ABSTRACT
Background: Different techniques are employed to assess the endpoint of fluid therapy in hypovolemic patients. Lung ultrasound (US) is increasingly becoming a diagnostic tool in the critical care setting, providing standardized data. The present study aimed to evaluate the role of lung US in comparison to central venous pressure (CVP) in assessment of endpoint of fluid therapy in patients with hypovolemic shock.
Patients and methods: Observational cross-sectional study carried in 60 adult patients with hypovolemic shock admitted to the intensive care unit received lactated Ringer’s solution. CVP, blood pressure (BP), urine output (UOP), and lung US score were recorded on admission and during the period of resuscitation. Lung US score was correlated with CVP, BP, and UOP and evaluated in assessment of endpoint fluid therapy in comparison to CVP as a gold standard.
Results: There was a significant increase in CVP, BP, UOP,and lung US score during fluid resuscitation. Lung US showed a significant positive correlation with CVP, BP, and UOP at different stages of fluid resuscitation. Lung US score showed a sensitivity of 95.7%, specificity of 92.9% with a positive predictive value of 97.8% negative predictive value of 86.7%, and the total accuracy was 95%.
Conclusion: Lung US provides a simple noninvasive approach in assessment of endpoint (score ≥16) of fluid therapy in patients with hypovolemic shock with high sensitivity and specificity.
1. Introduction
Shock is a common condition in critical care. The diagnosis of shock is based on clinical, hemodynamic, and biochemical signs. It is manifested with systemic arterial hypotension with mean arterial pressure less than 70 mm Hg, with reflex tachycardia. Also, there are clinical signs of tissue hypoperfusion, including cutaneous hypoperfusion with cold clammy skin, renal hypoperfusion with resulting oliguria (urine output [UOP] <0.5 ml/kg/h) and neurologic hypoperfusion with altered mental state. Tissue hypoperfusion leads to anaerobic tissue metabolism with hyperlactatemia (>1.5 mmol/L) [Citation1].
Assessment of hemodynamic status and lines of management of the acute circulatory shock remains a challenging issue in emergency medicine and critical care. As the use of invasive hemodynamic monitoring declines, bedside-focused ultrasound (US) has become a valuable tool in the evaluation and management of patients in shock [Citation2].
Different techniques are employed to assess the endpoint of fluid therapy which include physical examination (blood pressure [BP], UOP, dryness of tongue, etc.), central venous pressure (CVP) measurement, biochemical markers, estimate of the vascular pedicle width, pulmonary artery catheters, sonographic inferior vena cava (IVC) diameter assessment, and appearance of B-lines in lung sonar [Citation3]. Lung US is increasingly becoming a diagnostic tool in the critical care setting, providing standardized data [Citation4]. This study aimed to evaluate the role of lung US in comparison to CVP in assessment of endpoint of fluid therapy in patients with hypovolemic shock.
2. Patients and methods
Study design
This observational cross-sectional study was carried out in the intensive care unit (ICU), Benha University Hospital and approved by The Ethical Committee of Benha University. The study included 60 patients with hypovolemic shock admitted to the ICU in Benha University Hospital, from June 2019 till August 2020. A written informed consent was taken from patients relatives.
Inclusion criteria: ASA I–III, ICU patients above 18 years old, non-intubated, non-ventilated with hypovolemic nonhemorrhagic shock (mean arterial BP <65 mmHg and tachycardia (defined as heart rate >100 beats/minute) [Citation5].
Exclusion criteria: Patients under 18 years, patients with obstructive shock, cardiogenic shock, and morbid obesity (body mass index above 50 kg/m2), suspected or diagnosed raised intra-abdominal or intrathoracic pressures as pregnancy, portal hypertension or mediastinal mass, intracerebral hemorrhage or increased intracranial pressure, valvular heart disease or atrial fibrillation.
Patients′ demographic data: age, sex, body weight, and height were reported.
Patient assessment and hemodynamic monitoring
Full history taking, complete clinical examination, cause of hypovolemia were recorded. Laboratory investigation including complete blood count, liver function tests, kidney function tests, random blood sugar (RBS) were performed on admission. Arterial blood gas (ABG) and serum lactate were recorded on admission and at the end of fluid resuscitation. Supine chest radiography and transthoracic echocardiography are to exclude cardiogenic and obstructive shock.
Noninvasive arterial BP measurement (systolic, diastolic, and mean), electrocardiography, and pulse oximetry using the multichannel monitor were applied.
Central venous catheter was inserted to measure CVP, and urinary catheter was inserted to calculate UOP.
Fluid resuscitation: according to Messina et al. [Citation6]
All patients received 1000 ml of lactated Ringer’s solution rapidly infused within 10–15 min and then reassessment of patient was done using chest US. CVP measurement, BP measurement, and UOP were calculated and compared with each other.
According to the patient condition (CVP measurement), additional 1000 ml of lactated Ringer’s solution was infused within 1 h. During this infusion, reassessment of the patient was done every 15 min. Then, according to the patient condition (CVP measurement), 200 ml of lactated Ringer’s solution was infused within 10 min; then, reassessment was done. According to the assessment results, additional 200 ml was infused and final reassessment was done.
Fluid infusion was stopped if CVP increased to a value ≥12 cm H2O [Citation7].
Lung US and quantification of B-line score: according to Enghard et al. [Citation4]:
Lung US was done for all patients before giving any fluids using (Philips Hd5 color Doppler US machine, 2013 with 17-mm curved probe 1–5 MHz, 21 mm phase array), and patients were given a score according to the simplified protocol (). This protocol entails that the patients should be in the supine position while being scanned. Four areas are determined anatomically, and then scanned by the US: the third and the fourth intercostal spaces (ICSs) in the right side, and the sixth and the seventh spaces in the left side between the parasternal and midclavicular line (). Counting B-lines (either one or more) of the four ICSs was done, and a total score was given from 0 to 32. After giving a score, fluid resuscitation was started.
Table 1. Ultrasound-scoring system [Citation4]
Figure 1. Scheme of the four parasternal points corresponding to the intercostal spaces between the third and fourth ribs and between the sixth and seventh ribs used to calculate the ultrasound score [Citation4]
![Figure 1. Scheme of the four parasternal points corresponding to the intercostal spaces between the third and fourth ribs and between the sixth and seventh ribs used to calculate the ultrasound score [Citation4]](/cms/asset/9bc1b9ca-fcef-44a3-b616-bc723654eef8/teja_a_1906566_f0001_b.gif)
Data collection
Reassessment and scoring of B-lines were repeated during fluid resuscitation. Chest US score was correlated with CVP and BP measurements and UOP calculations on admission, during fluid resuscitation, and at the end of fluid resuscitation. The US score was evaluated in comparison to CVP at the end of fluid resuscitation to assess the endpoint of fluid therapy.
Statistical analysis
Data were analyzed using SPSS software, version 22.0 (IBM, Armonk, NY, USA) for Windows. Categorical data were presented as number and percentages. Quantitative data were tested for normality using the Shapiro–Wilks test assuming normality at P > 0.05. Normally distributed variables were expressed as mean ± standard deviation, while nonparametric ones were presented as median and interquartile range (IQR), and analyzed by Wilcoxon test for matched variables. Linear association between variables was assessed by Spearman’s correlation coefficients for nonparametric variables.
(rho) Spearman’s correlation coefficient: it evaluates the linear association between two quantitative variables (one is the independent variable X, and the other is the dependent variable, Y).
Receiver operating characteristic curves (ROC) were used to assess the validity and predictively of lung US for fluid end point. Two-sided P ≤ 0.05 was considered significant
P value >0.05 = insignificant
P < 0.05 = significant
P < 0.001 = highly significant
Sample size calculation
MedCalc software version 16.1(© 19,932,016 MedCalc Software bvba) was used to calculate the required sample size using area under the curve (AUC) of US parameter according to Shalaby et al. [Citation8] where the following parameters were entered in the program:
Level of significance (type I error) = 0.05,
Type II error (1-level of power) = 0.2
AUC = 0.746,
Null hypothesis value of 0.53
So, the sample size is at least 52 patients.
3. Results
The study initially included 72 patients assessed for eligibility, 12 patients were excluded (5 patients not meeting the inclusion criteria, 3 patients relatives refused participation, and 4 patients had valvular heart disease). The study finally included 60 patients. Patients aged from 37 to 91 years with a mean value of 68.6 ± 11.4. The number of male cases were 40(66.7%) and the number of female cases were 20(33.3%).
ABGs and serum lactate were recorded on admission and at the end of resuscitation. PH on admission ranged from 7.23 to 7.32 (median 7.29) and significantly increased (p < 0.001) at the end of resuscitation to range from 7.39 to 7.42 (median 7.39). Serum lactate on admission ranged from 2.3 to 2.6 mmol/L (median 2.5) and significantly decreased (p < 0.001) to range from 1 to 1.4 mmol/L (median 1.1) at the end of resuscitation (data not shown).
Patients’ CVP on admission ranged from −3 to 5 cm H2O and gradually increased during fluid resuscitation. Lung US score at admission ranged between 0 and 3 and gradually increased from 16 to 17 at the end of resuscitation. Patients’ UOP was zero in all patients on admission and it gradually increased with fluid resuscitation.
These data are shown in .
Table 2. Descriptive statistics for CVP values, lung US score, and UOP values over the period of resuscitation
On admission, patient’s SBP ranged between 40 and 80 mmHg and diastolic blood pressure (DBP) ranged between 30 and 50 mmHg. At the end of resuscitation, it gradually increased with fluid therapy to be 110–120 mmHg SBP and 80–90 mmHg DBP. These data are shown in .
Table 3. Descriptive statistics for blood pressure values over the period of resuscitation
CVP, UOP, and BP measurements were correlated with lung US score. There is a highly significant positive correlation (p < 0.001) between lung US and CVP at admission, after the first 1000 ml; after 15, 30, 45 and 60 min of the second 1000 ml infusion; and after the second additional 200 ml infusion. In addition, there is a significant positive correlation of lung US with BP and UOP measurements at many different stages of fluid resuscitation. These data are shown in .
Table 4. Correlation of lung US with CVP, blood pressure, and UOP
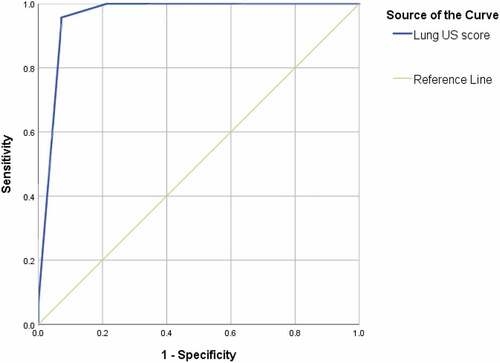
ROC curve analysis () was done for assessment and validation of endpoint (score ≥16) of fluid therapy using US in patients with hypovolemic shock. The AUC was 0.961, the sensitivity was 95.7%, specificity was 92.9%, the positive predictive value was 97.8%, the negative predictive value was 86.7%, and the total accuracy was 95%.
4. Discussion
Shock is a common life-threatening, generalized form of acute circulatory failure in critically ill patients, which is usually managed by infusing fluids to increase cardiac output and supply the systemic oxygen. International guidelines recommend use of an aggressive fluid resuscitation in the early phases of shock. In this context, crystalloids, including balanced solutions are suggested as first-line fluid therapy. Fluid therapy should be paired with timely monitoring of clinical and metabolic signs of shock [Citation6].
Patients with hypovolemic shock have severe hypovolemia with decreased peripheral perfusion. If left untreated, these patients can develop ischemic injury of vital organs, leading to multisystem organ failure. When etiology of hypovolemic shock has been determined, replacement of blood or fluid loss should be carried out as soon as possible to minimize tissue ischemia [Citation9].
Assessing hemodynamic function in acute circulatory failure is the routine work of the intensivist. Assessment tools include CVP measurement, analysis of IVC, continuous cardiac output devices, esophageal doppler, pulse pressure variation, oxygen transport assessment, analysis of tissue oxygenation, gastric tonometry, transthoracic echocardiography, laser doppler flowmetry, near-infrared spectroscopy, and the less commonly used pulmonary artery catheterization [Citation10].
The CVP remains the most frequently used variable to guide fluid resuscitation in critically ill patients [Citation11]. It is an indicator of right ventricular and, to a lesser extent, left ventricular preload. It reflects the limit to venous return and informs about right ventricular function. CVP is affected by thoracic, pericardial, and abdominal pressures making its interpretation more complicated [Citation12]. All these factors were excluded in the current study.
Lung US was introduced in the critical care practice since 1989, due to the pioneering work performed in Francois Jardin’s ICU [Citation10]. The FALLS-protocol (Fluid Administration Limited by Lung Sonography) is a tool proposed for the management of unexplained shock using lung US [Citation13]. It exploits the ability of US to detect interstitial syndrome which always precedes alveolar edema. The B-line is a certain comet-tail artifact. Multiple B-lines in one view are called lung rockets. Disseminated lung rockets define interstitial syndrome. The normal lung surface displays horizontal artifacts called A-lines [Citation14].
Lung US is not only a faster way of assessing EVLW but it can also differentiate between adult respiratory distress syndrome (ARDS) (as a prototypic form of permeability-type edema) and cardiogenic edema [Citation15]. As cardiogenic edema presents as a uniform distribution of B-lines with normal lung sliding and homogenous pleural effusions, patients with ARDS present heterogeneous distribution of B-lines with pleural line abnormalities, lack of lung sliding, uneven tissue patterns such as “spared areas” and consolidations [Citation16].
Other advantages of lung US include decreasing medical irradiation (most CTs in ARDS or trauma can be postponed), a use in traumatology, ICU, neonates (the signs are the same as in adults), poor countries, and a help in any procedure as thoracentesis. Lung US can be simply used by the intensivist, anesthesiologists, neonatal intensivists, pediatricians, emergency physicians, and others (pulmonologists, cardiologists, nephrologists, etc.) as the lung is a common target in these disciplines [Citation17,Citation18].
The aim of the present study was to evaluate the role of lung US in assessment of endpoints of fluid therapy in comparison to CVP as a gold standard in patients with hypovolemic shock.
An observational cross-sectional study was conducted on 60 patients with hypovolemic shock admitted to the ICU in Benha University Hospital. Patients age ranged from 37 to 91 years, with a mean value 68.6 ± 11.4 years. Male cases were more than female cases (40, 66.7%) versus (20, 33.3%).
Also in Enghard et al.’s [Citation4] study, the majority of their studied group were males (64%) with a mean age of 62 years. This can be explained by Piras [Citation9], who stated that elderly patients are more likely to experience hypovolemic shock as they have a less physiologic reserve.
ABGs and serum lactate were recorded on admission and at the end of resuscitation. Metabolic acidosis and serum lactate significantly improved at the end of resuscitation (p < 0.001 for both).
Ziglar [Citation19] reported that tissue hypoperfusion leads to metabolic acidosis and increased lactate level which rapidly normalize after adequate fluid resuscitation.
In the current study, patients’ CVP on admission ranged from −3 to 5 cm H2O and gradually increased during fluid resuscitation. Patients UOP was zero in all patients on admission and it gradually increased with fluid resuscitation. On admission, systolic blood pressure (SBP) ranged between 40 and 80 mmHg and DBP ranged between 30 and 50 mmHg. At the end of resuscitation, it gradually increased with fluid therapy to be 110–120 mmHg SBP and 80–90 mmHg DBP.
Our results are supported by the study by Moussa et al. [Citation20] who reported that, fluid administration resulted in significant increase in mean arterial pressure, pulse pressure and UOP (p < 0.01). Changes in BP were positively correlated with changes in UOP and mean arterial pressure.
In the current study, lung US score at admission ranged between 0 and 3 and start to increase to range from 16 to 17 at the end of resuscitation. There was a high significant correlation of lung US score with BP, CVP, and UOP measurements. The sensitivity of lung US score ≥16 in assessment of endpoints of fluid therapy in relation to CVP is 95.7%, specificity is 92.9%, PPV is 97.8%, and NPV is 86.7% with an accuracy of 95%.
Our results were supported by Enghard et al. [Citation4]. They stated that the CVP was significantly correlated (P = 0.4924) with the presence and extent of pulmonary B-lines and concluded that the US score ≥15 had a sensitivity and specificity of 92.1% and 91.7%, respectively, for diagnosing an extravascular lung water (EVLW) index above the normal value of 7 ml/kg (AUC = 0.9419).
Our results were also supported by the study of Ismail et al. [Citation21]. They concluded that the best cutoff value for US score that best represents the endpoint of fluid resuscitation was more than 10 with a significant correlation with hypoxic index and CVP readings (P = 0.001), and it showed sensitivity and specificity of LU of 84.21% and 90.48%, respectively.
Other studies revealed good sensitivity and specificity regarding the correlation of B-lines with EVLW using US waves [Citation22,Citation23].
Mayr et al. [Citation24] evaluated the scoring of 28-sector and 4-sector B-Lines US protocols in 50 critically ill patients. They reported significant positive correlation of the 4-sector B-Lines protocol with patient CVP (p = 0.039), EVLW index (p < 0.001), and cardiac index (p = 0.120). The 4-sector B-Lines protocol at endpoint ≥15 shows a sensitivity of 91.7%, AUC of 0.978 and specificity of 92.1% for identification of EVLW index ≥15 ml/kg (severe edema). They concluded that both B-line protocols provide accurate noninvasive evaluation of lung water in critically ill patients.
Multiple anterior diffuse B-lines with lung sliding indicated pulmonary edema with a sensitivity of 97% and a specificity of 95%. The same authors were able to discriminate patient with chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism, pneumothorax, or pneumonia with an overall correct diagnosis in 90.5% of cases when compared with conventional diagnostic tools [Citation25].
LU has proven its superiority over other diagnostic invasive and noninvasive imaging techniques. Specifically, it provides a higher diagnostic value, cost-effective, and easy to perform directly at the patient’s bedside [Citation25–27].
In addition, Agricola et al. concluded that the presence and the number of comet-tail images provide reliable information on interstitial pulmonary edema. Therefore, ultrasonography represents an attractive, easy-to-use, bedside diagnostic tool for assessing cardiac function and pulmonary congestion [Citation28].
Saad et al. [Citation29] highlight the potential role of lung US to guide fluid therapy and early diagnosis of overhydration. Volume overload poses an independent risk factor for death due to cardiovascular events in ICU patients.
The BLUE (Bedside Lung US in Emergency) protocol is a fast protocol (<3 min), which allows diagnosis of acute respiratory failure. It can discriminate patients with clinical emergencies including pulmonary edema, pulmonary embolism, pneumonia, COPD, asthma, and pneumothorax; each yields a specific profile [Citation17].
5. Conclusion
Lung US provides a simple noninvasive approach in assessment of endpoint (score ≥16) of fluid therapy in patients with hypovolemic shock with high sensitivity and specificity.
Financial support and sponsorship
This paper was not funded.
Conflicts of interest
There are no conflicts of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jl V, De BD. Circulatory shock. N Engl J Med. 2015;369(18):1726–1734.
- Ga O, Rl C, Jl V. What type of monitoring has been shown to improve outcomes in acutely ill patients? Intensive Care Med. 2008;34:690–800.
- Durairaj L, Ga S. Fluid therapy in resuscitated sepsis less is more. Chest. 2008;133(1):252–263.
- Enghard P, Rademacher S, Nee J, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19(36):1–8.
- Cherpanath TG, Aarts LP, Groeneveld JA, et al. Defining fluid responsiveness: a guide to patient-tailored volume titration. J Cardiothorac Vasc Anesth. 2014;28(3):745–754.
- Messina A, Greco M, Cecconi M. Fluids in shock. 2018;18(3):154–157. ICU Management & Practice.
- Cole E. Measuring central venous pressure. Nurs Stand. 2007;22(7):40–42.
- Shalaby MI, Roshdy HM, Elmahdy WM, et al. Correlation between central venous pressure and the diameter of inferior vena cava by using Ultrasonography for the assessment of the fluid status in intensive care unit patients. Egypt J Hosp Med. 2018;72(10):5375–5384.
- Piras C. Hypovolemic shock. International Physical Medicine and Rehabilitation Journal. 2017;2(3):240–242.
- Lichtenstein D. Lung Ultrasound, a Holistic Discipline. The example of the BLUE-Protocol. Ultrasound Med Biol. 2017;43:S30.
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529–1537.
- Chen KP, Cavender S, Lee J, et al. Peripheral edema, central venous pressure and risk of AKI in critical illness. Clin J Am Soc Nephrol. 2016;11(4):602–608.
- Da L. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147(6):1659–1670.
- Copetti R, Copetti P, Reissig A. Clinical integrated ultrasound of the thorax including causes of shock in non-traumatic critically ill patients. A practical approach. Ultrasound Med Biol. 2012;38(3):349–359.
- Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6(1):16.
- Grune J, Beyhoff N, Hegemann N, et al. From bedside to bench: lung ultrasound for the assessment of pulmonary edema in animal models. Cell Tissue Res. 2020;380(2):379–392.
- Da L. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1–12.
- Lichtenstein D, Mauriat P. Lung ultrasound in the critically ill neonate. Curr Pediatr Rev. 2012;8(3):217–223.
- Mk Z. Application of base deficit in resuscitation of trauma patients. International Journal of Trauma Nursing. 2000;6(3):81–84.
- Moussa MD, Scolletta S, Fagnoul D, et al. Effects of fluid administration on renal perfusion in critically ill patients. Crit Care. 2015;19(1):230–250.
- Ismail RM, Dahroug AH, Zaytoun TM. Determination of endpoint of fluid resuscitation using simplified lung ultrasound protocol in patients with septic shock. 2019;68(1):102–107. The Egyptian Journal of Chest Diseases and Tuberculosis.
- Shyamsundar M, Attwood B, Keating L, et al. Clinical review: the role of ultrasound in estimating extra-vascular lung water. Crit Care. 2013;17(5):237.
- Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19(3):356–363.
- Mayr U, Lukas M, Habenicht L, et al. B-Lines scores derived from lung ultrasound provide accurate prediction of extravascular lung water index: an observational study in critically ill patients. J Intensive Care Med. 2020;088506662096765. First published online. doi:10.1177/2F0885066620967655
- Lichtenstein DA, Ga M. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117–125.
- Abdalla W, Elgendy M, Abdelaziz AA, et al. Lung ultrasound versus chest radiography for the diagnosis of pneumothorax in critically ill patients: a prospective, single-blind study. Saudi J Anaesth. 2016;10(3):265–269.
- Brogi E, Bignami E, Sidoti A, et al. Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound. 2017;15(1):23.
- Agricola E, Bove T, Oppizzi M, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extavascular lung water. Chest J. 2005;127(5):1690–1695.
- Mm S, Kamal J, Moussaly E, et al. Relevance of B-lines on Lung ultrasound in volume overload and pulmonary congestion: clinical correlations and outcomes in patients on hemodialysis. Cardiorenal Med. 2018;8(2):83–91.