Then, patient was put in exaggerated lithotomy position for cystoscopy fixation of a ureteric catheter. It usually takes 10–20 min. Finally, prone position is assumed for PNL. Patients received 1000 ml Ringers acetate co-load solution over 30 min. Ringer’s infusion started 10 min before anesthesia and continued during the supine and lithotomy position and was completed prior to assuming the prone position.
4. Intra operative Management
Patients were adjusted in the prone position under restricted precautions. Patient lied over two bellows; one under the chest and one under the pelvis with pliable free moving abdomen. A jell ring was under the patient head knees and in front of heels. Then, the COP bio-impedance Cardiometer monitor [ICON C3 0815001–OSYPKA MEDICAL 5 M-17-011x-B] labelled clips electrodes(A,B,C,D) were connected on the neck and thorax of the patient left side in the following manner; electrode (A) 5 cm above the base of the patient neck, electrode (B) at the base of the patient neck, electrode (C) at the xiphoid level of the left thorax mid axillary line and electrode (D) mid axillary line 10 cm caudal to the electrode (C) then plug the EV patient cable in the terminal CO/CI of ICON and the electricity cable to the connector ICON power supply. Data were recorded according to the study predefined outcome variables every 10 min for basal reading plus another 12 readings (every 10 min) over the next 120 min after prone position adjustment.
5. Measurable outcomes
Primary measurable outcome was stroke volume index (SVI). While secondary outcomes included cardiac output (COP) – Stroke volume (SV) – Cardiac performance index (CPI) – Oxygen delivery DOI- Oxygen delivery (DO2) – cardiac Index (CI) and systolic tome ratio (STR).
Non-invasive basic haemodynamic; systolic blood pressure (SBP), mean blood pressure (MAP), heart rate (HR) and O2 saturation (SaO2). In addition, Haemoglobin (HB) level was routinely estimated from blood sampling at basal and after 2 hours.
6. Adverse events; definitions and management
Episode of perioperative hypotension was defined as systolic blood pressure drop >20% of the basal value or mean arterial blood pressure (MBP) less than 65 mmHg. Hypotension was managed by using bolus doses of ephedrine 6 mg, fluids and blood transfusion according to patients HB level with blood transfusion. HB level less than 8 mg/dl was considered cut point for indication of blood transfusion. Bradycardia was defined as HR drop >20% of the basal value or less than 60 b/m. It was managed by atropine 0.5 mg bolus. Desaturation was defined as SaO2 < 90%. It was managed by stop surgery, increase O2 flow via the oxygen face mask from 5 to 10 l/min, chest auscultation and verbal patient examination with shift to lithotomy position. Nausea was managed by treatment of hypotension as a common cause as discussed before. Vomiting was treated by dexamethasone 0.1 mg/Kg with metoclopramide 0.1 mg/Kg.
According to designed protocol; in case of early fading of spinal anesthesia, intraoperative pain to be managed by fentanyl 0.5 mcg/kg, IV infusion of paracetamol 10 mg/kg, propofol 50 mg increments. If the time to end of PNL was expected to be more than 15 min, general anesthesia was induced and cases passed with these situations were excluded from the study and replaced as a dropout case.
7. Sample size calculation
The sample size was calculated using Power Analysis and Sample Size software program (PASS) version 15.0.5 for windows (2017) using results obtained by a pilot study conducted on 5 patients with the difference in reliability (as measured by Cronbach’s alpha) between mean non-invasive blood pressure (MAP) and stroke volume index (SVI) as the primary outcome. A sample size of 32 patients was needed to achieve 90% power and detect the difference between the Cronbach’s alpha of the MAP (considered to be the null hypothesis) of 0.974 and the Cronbach’s alpha for the SVI of 0.988 using a two-sided F-test with a significance level of 0.05. The expected number of drop-outs was 8, so a total of 40 patients were enrolled into the study.
8. Statistical analysis
IBM’s SPSS statistics (Statistical Package for the Social Sciences) for windows (version 25) was used for statistical analysis of the collected data. We have studied single group of 40 patients which were further divided into two groups of patients; 1st group with significant blood loss included 14 (35%) patients while other 2nd group included 26 (65%) patients. Shapiro-Wilk test was used to check the normality of the data distribution. Normally distributed continuous variables were expressed as mean ± SD while categorical variables and the abnormally distributed continuous ones were expressed as median and inter-quartile range or number and percentage (as appropriate). Student t test and Mann–Whitney were used for normally and abnormally distributed continuous data, respectively. Chi-square test was used for categorical data using the crosstabs function. All tests were conducted with 95% confidence interval. P (probability) value < 0.05 was considered statistically significant.
9. Results
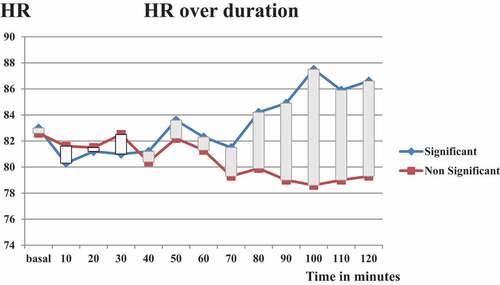
A total of 40 patients were divided into two groups. Group 1 included 14 (35%) patients with significant blood loss. Group 2 included 26 (65%) patients with insignificant blood loss. Blood transfusion was not in any patient. The procedure was completed under spinal anesthesia in all patients. No difference was detected between groups in demographic parameters, basic noninvasive measures as well as bio-impedance Cardiometer parameters (). Similarly, by end of 2 hours, all parameters were comparable between both groups (). O2 saturation was stable between 98% and 100% in both groups throughout procedures. No difference was detected in recorded data per 10-min interval in both groups throughout procedure except for heart rate and STR. Interestingly, recording of changes in STR were noticed 20 min prior to HR changes (at 80 min and 100 min, respectively) (, ). Record of mean±SD of STR at minute-80 was higher in group of significant blood loss (0.44 ± 0.07 Vs 0.38 ± 0.05, P 0.018). Record of HR started to be significantly higher at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05). With correction of blood loss, these differences disappeared in subsequent reading of heart rate but there was persistent significant difference in STR till record at minute-110. Patients demographics; including 21 (52.5%) females and 19 (47.5%) males underwent PNL under spinal anaesthesia. Mean age ± SD was 50.7 ± 10 (range 31–65 years). Mean BMI±SD was 30.5 ± 3.3 (range 23.6–35). Twenty-six (65%) were ranked as GII ASA score, remaining 14 (35%) were GI. Basal Hb level Mean ± SD was 13.4 ± 1.4 (range 10.4–16.4 gr). Mean ABP±SD was 87.6 ± 13 (range 61–107 mmHg).
Table 1. Baseline characteristics of patients with significant blood loss in comparison with patients with non-significant loss
Table 2. Patients'’ characteristic at minute-120
Figure 1. Pattern of changes of STR over duration of procedure. Record of mean±SD of STR at minute-80 was higher in group of significant blood loss (0.44 ± 0.07 Vs 0.38 ± 0.05, P 0.018). Abbreviations; STR systolic time ratio (STR), significant (significant blood loss), Non-significant (Non-significant blood loss)
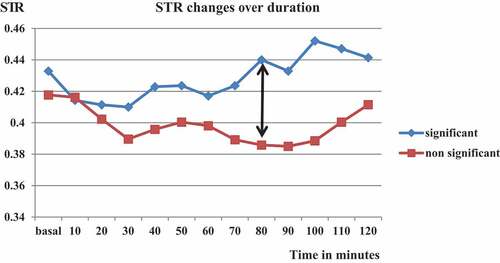
Figure 2. Pattern of changes of heart rate (HR) over duration of procedure. Record of mean±SD of HR over time. Record of HR started to be significantly higher at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05) Abbreviations; HR (heart rate), significant (significant blood loss), Non-significant (Non-significant blood loss)
10. Discussion
In this present study, we hypothesized that ICON Cardiometer parameters can detect haemodynamic collapse earlier than the routinely used basic haemodynamic monitoring variables response, targeting minute to minute detection of the haemodynamic changes.
The 40 patients as single group to detect the changes over time for each variable of both non-invasive bioimpedance cardiometer and the basic haemodynamic variables, furthermore the collected data were subdivided according to blood loss into two groups; Group 1 (significant group) included 14 (35%) patients with significant blood loss. Group 2 (non-significant group) included 26 (65%) patients with insignificant blood loss.
Recording of changes in STR were noticed 20 min prior to HR changes (at 80 min and 100 min, respectively). Record of mean ± SD of STR at minute-80 was higher in group of significant blood loss (0.44 ± 0.07 Vs 0.38 ± 0.05, P 0.018). Record of HR started to be significantly higher at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05). With correction of blood loss according to the protocol methodology, these differences disappeared in subsequent reading of heart rate but there was persistent significant difference in STR till record at minute 110. (, ).
American Society of Anesthesiologists [Citation16] and the World Health Organization’s [Citation17] recommends blood pressure monitoring in all anesthetized persons at least at 5-min intervals. Intermittent automated non-invasive oscillometric cuffs are mostly used for this purpose. Motion artifacts, adequate cuff size, and prolonged inflation/deflation times can pose significant drawbacks. [Citation18] However, even among high-risk surgical patients in about 50% the NIBP is used. [Citation19]
Using the intermittent cuff, NIBP monitoring may leave BP fluctuations undetected or may lead to late recognition and delayed correction. [Citation20,Citation21] Several recent large-scale observational studies have demonstrated, that not only the “intensity” (depth of hypotension) but also the “dose” (cumulative time spent in hypotension) are associated with severe postoperative complications [myocardial infarction, stroke, or acute kidney injury (AKI)]. [Citation22–25] Not only fast recognition but also prediction of further BP course would decrease the risk of hypotension-related complications.
One of the most bothersome complications of PNL is haemorrhage. Direct access to the pelvicalyceal system and intrarenal manipulation during PNL procedures cause injury to the renal vasculature, particularly to the segmental and interlobar arteries. The renal-collecting system is rich in vascularization, covering 20% of the total cardiac output, and often results in haemorrhage during PCNL [Citation26] many of the haemorrhage cases during PNL could be managed conservatively, however, 0.8% patients required a more invasive procedure to deal with the bleeding [Citation27]. PNL operation caries risk of other haemodynamic instability saturations due to many factors such as; it necessitates high-level spinal anaesthesia, it is associated with progressive creeping silent unmeasurable arterial bleeding, it is done in prone position which augments haemodynamic instability in a difficult resuscitation position.
As regard the significant increase in the HR at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05-not to the extent of real clinical tachycardia but judged relatively by 20% increase of the basal HR value) this can be explained by an early cardiac compensatory mechanism for the cumulative arterial bleeding induced hypovolemia during PNL endoscopic renal cortical arterial injury which was not yet detected by the NIBP wither systolic NIBP or even mean NIBP records which showed no significant change in between both study groups all over the study records due to minute to minute COP bioimpedance monitoring of vital signs with fluid resuscitation therapy. On the other hand Atici S and coworkers 2001 [Citation28] documented that the mean systolic and diastolic blood pressure levels were significantly higher during PNL due to irrigation fluid overload while heart rate remained constant. On the other hand, Koroglu et al.2003[Citation29] couldn’t find significant changes in blood pressure, heart rate before and after irrigation.
STR normal value is tightly inversely linked to the cardiac ejection fraction that maintains the COP and the coronary perfusion [with normal EF >55% the decreases STR<0.35, If EF decrease<55% STR increases>0.35, if EF further decrease < 35% STR more increases >0.65] [Citation30], Interestingly, recording of changes in STR noticed 20 min prior to HR changes (at 80 min and 100 min, respectively) (, ). Record of mean±SD of STR at minute-70 was higher in group of significant blood loss (0.44 ± 0.07 vs. 0.38 ± 0.05, P 0.018). Record of HR started to be significantly higher at minute-100 (87.5 ± 13 Vs.78.6 ± 12, P 0.05). With correction of blood loss, these differences disappeared in subsequent reading of heart rate but there was persistent significant difference in STR till record at minute 110. STR is the ratio of electrical and mechanical systole STR = PEP/LVET. Pre-ejection period (PEP) Time interval from the beginning of the electrical stimulation of the ventricles to the beginning of the opening of the aortic valve (electric systole), Left ventricular ejection time (LVET) is the time interval from opening to closing of the aortic valve (mechanical systole). When The PEP decreases [corresponding to the interval between the depolarization onset (Q-wave) and aortic valve opening that includes the excitation contraction coupling and iso-volumetric contraction of the LV], with increasing LV pressure at the end of diastole diastolic dysfunction occur. [Citation30]
In this present study, the increased STR values 20 min before HR elevation can be explained by the fact that; direct access to the pelvi-calyceal system and intrarenal manipulation during PCNL procedures cause injury to the renal vasculature, particularly to the segmental and interlobar arteries. The renal-collecting system is rich in vascularization, covering 20% of the total cardiac output, and often results in haemorrhage during PCNL, up to 11% of patients who underwent PCNL required blood transfusion [Citation26] leading to reduction of coronary blood flow leads to prolongation of the PEP meanwhile LVET relatively shortened resulting in the increase (STR = PEP/LVET), in line with this explanation to our result; Chen S J and colleagues 2014 (27) documented that STR is a sensitive and liable indicator in the diagnosis of CHD.
Therefore, STR is an early indicator of haemodynamic collapse due to bleeding even precedes the earliest cardiac compensatory HR elevation by significant 20 minutes’ period fair enough to initiate active resuscitation and never face the sudden drop of MAP below 60 mmHg at which the vital organs become already compromised in response to hidden active arterial blood loss.
The current study has many limitations that should be acknowledged. Central venous pressure catheter couldn’t be inserted because of the local hospital policy protocol so systemic vascular resistance and its related variables was excluded from this present study. SVV was also excluded from this present study as SVV as it is not reliable during spinal anesthesia. It is only a reliable indicator of fluid responsiveness in mechanically ventilated patients with adequate tidal volumes a situation which is not available during spinal anesthesia. [Citation3,Citation4]
Haemoaemoglobin (HB) level sampling after 1 h of the start of surgery was omitted during the study as there was no major haemodynamic incident necessitates intraoperative HB measurement, no patient was in need for blood TX because of rapid detection of haemodynamic instability. Irrigation fluid absorption can’t be estimated hindering Intravenous fluids infused volume calculation to be useless. Finally, we could not estimate blood loss in ml since continuous irrigation during PNL prevents us from accurate estimation. However, we could consider last two limitations to be inevitable. Indeed, last 2 limitations were the actual promoters for conduction of the current work.
11. Conclusion:
STR is a more reliable tool than MBP and HR for early detection of haemodynamic collapse in case of non-measurable bleeding during PNL. STR should be integrated as a routine monitoring tool during PNL.
Acknowledgments
The authors report no acknowledgment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yu J, Park HK, Kwon HJ, et al. Risk factors for acute kidney injury after percutaneous nephrolithotomy: implications of intraoperative hypotension. Medicine (Baltimore). 2018;97(30):e11580.
- Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. British Journal of Anesthesia. 2008;100(2):165–183.
- Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18(6):644.
- Malmqvist LA, Bengtsson M, Björnsson G, et al. Sympathetic activity and haemodynamic variables during spinal analgesia in man. Acta Anaesthesiol Scand. 1987;31(6):467–473.
- Mascha EJ, Yang D, Weiss S, et al. Intraoperative mean arterial pressure variability and 30-day mortality in patients having non-cardiac surgery. Anesthesiology. 2015;123(1):79–91.
- Atkinson CJ1, Turney BW, Noble JG, et al. Supine vs prone percutaneous nephrolithotomy: an anesthetist’s view. BJU Int. 2011;108(3):306–308.
- Poon KS1, Wu KC, Chen CC, et al. Jun, 2008. Hemodynamic changes during spinal surgery in the prone position. Acta Anesthesiol Taiwan., 46(2): 57–60.
- Kirov MY, Kuzkov VV, Molnar Z. Perioperative hemodynamic therapy. Curr Opin Crit Care. 2010;16(4):384–392.
- Jakovljevic DG, Trenell MI, MacGowan GA. Bioimpedance and bioreactance methods for monitoring cardiac output. Best Pract Res Clin Anesthesiol. 2014;28(4):381–394.
- Zoremba N, Bickenbach J, Krauss B, et al. Comparison of electrical velocimetry and thermodilution techniques for the measurement of cardiac output. Acta Anesthesiol Scand. 2007;51(10):1314–1319.
- Schmidt C, Theilmeier G, Van Aken H, et al. Comparison of electrical velocimetry and trans-esophageal doppler echocardiography for measuring stroke volume and cardiac output. Br J Anaesth. 2005;95(5):603–610.
- McGovern -M, Miletin J. Cardiac output monitoring in preterm infants. Front Pediatr. 2018;6:84.
- Singh Y. Echocardiographic evaluation of hemodynamics in neonates and children. Front Pediatr. 2017 2017;5:201.
- Rauch R, Welisch E, Lansdell N, et al. Non-invasive measurement of cardiac output in obese children and adolescents: comparison of electrical cardiometry and transthoracic doppler echocardiography. J Clin Monit Comput. 2013;27(2):187–193. Apr Epub 2012 Nov 21.
- Norozi K, Beck C, Osthaus WA, et al. Electrical velocimetry for measuring cardiac output in children with congenital heart disease.Br J Anesth. 2008 [Jan];100(1):88–94.
- Stenglova A, Benes J, Continuous non-invasive arterial pressure assessment during surgery to improve outcome. Front Med (Lausanne). Published online 2017 2017 Nov 17;4: 202.
- World Health Organization. Guidelines for safe surgery (2009). WHO; (2009). 125 p. Available from: http://apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf.]
- Wax DB, Lin H-M, Leibowitz AB. Invasive and concomitant noninvasive intraoperative blood pressure monitoring. Anesthesiology. 2011;115(5):973–978. 10.1097/ALN.0b013e3182330286
- Cannesson M, Pestel G, Ricks C, et al. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among north american and european anesthesiologists. Crit Care. 2011;15(4):R197.
- Chen G, Chung E, Meng L, et al. Impact of non invasive and beat-to-beat arterial pressure monitoring on intraoperative hemodynamic management. J Clin Monit Comput. 2012;26(2):133–140.
- Benes J, Simanova A, Tovarnicka T, et al. Continuous non-invasive monitoring improves blood pressure stability in upright position: randomized controlled trial. J Clin Monit Comput. 2015;29(1):11–17.
- Walsh M, Kurz A, Turan A, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery. Anesthesiology. 2013;119(3):507–515.
- Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after non-cardiac surgery. Anesthesiology. 2017;126(1):47–65.
- Bijker JB, Persoon S, Peelen L, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery. Anesthesiology. 2012;116(3):658–664.
- Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective non-cardiac surgery. Anesthesiology. 2015;123(3):515–523.
- Kukreja R, Desai M, Patel S, et al. Factors affecting blood loss during percutaneous nephrolithotomy: prospective study. J Endourol. 2004;18(8):715–722.
- S J C, Gong Z, Duan Q. Evaluation of heart function with impedance cardiography in acute myocardial infarction patients. Int J Clin Exp Med. 2014;7(3):719–727.
- Atici S, Zeren S, Ariboğan A. Hormonal and hemodynamic changes during percutaneous nephrolithotomy. Int Urol Nephrol. 2001;32(3):311–314.
- Koroglu A, Togal T, Cicek M, et al. The effects of irrigation fluid volume and irrigation time on fluid electrolyte balance and hemodynamics in percutaneous nephrolithotripsy. Int Urol Nephrol. 2003;35(1):1–6.
- R N L, P M S, Branco L, et al. Systolic time ratio measured by impedance cardiography accurately screens left ventricular diastolic dysfunction in patients with arterial hypertension. Clin Hypertens. 2017;23(1):28.
