ABSTRACT
Background
Intrathecal morphine (ITM) has proven to be excellent in reducing postoperative pain. However, its use is associated with the occurrence of postoperative nausea and vomiting (PONV). In this study, we wish to compare the efficacy between the combination therapy of granisetron and dexamethasone versus granisetron alone on the occurrence of postoperative nausea and vomiting (PONV) in parturients undergoing elective Caesarean delivery.
Method
This is a prospective double-blinded, randomised controlled trial (RCT) involving 126 parturients of American Society of Anesthesiologist (ASA) physical status I and II undergoing elective Caesarean deliveries. Subjects were randomly allocated into 2 groups (n = 63), to either receive a combination of 1 mg intravenous (IV) granisetron plus 4 mg IV dexamethasone (Group A) or to receive 1 mg IV granisetron (Group B). They were assessed at 1, 4, 8, 12 and 24-hour postoperatively. Episodes of nausea, retching, vomiting and the requirement of rescue antiemetics at these time intervals were recorded.
Results
There are no statistically significant differences in the occurrence of nausea, retching and vomiting between both groups at 1, 4, 8, 12 and 24 h postoperatively. The usage of rescue antiemetics were similar in both groups of subjects.
Conclusion
The use of granisetron is comparable with the use of granisetron and dexamethasone in the prevention of PONV in parturients receiving intrathecal morphine for elective Caesarean section.
1. Introduction
Low dose intrathecal morphine is proven efficient as a mode to reduce postoperative pain in many surgical areas including Caesarean delivery [Citation1–4]. In recent years, hospitals in Malaysia adapt to the administration of a single low-dose intrathecal morphine (ITM) routinely during Caesarean deliveries.
The supplementation of morphine, however raises the occurrences of postoperative nausea and vomiting (PONV) in these patient [Citation5]. To tackle this problem, we comply to the combination therapy of antiemetics [Citation6–8]. It is a standard practice in Malaysian hospitals whereby a serotonin receptor antagonist of either intravenous (IV) ondansetron or granisetron [Citation7] with IV dexamethasone is administered after the administration of intrathecal morphine.
Despite being routinely used, the evidence in the efficacy of a serotonin antagonist and corticosteroid combination in parturients are conflicting and lacking. In this study, we would like to assess and compare the occurrences of PONV in patients undergoing Caesarean deliveries supplemented with ITM in those given a combination of IV granisetron-dexamethasone versus that of IV granisetron alone.
2. Methods
This prospective, double-blind, randomised study conducted in Hospital Universiti Sains Malaysia, Kelantan was approved by the Human Research Ethics Committee USM (HREC), JEPeM Code: USM/JEPeM/18090426 and registered at ClinicalTrials.gov (ID NCT04570592)
Informed and written consents were obtained from 126 parturients aged between 18 to 40 years old who were American Society of Anesthesiology (ASA) physical status I and II undergoing elective Caesarean deliveries from the 1st of June 2019 till the 30th of January 2020. Subjects who had coagulopathy, uncorrected hypovolemia, neurologic disease, infection at site of injection and raised intracranial pressure were excluded from the study. Others who were contraindicated for either morphine, granisetron or dexamethasone administration, morbidly obese patients with BMI of 40 kg/m2 or more were also excluded. Patients’ information including gravity, parity, age, weight and height were obtained. Their smoking status, history of PONV or motion sickness were also elicited to allow the calculation of the APFEL score [Citation9]. This score includes four predictors which are the female gender, nonsmoking, history of PONV or motion sickness and postoperative opioids use. Higher sum score correlates with higher risk of PONV. Those who needed conversion to general anaesthesia or admitted to ICU post-surgery were withdrawn from the study.
Subjects were randomised using a computer-generated randomisation software using block size 3 into two groups: group A and group B each consisting of 63 subjects. The order of interventions within each block was random as determined by computer random number generator. This technique was chosen to ensure similar numbers of patients in each group at any point during the study. Both patients and the assessors of PONV which are the Acute Pain Service (APS) team were blinded.
Patients were then subjected to fasting 6 hours prior to surgery. IV metaclopromide 10 mg, IV ranitidine 50 mg and per oral sodium citrate 0.3 M 30 mls were administered as part of the acid aspiration prophylaxis.
Standard monitoring with continuous noninvasive blood pressure monitoring (NIBP), electrocardiogram (ECG), respiratory rate and pulse oximetry (SpO2) was undertaken throughout anaesthesia.
Ringer’s lactate solution 10 ml/kg was infused for co-loading [Citation10,Citation11]. Spinal anaesthesia was administered in the sitting position. We implemented a height-based dose for 0.5% heavy bupivacaine administration. Based on the subject’s height, those who were less than 150, 151–154 cm and those who were more than 155 cm received 1.5mls, 1.7mls and 1.9mls heavy bupivacaine 0.5% (Astra Zeneca ®), respectively. Intrathecal fentanyl 15–20 mcg and morphine 0.1 mg were added to supplement the spinal anaesthesia.
On the completion of spinal anaesthesia, 4 mg of IV dexamethasone was administered to parturients in group A while group B received 1 ml of normal saline injected intravenously. IV granisetron 1 mg was administered to both groups of subjects after cord clamping. These drugs were given by the anaesthetist administering the spinal anaesthesia. The anaesthetist in charge was not blinded as this was important to ensure safety of patients should any complication arises during the delivery of anaesthesia. Patients were given Diclofenac suppository 100 mg as long as the parturient weighed 50 kg or more and Diclofenac suppository 50 mg if the parturient weighed 50 kg or less. The weight was measured before delivery during the preoperative assessment of the vital signs of the patient.
Subjects were then transferred to the recovery room and monitored for an hour. Tablet (t.) Paracetamol 1 g qid and t. Voltaren 50 mg tds were provided to patients to postoperatively to supplement ITM as the postoperative analgesia. The use of opioids was avoided during the first 24 hours of intrathecal morphine administration. Both patients and the assessors of PONV who are the Acute Pain Service (APS) team were blinded to the study drugs. Subjects were monitored for complications of spinal ITM for the first 24 hours.
Assessment was made at 1-h post-surgery, 4 hourly for the next 12 h and at 24 h after surgery. Episodes of nausea, retching and vomiting were recorded at each assessors’ visit. Nausea was described as an unpleasant sensation related to the urge to vomit. Retching was defined as an involuntary effort to vomit but without the expulsion of the gastric contents, while vomiting is when expulsion of gastric contents occurs.
The requirement of rescue antiemetics at each time interval was also documented. Rescue antiemetic was given when a patient developed one or more episodes of vomiting. IV ondansetron 4 mg is given to group A while either IV dexamethasone 4 mg or iv ondansetron is given to group B.
Sample size was calculated based our assumption that the combination of a serotonin receptor antagonist with a corticosteroid would be superior than its monotherapy and thus, the addition of IV dexamethasone to granisetron would reduce the incidence of PONV from 20% to 3.3% [Citation12,Citation13]. Calculation was made using the PS software implementing a dichotomous, independent, prospective, 2 proportions testing to compare prevalence with significance level, ⍺ = 0.05 and power of 80%. The calculated sample required for each group is 57, and with the additional 10% for dropout rate, this study required 63 patients in each group, giving a total of 126 patients.
Data analysis was performed using SPSS version 24 for MAC. The incidences of nausea, retching, vomiting and the use of rescue antiemetic were analysed using Chi-Square test. Data are presented as percentage and a p-value of <0.05 is considered statistically significant.
3. Results
A total of 126 parturients were randomly allocated into group A and B. Data from 114 patients were analysed as in both groups of patients, 6 were excluded from analyses as they were loss to follow-up.
The baseline characteristics between both groups of subjects were comparable. The mean age, weight, height and BMI, gravidity and parity and ASA classification in group A and group B has a p-value of > 0.05. All participants had an APFEL score of 3, thus no comparison was computed as this variable was a constant ().
Table 1. Patients’ baseline characteristics
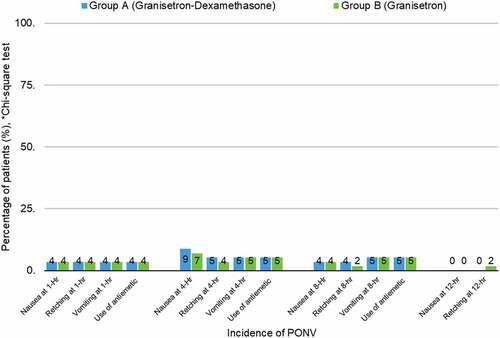
From the analysis, there is no statistically significant differences on the occurrences of PONV between both groups at 1, 4, 8 and 12 h postoperatively with a calculated p-value >0.999. It is observed that the occurrence of PONV at 1-h postoperatively was similar between both groups.
At 4-h postoperatively the occurrence of nausea and retching in group A was slightly more than in group B (8.8% vs 7%) but the incidence of vomiting was again similar between both groups (p = 0.999). At 8-h postoperatively, group A recorded a slightly higher occurrence of retching than group B (3.5% vs 1.8%) but then again, this is also statistically insignificant (p = 0.999). There was an incident of retching in group B, but there was no episode of PONV seen in patients in group A (1.8% vs 0, p = 0.999).
The use of rescue antiemetic between both groups were equal at 1, 4, and 8-h post-surgery. No episodes of vomiting were recorded during the 12-h surgery and thereafter ( and ).
Table 2. Incidence of PONV at 1, 4, 8, 12 and 24-hour postoperatively
4. Discussion
From this study, it is revealed that there are no significant differences in the occurrence of PONV after spinal anaesthesia supplemented by ITM between parturients who received the combination of IV granisetron and dexamethasone to those who received IV granisetron alone. Other than an episode of retching in group B at 12-h postoperatively, there was no further documented episode of PONV at 12-h post-surgery and thereafter.
PONV is one of the complex entities in anaesthesia. Its pathophysiology involves various receptors and afferent pathways, in which the primary ones are the stimulation of vagal mucosal pathway of the gastrointestinal system by paracrine activity, the stimulation of the chemoreceptor trigger zone (CTZ) [Citation14], the vestibular system [Citation15], area postrema of the midbrain [Citation16] and afferent pathways from the cerebral cortex. Inhibition of any of these pathways would prevent transmission of impulses to the nucleus tracts solitarius (NTS), thus avoiding emesis.
In this study, all parturients had an APFEL score of 3 meaning that they were high risk in developing PONV. Despite this, those who received IV granisetron alone had comparable incidences of PONV to those received both IV granisetron and dexamethasone.
This finding could partly be explained by the pharmacology of granisetron. Granisetron is a potent and selective serotonin receptor antagonist acting on the 5-HT3 receptor. The antiemetic effect is through the blockade of the vagal efferent nerves in the gut which in turn inhibits the visceral afferent stimulation of the vomiting center. As it causes irreversible blockade of the 5-HT3 receptors when administered as a single dose for the provision of PONV, its duration of action is prolonged and effective up to 24 hours [Citation17].
Secondly, this result may be explained by the structure and methodology of this study which included careful subject selection, a homogenous study population and a meticulous methodology. By controlling the above parameters, we were allowed to control our confounding factors that may attribute to the occurrence of PONV. The causes of PONV are multiple. It can be influenced by factors related to the patients, anesthesia, and surgery. Females, non-smoker with history of PONV or motion sickness are considered those bearing high risks for the development of PONV [Citation9,Citation18]. Anesthetic-related factors include the use of nitrous oxide, inhalational agents, perioperative opioids, hypotensive episodes [Citation19] and protracted surgery [Citation20]. The use of spinal anesthesia in this study negates the effects of general anesthesia that would include the use of inhalation agents and nitrous oxide.
Patients enrolled were parturients aged between 18 and 45 years of either ASA I or ASA II. Patients who were classified as ASA III or IV including those with cardiac or chronic lung diseases were not recruited. The selection of these cohort of parturients would allow us to reduce the possibility of hemodynamic instabilities under spinal anaesthesia. Furthermore, subjects were co-loaded with crystalloids as preoperative corrections of intravascular volume deficits attenuates both hypotension and PONV. In addition, the dose for spinal anesthesia administration is controlled. In this study, we adopted the height-based dose of heavy bupivacaine 0.5% for spinal anesthesia to provide sufficient anesthesia at the same time without giving rise to significant hypotension.
We do acknowledge the limitation in terms of accurate comparison in terms of rescue antiemetics protocols received by parturient. Group A received IV Dexamethasone 4 mg and IV Granisetron 1 mg intraoperatively as opposed to Group B that received only IV Granisetron 1 mg intraoperatively. Hence, in Group B the rescue protocol differed to include an option of either IV Dexamethasone or IV Ondansetron. Although less than ideal, there may be bias in terms of group blindness and accurate comparison.
Dosage of Granisetron that is used in this study is a IV Granisetron 1 mg bolus dose. This is the recommended by the Ministry of Health Malaysia drug formulary for prevention of postoperative nausea vomiting [Citation21]. Hence, it is used as we are running a clinical trial of parturients and safety is of our utmost concern.
The results of our study were found to be similar to several published studies. In 2013, Ryu et al [Citation22] compared the incidence of PONV in 72 patients who underwent laparoscopic surgeries. They revealed that the incidence of PONV was comparable in those received ramosetron alone to those receiving ramosetron and dexamethasone. Likewise, in 2016 a meta-analysis of published randomised controlled trials showed that the combination of either ramosetron or granisetron to dexamethasone was none superior than the 5-HT3 antagonist alone [Citation23] in the attenuation of PONV episodes.
In addition, several interventions pertaining to parturients drew similar conclusions. Demirhan and colleagues in 2013 concluded that the combination of ondansetron and dexamethasone does not seem to increase the antiemetic efficacy in a study involving 86 parturients [Citation24]. Another study assessing the efficacy of a combination therapy of 5-HT3 receptor antagonist and dexamethasone to a 5-HT3 antagonist monotherapy in preventing PONV was conducted by Swaro et al [Citation25]. They compared palonosetron and dexamethasone to palonosetron alone in parturients undergoing Caesarean deliveries and concluded that the combination of palonosetron and dexamethasone proved no better than palonosetron alone (p = 0.29) [Citation25].
From this study, we could safely conclude that the combination of granisetron and dexamethasone is comparable to granisetron monotherapy in the prevention of PONV and with the evidence at present, it is sufficient to say that granisetron may be administered alone without the addition of dexamethasone when being used as PONV prophylaxis.
This is profoundly captivating and clinically significant. Firstly, the unnecessary exposure of dexamethasone to the patients could be completely avoided. The role of dexamethasone in clinical practice is established owing to its potent glucocorticoid activity. It is cheap and readily available. Dexamethasone is used across multiple medical discipline ranging from the treatment of acute and emergency conditions of bronchial asthma and anaphylaxis to treating the chronic conditions of rheumatoid arthritis. It is also being administered to accelerate lung maturation in fetuses in women at risk of premature labour. Whilst the side-effect of dexamethasone is related to its long-term application, the safety on its single-dose perioperative usage is not without concern. The administration of dexamethasone was associated with raised glucose level in both non-diabetic and diabetics [Citation26–28] despite being given as a single intravenous dose. Others found its association with delayed wound healing and possible postoperative infection [Citation29,Citation30].
Any intervention toward the parturient could indirectly affect the fetus. The submission of intravenous dexamethasone to fetuses in mid-late pregnancy may lead to intrauterine-growth restriction [Citation31] and neonatal hypoglycaemia [Citation32] although these are seen with repeated doses. Whilst the benefits of dexamethasone are obvious, the nonessential administration of this drug should be avoided to negate any possible side effects to both the patients and fetuses.
More areas are remained to be explored with regards to this study. Firstly, whilst this study suggests that granisetron monotherapy is comparable to the combination therapy of granisetron and dexamethasone for PONV prevention in parturients, one question remains. Can this result translate with the use of other 5-HT3 antagonist? As far, sparse and conflicting evidence from the literature were seen as earlier studies and recommendations supported the use of the combination therapy [Citation7]. However, one metanalysis of RCTs conducted by Awad and colleagues in 2016 [Citation23] revealed that 5-HT3 antagonist monotherapy with either granisetron and ramosetron were comparable to their combination therapy with dexamethasone but this result were not seen with other 5-HT3 antagonists.
Furthermore, with dexamethasone may be dispensable from the combination therapy, studies should be conducted to assess the cost-effectiveness of using granisetron as single antiemetic to prevent PONV. If it is found to be beneficial, this could lift a part of the economic burden to low and middle resourced countries.
5. Conclusion
The combination of intravenous granisetron and dexamethasone is non-superior than intravenous granisetron alone, thus the use of granisetron is comparable to the combination of granisetron and dexamethasone on diminishing the occurrence of PONV in parturients receiving ITM for Caesarean deliveries.
Acknowledgments
We would like to acknowledge funding received from Universiti Sains Malaysia Short Term Grant 304/PPSP/6315006
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abouleish E, N Rawal, Fallon K, Hernandez D. Combined intrathecal morphine and bupivacaine for cesarean section. Anesth Analg. 1988;67(4):370–374.
- Gwirtz KH, Young JV, Byers RS, et al. The safety and efficacy of intrathecal opioid analgesia for acute postoperative pain: seven years’ experience with 5969 surgical patients at Indiana University Hospital. Anesth Analg. 1999;88(3):599–604. .
- Cummings A, Orgill BD, Fitzgerald BM. Intrathecal Morphine. In: StatPearls. Treasure Island (FL); 2020.
- Sharpe EE, Molitor RJ, Arendt KW, et al. Intrathecal morphine versus intrathecal hydromorphone for analgesia after cesarean delivery: a Randomized Clinical Trial. Anesthesiology. 2020;132(6):1382–1391. .
- Dahl JB, Jeppesen IS, Jorgensen H, et al. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91(6):1919–1927.
- Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350(24):2441–2451. .
- Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. .
- Gupta P, Jain S. Postoperative nausea and vomiting prophylaxis: a comparative study of ondansetron, granisetron and granisetron and dexamethasone combination after modified radical mastectomy. Saudi J Anaesth. 2014;8(Suppl 1):S67–71.
- Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700.
- Maharaj CH, Kallam SR, Malik A, et al. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. 2005;100(3):675–682.
- Ni HF, Liu HY, Zhang J, et al. Crystalloid coload reduced the incidence of hypotension in spinal anesthesia for cesarean delivery, when compared to crystalloid preload: a meta-analysis. Biomed Res Int. 2017;2017:3462529.
- Gombar S, Kaur J, Kumar Gombar K, et al. Superior anti-emetic efficacy of granisetron-dexamethasone combination in children undergoing middle ear surgery. Acta Anaesthesiol Scand. 2007;51(5):621–624.
- Sinha R, Shende D, Maitra S, et al. Granisetron versus granisetron-dexamethasone for prevention of postoperative nausea and vomiting in pediatric strabismus surgery: a Randomized Double-Blind Trial. Anesthesiol Res Pract. 2016;2016:4281719.
- Minami M, Endo T, Hirafuji M, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99(2):149–165. .
- Catanzaro MF, Miller DJ, Cotter LA, et al. Integration of vestibular and gastrointestinal inputs by cerebellar fastigial nucleus neurons: multisensory influences on motion sickness. Exp Brain Res. 2014;232(8):2581–2589.
- Jovanovic-Micic D, Strbac M, Krstic SK, et al. Ablation of the area postrema and emesis. Metab Brain Dis. 1989;4(1):55–60.
- Wilson AJ, Diemunsch P, Lindeque BG, et al. Single-dose i.v. granisetron in the prevention of postoperative nausea and vomiting. Br J Anaesth. 1996;76(4):515–518. .
- Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. .
- Pusch F, Berger A, Wildling E, et al. The effects of systolic arterial blood pressure variations on postoperative nausea and vomiting. Anesth Analg. 2002;94(6):1652–1655.
- Koivuranta M, Laara E, Snare L, et al. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52(5):443–449.
- Cipta H Kementerian Kesihatan Malaysia.
- Ryu J-H, Chang J-E, Kim H-R, et al. Ramosetron vs. ramosetron plus dexamethasone for the prevention of postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: prospective, randomized, and double-blind study. Int J Surg. 2013;11(2):183–187.
- Awad K, Ahmed H, Abushouk AI, et al. Dexamethasone combined with other antiemetics versus single antiemetics for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: an updated systematic review and meta-analysis. Int J Surg. 2016;36(Pt A):152–163. .
- Demirhan A, Tekelioglu YU, Akkaya A, et al. Antiemetic effects of dexamethasone and ondansetron combination during cesarean sections under spinal anaesthesia. Afr Health Sci. 2013;13(2):475–482. .
- Swaro SKD, Karan D, Banerjee A. Comparison of palonosetron, dexamethasone, and palonosetron plus dexamethasone as prophylactic antiemetic and antipruritic drug in patients receiving intrathecal morphine for lower segment cesarean section. Anesth Essays Res. 2018;12(2):322–327.
- Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200.
- Toner AJ, Ganeshanathan V, Chan MT, et al. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–248.
- Polderman JA, Farhang-Razi V, Van Dieren S, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018;11:CD011940.
- Percival VG, Riddell J, Corcoran TB. Single dose dexamethasone for postoperative nausea and vomiting–a matched case-control study of postoperative infection risk. Anaesth Intensive Care. 2010;38(4):661–666.
- Snall J, Kormi E, Koivusalo AM, et al. Effects of perioperatively administered dexamethasone on surgical wound healing in patients undergoing surgery for zygomatic fracture: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(6):685–689. .
- McKinlay CJ, Crowther CA, Middleton P, et al. Repeat antenatal glucocorticoids for women at risk of preterm birth: a cochrane systematic review. Am J Obstet Gynecol. 2012;206(3):187–194.
- Gyamfi-Bannerman C, Thom EA. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;375(5):486–487.