?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective: Evaluation of the analgesic efficacy and safety of ultrasound-guided pterygopalatine fossa (PPF) block in patients undergoing maxillofacial cancer surgeries under general anesthesia.
Methods: Forty-eight patients scheduled for maxillofacial cancer surgeries enrolled in the study were randomly allocated into group (A): ultrasound-guided bilateral PPF block using local anesthetic; and group (B): ultrasound-guided bilateral PPF injection with saline. Our primary outcome was assessing postoperative analgesia using visual analog scale and the amount of nalbuphine used for rescue analgesia.
We recorded the operative field’s quality, end-tidal sevoflurane concentration, the total amount of nitroglycerin used to achieve the target mean arterial pressure (MAP) of 60–65 mmHg, the frequency of propranolol usage, emergence time and Aldrete score.
Results: The VAS score was significantly lower in group A than group B until the 18th postoperative hour (P < 0.0001). The number of patients required nalbuphine (12 versus 24 patients) and total nalbuphine doses were significantly less in group A (10 ± 2 mg) versus group B (20 ± 5 mg) (P < 0.01). Sevoflurane mean end-tidal concentration was significantly less in group A (2.2 ± 0.53%) than in Group B (2.7 ± 0.48%), P-value = 0.019. Total nitroglycerine dose was significantly lower (2.45 ± 0.63 µg/kg/min) in group A than (3.58 ± 0.77 µg/kg/min) in group B (P value<0.05).
Conclusions: ultrasound-guided PPF block combined with general anesthesia is a safe technique and helps in providing better operative field by adequate control over the blood pressure. It is effective for decreasing the postoperative pain and analgesic requirements in patients undergoing maxillofacial cancer surgery.
1. 1.Introduction
Inadequate treatment of postoperative pain is common in surgical patients. It has been found that approximately 40% of surgical patients exhibited moderate to severe pain during the first 24 h postoperatively in a general surgical setting [Citation1].
Bleeding from maxillofacial surgery of any type can be extensive. Head-up positioning, infiltration of adrenaline-containing local anesthetic solutions, and avoidance of hypertension can all help reduce blood loss. Regional anesthesia and remifentanil target-controlled infusions provide a responsive method of preventing stimulation-driven hypertension. Extensive maxillofacial surgery will usually require strong opioids in the immediate postoperative period. Patients with cancer or temporomandibular dysfunction may have chronic pain and baseline opioid requirements, making perioperative analgesia management more challenging. Early involvement of the acute pain service is invaluable for these patients [Citation2].
The sphenopalatine ganglion (SPG) is located within the pterygopalatine fossa (PPF). This ganglion has sympathetic activity in the form of visceral motor functions through its connection to the cervical sympathetic chain via the deep petrosal nerve and parasympathetic activity via the superficial petrosal nerve. The primary sensory distribution is the palate, buccal mucosa, nose, and orbit () [Citation3].
Figure 1. Redrawing diagram depicting relationships of trigeminal nerve, sphenopalatine ganglion (SPG) and the three divisions of the trigeminal nerve [Citation3]
![Figure 1. Redrawing diagram depicting relationships of trigeminal nerve, sphenopalatine ganglion (SPG) and the three divisions of the trigeminal nerve [Citation3]](/cms/asset/f1586322-c165-4c6d-93f2-297d7319c4da/teja_a_1903667_f0001_oc.jpg)
Sphenopalatine ganglion blockade is commonly utilized to manage chronic pain syndromes such as trigeminal neuralgia, atypical facial pain, and headaches. There are many available approaches to blocking this ganglion. One of the described approaches is the sinoscopic guided technique. In this technique, local anesthetics are deposited superior-posterior to the middle turbinate [Citation4–7].
One of the most recently described approaches to block the PPF is via ultrasound-guided technique. The use of ultrasound-guided anesthesia is becoming increasingly popular, so the pterygopalatine fossa, the lateral pterygoid plate, and the maxillary artery can be visualized in real-time. This technique allows easy access to the pterygopalatine fossa and its contents, including the maxillary nerve, the sphenopalatine ganglion, and the deep and superficial petrosal nerves [Citation8]. According to Nader et al., all patients experienced pain relief within 5 min of PPF block. Eighty percent of patients had complete anesthesia within 15 min of PPF block in V1, V2, and V3 distributions [Citation9].
Our study was designed to evaluate the hypothesis that a PPF block under ultrasound guidance performed under general anesthesia could have a perioperative analgesic effect, provide good surgical conditions, and improve patient recovery characteristics.
2. Patients and methods
This study was conducted at the National Cancer Institute at Cairo University from June 2017 till March 2018, after approval of the local ethical committee, informed consent was obtained from all patients. It was registered at www.clinicaltrials.gov at no.: NCT03171090. The study was conducted according to the most recent version of the Declaration of Helsinki. We enrolled adult patients scheduled for maxillofacial cancer surgeries (maxilla, mandible, alveolar margin, and tongue) in this prospective, randomized study. The study included participants with ASA (American Society of Anesthesiologists) physical status I or II, and between 20 and 70 years. A detailed history and preoperative assessment of the tumor and disfigurement both clinically and radiologically.
Patients with disfigurement and disturbed anatomy prevented easy access to the sphenopalatine ganglion, history of bleeding disorders, or receiving anticoagulant therapy were excluded. Patients with a history of hepatic, renal, or cardiopulmonary dysfunction and patients with infection at the injection site or previous allergy to local anesthetics were excluded.
The night before surgery, we explained the block technique to the patients. All patients were instructed how to assess their pain using a 10 cm visual analog scale (VAS), where score 0 denotes no pain and score10 indicates the maximum pain felt. A 20-gauge radial artery cannula was placed and, Intravenous lines were inserted for administration of anesthetic drugs and fluids. All patients were premedicated with 0.02 mg/kg midazolam intravenously (IV) 2 h before surgery. On arrival to the operating room, a pulse oximeter and five-lead ECG were attached, and invasive arterial blood pressure was inserted to monitor SpO2, ECG and arterial BP. EtCO2, heart rate, temperature, BIS monitoring, and train of four (TOF) were also monitored throughout the surgery (Infinity SC 8000, Drager Medical Inc., USA).
Patients were randomly allocated according to computer-generated tables to one of the two treatment groups. Group A, ultrasound-guided PPF block using a local anesthetic, and group B, ultrasound-guided PPF injection using normal saline 0.9%.
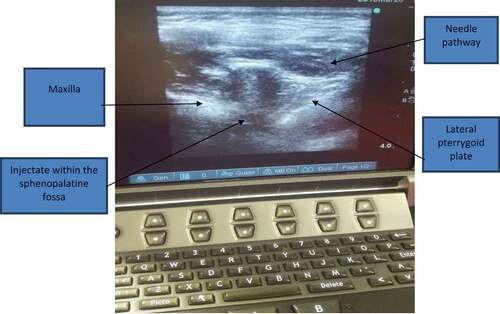
Before induction of general anesthesia, starting from the side of the tumor, the patients were placed in a lateral decubitus position to perform the block with the aid of ultrasonography (Sonosite, M-Turbo, USA). Using aseptic precautions, the linear transducer probe was positioned longitudinally on the lateral side of the face below the zygomatic bone, above the mandibular notch, and in front of the mandibular condyle with a cephalad angulation. The maxilla, lateral pterygoid plate, and lateral pterygoid muscle were identified. The surrounding vasculature, including the maxillary artery, was visualized by using Color Power Doppler in the pterygopalatine fossa. In order to optimize the “angle of insonation” (needle to probe angle), the transducer probe was placed closer (just anterior) to the mandibular condyle.
An insulated echogenic needle (22-G 50 mm, Pajunk, Sonoplex) was inserted in plane parallel to the transducer probe and advanced in a lateral to medial and posterior to anterior direction toward the pterygopalatine fossa () (8). Following negative aspiration, the local anesthetic was deposited just deep to the lateral pterygoid muscle and plate. In group A, 4 mL of bupivacaine 0.25%was deposited on each side, and in group B, 4mL0.9% saline was deposited on each side. The time required to perform the technique, defined as the time from the beginning of scanning until the local anesthetic injection, was recorded.
In group A, sensory assessments of the patient to pinprick were performed using a Neurotips examination pin in the V1, V2, and V3 trigeminal nerve distributions, by an observer blinded to the technique (maximum time till induction of anesthesia was 15 min). In group B, induction of anesthesia started 15 min after the injection of saline. According to the manufacturer guidelines, standard BIS® monitoring strip (BISX®, Aspect Medical Systems, Norwood, MA, USA) was placed on the patients’ forehead of the dominant hemisphere.
General anesthesia was induced with intravenous fentanyl 2 µg/kg, and propofol 2–3 mg/kg. Tracheal intubation was facilitated with atracurium besylate 0.5 mg/kg. Anesthesia was maintained using sevoflurane in 100% oxygen and atracurium besylate 0.15 mg/kg every 20–30 min according to readings on train of four (TOF) watch. All patients were mechanically ventilated to maintain an end-tidal carbon dioxide level between 35 and 40 mmHg.
Target intraoperative MAP of 60–65 mmHg was maintained by the adjustment of the nitroglycerine infusion from 0.5 to 10ug/kg/min according to the patient’s response. Bradycardia (HR45 beats/min) was treated with a 300 ug bolus of atropine. Tachycardia (HR
100 beats/min) a 0.2 mg bolus of propranolol was given and repeated as needed. When MAP reached the desired level (60–65 mmHg) and maintained for at least 15 min, the surgeon blinded to hemodynamic parameters and group assignment was asked to evaluate the operative field’s quality (according to the severity of bleeding). They were asked about the quality every 30 min during the operation using a predefined average category scale (ACS) from 0 to 5 () [Citation10]. The same surgical team performed all operations to ensure consistency in evaluating the operative field for bleeding and surgical time.
Table 1. Average category scale (10)
Sevoflurane concentration was titrated intraoperatively to achieve a BIS value between 40 and 50. At the end of the surgery, all infusions together with sevoflurane were discontinued. The total amount of nitroglycerine used to achieve the target MAP was recorded.
Intraoperative blood loss was estimated and recorded by measuring blood volume in the suction canister minus the normal saline used to wash the surgical field. Also recorded the weight of all towels used minus the preoperative weight of towels/pads. One gram of fluid was converted to 1 mL of blood.
In both groups, 1 g of paracetamol was infused 30 min before emergence from anesthesia. The residual neuromuscular block was antagonized with neostigmine 0.05 mg/kg and atropine 0.01 mg/kg before extubation of the trachea. At emergence from anesthesia, 60 mg of ketorolac was infused.
Emergence time was recorded for all patients; it was defined as the time interval between anesthetic discontinuation to an eye-opening response following a verbal command [Citation11]. Following extubation, all patients were transferred to the post-anesthesia care unit (PACU). Postoperative recovery was evaluated using a modified Aldrete score (0–10) and the time required to reachnine was recorded [Citation12]. They were monitored for 24 h. Patients in both groups were provided 60 mg of ketorolac every 12 h and 1 g of paracetamol every 8 h through 24 h follow-up.
Our primary outcome of interest was postoperative pain, which was assessed using a VAS in the (PACU). Assessments were taken immediately after surgery, 30 min, 2, 6, 10, 14, 18, and 24 h postoperatively. A VAS score greater than 3 was managed with incremental intravenous dose of 0.07 mg/kg nalbuphine. The number of patients who received nalbuphine in the PACU was recorded, along with the overall total dose of nalbuphine received. Postoperative side effects such as nausea and vomiting (PONV), headache, visual disturbances, and bleeding were recorded.
2.1. Statistical analysis
For our study design, the sample size was calculated using G Power software (University of Düsseldorf, Düsseldorf, Germany); we set the power of 80% and assumed a5% significance. We estimated that 20 patients per group would be necessary and enrolled 24 patients in each group in case of dropout.
Data were analyzed using SPSS Statistics software (version 21.0, IBM Corp., Armonk, NY, USA). Numerical data were reported as mean and standard deviation. Data were tested for normality using the Kolmogorov–Smirnov test or Shapiro–Wilk test. The Student’s t-test was used to assess if the measurements were normally distributed between the two groups. If the measurements were not normally distributed, the Mann–Whitney test was performed. Categorical data are expressed as a number and percentage. Comparisons between the two groups were performed by a Chi-squared test or Fisher’s exact test as appropriate. A P value less than or equal to 0.05 was considered statistically significant.
3. Results
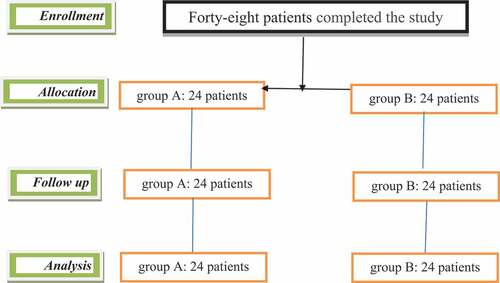
Patients who failed to meet the inclusion criteria and/or refused to sign the consent form were excluded from the study. Forty-eight patients completed the study, and their data were presented in the final analysis (). Patients in both groups of the study were comparable with respect to demographic data, type of the operation, and time required to perform the procedure ().
Table 2. Demographic and operative data in both groups [mean ±SD, number (%)]
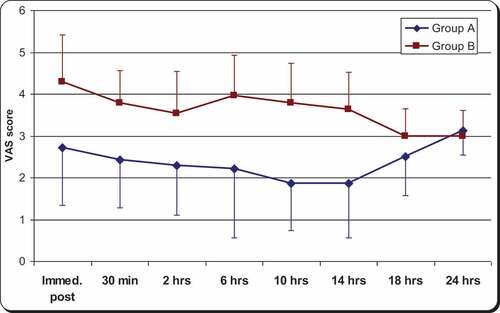
The visual analog pain score was significantly lower in group A than group B until the 18th hour postoperatively (P < 0.0001), at which point, there was no significant difference ().
Twelve patients (50%) in group A and 24 patients (100%) in group B received supplementary nalbuphine analgesia in the PACU (P < 0.01). The total nalbuphine doses given were significantly lesser in group A versus group B (P < 0.01) ().
Table 3. Postoperative analgesic requirements in the post-anesthesia care unit [mean ±SD, number (%)]
In group A, the three branches of the trigeminal nerve were blocked in 62.5% of cases on the right side and in 66.6% of cases on the left side. Sensory anesthesia with pinprick was achieved in all patients in V2 branch distribution. The time to onset of the block was 10.8 ± 1.52 min on the right side and 11.12 ± 1.42 min on the left side (P ˃ 0.05).
There was no statistically significant difference between the groups regarding the duration of surgery. Intra-operative blood loss was significantly less in group A (995.4 ± 321.7 mL) in comparison to group B (1417.9 ± 288.4 mL), P < 0.0001. The average category scale (ACS) for quality of the surgical field was significantly better in group A than in group B (P = 0.003), in the range of MAP between 60 and 65 mmHg in all studied time intervals ().
Table 4. Intraoperative data in both groups (mean ± SD)
Sevoflurane mean end-tidal concentration required to maintain a BIS value between 40 and 50 was significantly lesser in group A (2.2 ± 0.53%) than in Group B (2.7 ± 0.48%) (P-value = 0.019). All patients in both groups (100%) were supplemented with nitroglycerine to achieve the desired MAP (60–65 mmHg). There was a significant difference between the groups for mean dose of nitroglycerine required, group A (2.45 ± 0.63 μg/kg/min) versus Group B (3.58 ± 0.77 μg/kg/min) (P < 0.05). Propranolol administration was necessary for 5 patients (20.8%) in group A and10 patients (41.7%) in group B, (P < 0.05). Emergence time and time needed to achieve ≥9 of the modified Aldrete score were significantly shorter in group A than group B (6.96 ± 1.7 min and10.3 ± 1.58 min versus 9.85 ± 1.85 min and 13.6 ± 2.9 min, respectively; P < 0.0001) ().
The occurrence of postoperative complications and the number of patients who received an additional antiemetic were comparable in both groups ().
Table 5. Postoperative complications within 24 h of surgery [number (%)]
4. Discussion
This study utilized an ultrasound-guided technique to block the PPF bilaterally and evaluated its ability to control perioperative pain in patients undergoing maxillofacial cancer surgeries. We found that more patients reported significantly lower VAS scores in group A than in group B throughout the postoperative follow-up period. Patients’ nalbuphine dosage was significantly higher in group B than in group A. This finding is in line with previous studies where participants who received the block had decreased postoperative pain scores compared to the non-block group [Citation13–15].
The trigeminal nerve is the largest and most significant nerve innervating the oral mucosa, tongue, teeth, facial, skin, meningeal lining, and muscles of mastication. It is a mixed nerve composed of sensory and motor fibers. The sensory components travel from the periphery within the three main divisions of the nerve (ophthalmic, maxillary, and mandibular) to their cell bodies in the Gasserian ganglion located in the floor of the middle cranial fossa. The sensory nerve fibers then arise from the Gasserian ganglion and synapse with the trigeminal nuclei in the brainstem at the pons level [Citation16].
Nader et al., demonstrated that using fluoroscopy-guided injection of only 2 mL of contrast dye into the pterygopalatine fossa produced a retrograde passage of contrast into the middle cranial fossa and permitted gasserian ganglion visualization. They attributed the retrograde spread of the dye to the small volume of the pterygopalatine fossa and its connection with the middle cranial fossa through the foramen rotundum [Citation8,Citation9].
Recently, blocking of the SPG has been used in combination with general anesthesia to control perioperative pain [Citation14].
We found that the technique only required a short duration of time to administer, approximately 6 min. In a study by Nader et al., they used the ultrasound-guided PPF block technique in patients suffering from trigeminal neuralgia and atypical facial pain. They found that all PPF blocks were achieved within 5 min from needle insertion to needle withdrawal. All patients experienced complete sensory block to pinprick in the V2 branch distribution, and 80% achieved total sensory block in V1, V2, and V3 distributions within 15 min of receiving a block. They concluded that an ultrasound-guided injection of 5 mL 0.25% bupivacaine combined with steroid under the lateral pterygoid muscle in the pterygoid fossa resulted in immediate sensory analgesia in all branches of the trigeminal nerve. Most patients also experienced and sustained pain control [Citation9].
In this study, the combination of general anesthesia with ultrasound-guided PPF block in maxillofacial cancer surgeries resulted in improved outcomes and provided better surgical field visualization. Patients experienced decreased blood loss and intraoperative medication usage (sevoflurane, nitroglycerin, propranolol). Reduced recovery time, postoperative pain and rescue analgesia utilization, and reduced postoperative complications.
In our study, sevoflurane was titrated as guided by the patient monitor to produce the desired level of hypnosis, a BIS level between 40 and 50. In each group, the mean end-tidal sevoflurane dose was significantly lower in group A compared to group B. This finding coincides with the results of Hassan and Ehab (13) and the results of Ashgan et al., (14) in which the combination of bilateral sphenopalatine ganglion block (SPGB) with general anesthesia during sinonasal surgery and endoscopic transnasal resection of pituitary adenoma, respectively, decreased the anesthetic requirements. A study done by Hodgson and Liu demonstrated that the deafferentation produced by regional anesthesia resulted in supraspinal effects that modulate hypnotic anesthetic requirements [Citation17]. Our results support the findings of previous studies and explain the significant difference witnessed between the two groups for end-tidal sevoflurane utilization when a pterygopalatine fossa SPG blockade is combined with general anesthesia.
In our study, group A achieved the targeted blood pressure with lower doses of sevoflurane, nitroglycerine, and propranolol, compared to group B. The hemodynamic effects of PPF block could be related to the adequate profound anesthesia achieved in the sphenopalatine ganglion and its related nerves (maxillary greater and lesser palatine nerves). Blockade of the trigeminal ganglia via diffusion of local anesthetic through the foramen rotundum prevented a rapid fluctuation in blood pressure due to painful stimulation during surgical manipulation. Our findings agree with the results of Hassan and Ehab, Ashgan et al., and Chadha et al. [Citation13–15].
An oral surgery study that evaluated a regional nerve block with general anesthesia reported the dose of adenosine needed to maintain hypotension was reduced due to the hemodynamic stability produced by the nerve blocks [Citation18]. The main goal of controlling blood pressure during maxillofacial cancer surgery is to achieve a dry surgical field to improve visibility and facilitate the surgical approach. Studies evaluating the quality of the surgery field could be a better indicator than recording the absolute mean arterial blood pressure [Citation19,Citation20].
In our study, the visibility of the surgery field was better in group A than in group B because of significantly lower ACS in group A than in group B. Patient blood loss during surgery was significantly lesser in group A compared to group B. Our results are comparable to the studies by Hassan and Ehab and Ashgan et al. [Citation13,Citation14]. SPG is the main parasympathetic ganglion of the head and neck; blocking of its parasympathetic activity permits the unopposed sympathetic activity of the head and neck; this may explain the hemostasis and dry surgical field obtained with an SPG block [Citation21,Citation22].
The emergence time (time to respond to verbal command) and the time required to achieve an Aldrete score ≥9 in our study were significantly shorter in group A than in group B. Two prior studies utilized a bilateral SPG block in sinoscopic and trans-sphenoidal surgeries reported similar results. The authors attributed this to the significantly decreased sevoflurane utilization in the block group compared to the non-block group [Citation13,Citation15].
One of the important advantages of ultrasound guidance is visualizing vascular structures in real-time, which minimizes the potential of inadvertent needle puncture (22). We were able to visualize the maxillary artery in all our cases which improved the safety in our study.
In conclusion, a bilateral ultrasound-guided PPF block in association with general anesthesia helps in providing better operative field as evidenced by less bleeding, and a safe technique because no inadvertent intravascular injection, or exposure to radiation during the procedure. It is an effective technique for decreasing the postoperative pain and analgesic requirements in patients undergoing maxillofacial cancer surgery. It helps to provide adequate control over the blood pressure during the operative period with a low dose of nitroglycerin and sevoflurane. The patients also experience less intraoperative blood loss and a faster recovery.
Disclosures
Nothing to disclose
Disclosure statement
The authors declare no conflicts of interest.
References
- Beauregard L, Pomp A, Choinere M. Severity and impact of pain after day-surgery. Can J Anaesth. 1998;45(4):304–311.
- Jacqueline MC, Urmila R. Anesthesia for maxillofacial surgery. Anesth Intensive Care Med. 2020;21(9):457–462.
- Waxman S. Correlative neuroanatomy. 23rd ed. Stanford: Appleton & Lange; 1996. p. 265–266.
- Yang IY, Oraee S. A novel approach to transnasal sphenopalatine ganglion injection. Pain Physician. 2006;9(2):131–134.
- Peterson JN, Schames J, Schames M, et al. Sphenopalatine ganglion block: a safe and easy method for the management of orofacial pain. Cranio 1995; 13:177–181.
- Felisati G, Arnone F, Leon M, et al.,, . Sphenopalatine endoscopic ganglion block: a revision of a traditional technique for cluster headache. Laryngoscope. 2006;116(8):1447–1450.
- Varghese BT, Koshy RC. Endoscopic transnasal neurolytic sphenopalatine ganglion block for head and neck cancer pain. J Laryngol Otol. 2001;115(5):385–387.
- Nader A, Schittek H, Kendall MC. Lateral Pterygoid muscle and maxillary artery are key anatomical landmarks for ultrasound-guided trigeminal nerve block. Anesthesiology. 2013;118(4):957.
- Nader A, Kendall MC, De Oliveria GS, et al. Ultrasound-guided trigeminal nerve block via the pterygopalatine fossa: an effective treatment for trigeminal neuralgia and atypical facial pain. Pain Physician. 2013;16:537–545.
- Fromme GA, Mac Kenzie RA, Gould AB Jr, et al. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65(6):683–686.
- Chung F. Are discharge criteria changing? J Clin Anesth. 1993;5(6):64S.
- Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89.
- Ahmed HM, Abu-Zaid EH. Role of intraoperative endoscopic sphenopalatine ganglion block in sinonasal surgery. J Med Sci. 2007;7(8):1297–1303.
- Ali AR, Sakr SA, Ahmed Shawky MA, Rahman. Bilateral sphenopalatine ganglion block as adjuvant to general anesthesia during endoscopic trans-nasal resection of pituitary adenoma. Egypt J Anesth. 2010;26:273–280.
- Chadha R, Padmanabhan V, Rout A, et al.,MohandasK. Prevention of hypertension during trans-sphenoidal surgery –the effect of bilateral maxillary nerve block with local anesthetics. ActaAnesthesiolScand 1997; 41:35–40.
- Ong CKS, Seymour RA. Pathogenesis of postoperative oral surgical pain. Anesth Prog. 2003;50:5–17.
- Hodgson PS, Liu SS. Epidural lidocaine decreases sevoflurane requirement for adequate depth of anesthesia as measured by the bispectral index monitor. Anesthesiology. 2001;94(5):799–803.
- Noma T, Ichinohe T, Kaneko Y. Inhibition of physiologic stress responses by regional nerve block during orthognathic surgery under hypotensive anesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(5):511–515.
- Gemma M, Tommasino C, Cozzi S, et al.,, . Remifentanil provides hemodynamic stability and faster awakening time in transsphenoidal surgery. Anesth Analg. 2002;94(1):163–168.
- Prasant MC, Kar S, Rastogi S, et al. Comparative study of blood loss, quality of surgical field and duration of surgery in maxillofacial cases with and without hypotensive anesthesia. J Int Oral Health. 2014;6(6):18–21.
- Day M. Sphenopalatine ganglion analgesia. Curr Rev Pain. 1999;3(5):342–347.
- Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anesthesia. Br J Anaesth. 2005;94(1):7–17.