ABSTRACT
Background
Using of the pneumatic tourniquet is a common practice in many surgeries. While it creates bloodless field, it is not free of complications. Tourniquet deflation is a critical stage, greatly affecting hemodynamics. Till now, no strict guidelines have been developed for managing such a procedure.
Methods
Sixty patients, >40 years old, undergoing total knee arthroplasty enrolled in this study. Tourniquet deflation done over 3 min before complete release, either by gradual pressure release 50 mmHg/30 s in gradual deflation (G) group or by three cycles of deflation-reinflation (deflation for 10 s and reinflation for 50 s) in intermittent deflation (I) group.
Results
No significant difference was found regarding patient demographics and basal data. Mean arterial pressure was significantly lower in G group at times of deflation and post deflation for 5 min (P values 0.011, 0.023, 0.024, 0.001, and 0.013, respectively). Also, heart rate and acid base parameters were more stable and convergent to basal data in I group.
Conclusion
Compared to gradual deflation, intermittent deflation of tourniquet in middle and old aged, total knee arthroplasty patients resulted in more stability in hemodynamics and acid base parameters.
1. Introduction
Pneumatic tourniquet is a compressive device that occludes blood flow to the limbs to create a bloodless surgical field and decrease the operative blood loss [Citation1]. Side effects such as venous congestion, blood stagnation, ischemic reperfusion, and pressure effects are affected by patient’s age, medical status, tourniquet pressure, tourniquet time, and tourniquet deflation way [Citation2].
Despite many years of tourniquet use in different types of surgery, there are no specific and binding strict guidelines for inflation pressure, duration time, and way of deflation in tourniquet use [Citation3]. Tourniquet deflation is a critical stage because it causes a sudden drop in central venous pressure, mean arterial pressure (MAP) and HR variability. Cardiac arrests have been reported following cuff deflation [Citation4,Citation5].
These hemodynamic changes are due to the combination of back shifting of blood volume into the limb and washout of metabolites from the ischemic limb into the systemic circulation causing hypotension, metabolic acidosis, hyperkalemia, myoglobulinemia, myoglobinuria, and possible renal failure. It is also known as “myonephropathic metabolic syndrome” and depends on the size of the extremity, duration of tourniquet time, and overall physiological status of the patient [Citation6,Citation7].
In this clinical trial, we compared two techniques of tourniquet deflation and their clinical implication on hemodynamics and acid base parameters in orthopedic patients undergoing total knee arthroplasty surgery under neuroaxial anesthesia. MAP was adjusted as the primary outcome objective of this trial.
2. Materials and methods
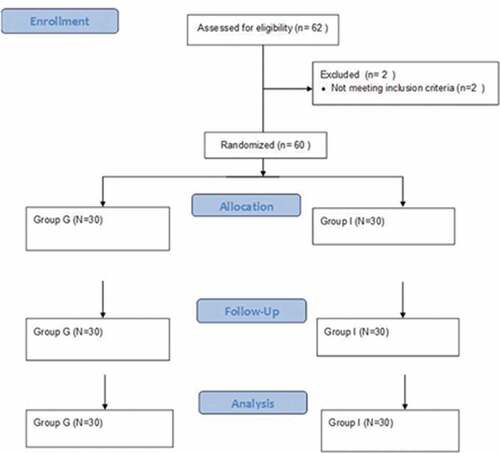
After approval of institutional research board (IRB-R.20.02.739, 12 February 2020), trial registration (PACTR202003900400089 March 17–2020) and obtaining a written informed consent from all participants, this study was carried out at Mansoura University Hospital. Sixty-two patients were enrolled in this study, which adheres to the applicable CONSORT guidelines (). Two patients were excluded due to complicated surgery (time exceeded 3 hdue to surgical difficulties). Included patients were adults of either sex, ASA I or II, and age >40 years undergoing total knee arthroplasty surgery. Exclusion criteria were patient refusal, major cardiopulmonary disorders, hypertension, hepatic or renal disorders, patient allergy to local anesthetic, risk dissemination of malignancy or infection, patient with relative contraindication for tourniquet use as peripheral vascular disease, sickle cell anemia, deep venous thrombosis, diabetic neuropathy and crushed injury, and complicated surgery extending more than 3 h. Random number generator with closed envelope technique randomized patients into two groups based on post-surgical deflation regimen: 30 patients for gradual deflation group (G group) and 30 patients for intermittent group (I group).
Preoperative anesthetic assessment was done at anesthesia clinic. ECG and ECHO were done to meet inclusion and exclusion criteria. Laboratory investigations included complete blood count, liver function test, kidney function test, International normalization ratio (INR), arterial blood gases, serum lactate, and serum potassium (K).
At operation suite, patients had second assessment for anesthetic fitness. A 20 G intravenous line was inserted in non-dominant hand to receive 500 ml ringer acetate as fluid co-loading before starting regional subarachnoid block, then transferring to operating room. After patients being connected to basic monitors, ECG, NIBP, SpO2, placed in sitting position to facilitate regional block. The researcher anesthesiologist started back scrubbing with betadine and alcohol, then introduced a 25 G spinal needle at L4-5 sub-arachnoid space and injected 12.5–15 mg heavy bupivacaine 0.5% (Marcaine spinal 0.5% heavy, Astrazeneca, England) plus 20 μg fentanyl (fentanyl Hameln, Hameln pharmaceuticals, Germany), according to patient’s height [2.5 ml heavy bupivacaine 0.5% (12.5 mg) for patients’ height <170 cm, 3 ml heavy bupivacaine 0.5% (15 mg) for patients’ height >170 cm]. Later, the patient was placed in supine position with slight head elevation using rubber pillow, under continuous monitoring and fluid replacement using ringer acetate at a rate 4 ml.kg−1.h−1. After insuring of motor block of spinal anesthesia and sensory levels reaching at least T10, surgeon started limb elevation, exsanguinating of blood and applying a double cuffed tourniquet at middle of the thigh over cotton padding, with pressure ranging 250–300 mmHg according to the patient's blood pressure (150 mmHg above systolic blood pressure). After completion of surgical procedure, researcher anesthesiologist checked the sensory level of subarachnoid block in both limbs before starting tourniquet deflation procedure. Tourniquet deflation was done by anesthesia technician in two ways according to randomization number received in closed envelop with patient file: in gradual group (G group) deflation by rate 50 mmHg/30 s till complete release within 3 min. In intermittent group (I group) complete deflation for 10 s then re-inflate for 50 s; this cycle was repeated three times till complete final tourniquet release. The researcher anesthesiologist was separated from the lower limb, tourniquet device, and the technician by surgical curtain to ensure full blinding of deflation’s technique. Hemodynamic management during deflation process done by the researcher anesthesiologist, drop in HR or MAP > 25% of pre-deflation value managed by 0.1 mg.kg−1 of ephedrine (ephedrine sulphate, USP, Amneal Pharmaceuticals Pvt. Ltd, India) and recorded (frequency, total dose).
Patient’s hemodynamic data were recorded by blinded researcher anesthesiologist, including HR, MAP, oxygen saturation, and respiratory rate (RR), and were recorded basally, post-induction, 1 min before deflation, every 1 min during deflation, and 1, 5, 30 min after deflation. Blood samples for serum lactate, serum K, and PH were taken 5–30 min after deflation. Mean arterial blood pressure was adjusted as primary outcome objective, while others including HR, RR, PH, serum lactate, HCO3, and serum potassium were secondary outcomes.
In this trial, we hypothesized that intermittent deflation of tourniquet will act as ischemic preconditioning (IPC) manner, enabling better tolerating ischemic reperfusion effects of tourniquet release more than gradual deflation manner, especially in our group of elderly patients.
3. Sample size and statistical analysis
A pilot study of 10 patients, five in each group, was conducted to detect the difference between the two groups regarding the difference between the MAP 1 min after deflation of the tourniquet. The study showed that the difference between the two groups was 20%. We used G*power software version 3.1.9.4 for sample size calculation. It was found that 58 patients, distributed equally between the study groups, were sufficient to achieve a study power of 0.8 with an alpha error of 0.05. Cases of pilot study were not included in the study. Additionally two patients were recruited to compensate for dropouts, reaching a total sample size of 60 patients.
Perioperative data will be tabulated and analyzed using IBM SPSS software version 22. Continuous data will be presented as mean SD or median IQR according to normality of distribution. Nominal and categorical data will be presented as numbers and percentages. Independent-sample t-test, Mann–Whitney test, or chi-square test will be utilized to detect statistical differences between the studied groups.
4. Results
This study enrolled 60 patients scheduled for elective total knee arthroplasty surgery, presented in . Patient demographics, basal data, intraoperative fluids, and duration of the tourniquet are presented in without significant difference between the studied groups.
Table 1. Patients’ demographics, basal data and intraoperative fluids. Data are presented as mean ± SD or numbers
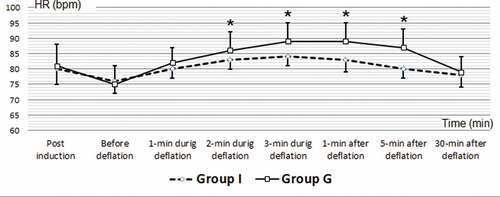
Intraoperative and postoperative (MAP) are illustrated in with significant difference between studied groups as: MAP 1 min during deflation (MAPD1, G 95.30 ± 3.87, I 98.63 ± 5.76, P 0.011), MAP 2 min during deflation (MAPD2, G 92.40 ± 3.43, I 94.90 ± 4.78, P 0.023), MAP 3 minute during deflation (MAPD3, G 90.30 ± 3.47, I 92.80 ± 4.79, P 0.024), MAP 1 min after deflation (MAP1, G 89.70 ± 4.08, I 93.90 ± 5.23, P 0.001), MAP 5 minute after deflation (MAP5, G 93.33 ± 4.49, I 96.83 ± 5.94, P 0.013).
Figure 2. Intraoperative and postoperative mean arterial pressure (MAP/mmHg) of the included patients
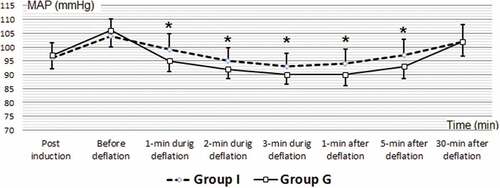
Intraoperative and postoperative (HR) are illustrated in with significant difference between studied groups as: HR 2 min during deflation (HRD2, G 86.53 ± 5.58, I 83.46 ± 3.04, P 0.011), HR 3 min during deflation (HRD3, G 89.63 ± 6.03, I 84.60 ± 2.94, P 0.000), HR 1 min after deflation (HR1, G 89.96 ± 6.04, I 83.00 ± 3.75, P 0.000), HR 5 min after deflation (HR5, G 87.16 ± 5.52, I 80.00 ± 3.18, P 0.000).
Intraoperative (RR) are illustrated in with significant difference between studied groups as RR 3 min during deflation (RRD3, G 17.40 ± 1.32, I 15.66 ± 0.75, P 0.000), RR 1 min after deflation (RR1, G 18.30 ± 0.83, I 15.53 ± 0.62, P 0.000), RR 5 minute after deflation (RR5, G 18.06 ± 0.78, I 15.80 ± 0.66, P 0.000).
Table 2. Intraoperative respiratory rate of the included patients .Data are presented as mean ± SD
After deflation laboratory data of the included patients are illustrated in with significant difference between studied groups as: PH 5 min after deflation (PH5, G 7.30 ± 0.03, I 7.32 ± 0.04, P 0.018), serum potassium 5 min after deflation (K5, G 4.48 ± 0.30, I 4.19 ± 0.35, P 0.001), serum potassium 30 min after deflation (K30, G 4.40 ± 0.30, I 4.12 ± 0.36, P 0.002), serum lactate 5 min after deflation (lactate 5, G 1.91 ± 0.33, I 1.29 ± 0.29, P 0.000), serum lactate 30 min after deflation (lactate30, G 1.58 ± 0.30, I 1.06 ± 0.23, P 0.000), while other parameters were comparable between patients of studied groups.
Table 3. After deflation laboratory data of the included patients.Data are presented as mean ± SD
Regarding patients of both groups, pre and postoperative sensory level of subarachnoid block, were comparable; no recorded patient needed vasopressor (bolus ephedrine) during deflation periods.
5. Discussion
In this study, 60 patients undergoing total knee arthroplasty were randomly allocated into two equal groups according to the regimen of tourniquet deflation. Intermittent deflation of the tourniquet resulted in more stable hemodynamics and acid base parameters compared to gradual deflation technique.
Pneumatic tourniquet application is a common practice in many surgeries. While it creates a bloodless field, decreasing blood loss and conserving blood transfusion, it is not free of complications; depending on pressure applied, duration of surgery and type of anesthesia used. Beside pressure injury, thrombosis and systemic congestion, the ischemia induced by tourniquet resulting in anaerobic metabolism with accumulation of metabolites, waste products and acids resulting in systemic acidosis, tachycardia, hyperlactemia, hyperkalemia, vasodilatation and hypotension [Citation2]. These drawbacks are augmented by sudden venous pooling of blood in the limb after tourniquet deflation, resulting in a serious hemodynamic instability, tissue hypoxia and surgical field infection on the long term [Citation8].
Also, tourniquet deflation has ischemic reperfusion effects with increase in oxygen-free radicals and lipid peroxides making the patients more labile to hemodynamics instability with acid base and electrolytes disturbance [Citation9,Citation10]. These effects are obvious in elderly patients and those with cardiopulmonary disorders due to a lack of cardiac reserve and compensatory mechanism [Citation11].
Many trials studied these effects and used multiple drugs as antioxidant to attenuate ischemic reperfusion injury. Dexmedetomidine, propofol, ketamine, lidocaine, pregabalin, hydroxyethyl starch, N-acetyl cysteine, nitric oxide, mannitol, vitamins C and E have been studied with variable results regarding hemodynamics, acid base parameters, analgesia and sedation effects. IPC technique seems superior in controlling these drawbacks without need to use systemic drugs [Citation12–22].
IPC is defined as an intervention whereby brief intermittent ischemic episodes are induced in a limb (usually three or four cycles, each cycle lasting for 5 min of arterial occlusion bouts, interspersed with 5 min of reperfusion) either at the site of interest (IPC) or at a distance from the site of interest [remote IPC (RIPC)]. This phenomenon has been used as a clinical tool in order to enhance a tissue tolerance to ischemia-induced injury [Citation23,Citation24]. Our technique is based on this idea with some modifications regarding timing and duration. After completion of the surgical procedure, we performed tourniquet deflation and re-inflation for three cycles in a range 3 minduration. The choice based on regional anesthesia used in this study does not allow us to use remote ischemia without applying local anesthesia in remote limb or systemic sedative or analgesic drugs, which may interrupt our results. Pre-application use may take time affecting the duration of anesthesia in our surgery. So, in this study the technique modification was done by gaining the benefits of allowing gradual blood pooling in the lower limb after surgical end and beginning of tourniquet deflation.
Intermittent tourniquet deflation resulted in more stable hemodynamics, less tachycardia and hypotension, less acidosis, less hyperlactemia and less hyperkalemia. This may be attributed to preconditioning action and slowly interrupted blood pooling in ischemic limb allowing compensatory mechanisms to take place and counteract these negative effects. Also, gradual release seems to have nearby effects, but we can conclude that continuous flow of negative metabolites into systemic circulation, and blood into the ischemic limb resulted in less protective action.
Jason et al. used a similar technique with some modification regarding times of deflation and reinflation. They concluded that the staggered tourniquet release was associated with greater hemodynamic stability and reduced the rate of acute systemic metabolic changes associated with limb reperfusion. The reapplication of a tourniquet seemed to halt further reperfusion, providing a window period for patient evaluation and management [Citation25].
Almeida et al. used the gradual technique for tourniquet deflation over 4 min compared to sudden release. They proved that gradual technique allowed more stable hemodynamics with better myocardial oxygen consumption [Citation26].
Using IPC technique had been evaluated in four trials, one of them in comparison with N-acetyl cysteine. All of these trials proved efficacy in better controlling and stability of hemodynamics and general conditions during deflation time [Citation12,Citation27].
This study had some limitations, while primary objective based on MAP we did not use invasive tools in measuring blood pressure, which is due to ethical issues of using invasive methods only for research purposes. We did not use inflammatory indicators for economic deficiency support. We had short-term monitoring while long-term monitoring may give a more obvious view of the efficacy of both techniques.
6. Conclusion
Intermittent deflation of tourniquet used in total knee arthroplasty resulted in more stable hemodynamics, acid base and metabolic parameters compared to gradual deflation technique.
Abbreviations
ASA: American Society of Anesthesiologists
ECG: electrocardiogram
ECHO: echocardiography
HR: heart rate
INR: international normalization ratio
IPC: ischemic preconditioning
MAP: mean arterial pressure
RIPC: remote ischemic preconditioning
RR: respiratory rate
SpO2: peripheral oxygen saturation
Author’s individual contribution
Rania Elmohamady Elbadrawy helped in the study design, data collection, writing the manuscript and submission.
Mohamed Adel Aboelela helped in study design, data collection, statistical analysis and reviewing the manuscript.
Clinical trial no
(PACTR202003900400089).
Financial disclosures
None.
Institutional review board
Mansoura Faculty of Medicine (IRB-R.20.02.739, 12 February 2020).
Acknowledgments
Staff nurses in orthopedic surgery operating rooms, Mansoura University Hospital, Egypt, participated in providing perioperative care for the patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Masri BA, Eisen A, Duncan CP, et al. Tourniquet-induced nerve compression injuries are caused by high pressure levels and gradients – a review of the evidence to guide safe surgical, pre-hospital and blood flow restriction usage. BMC Biomed Eng. 2020;2(1):1–7.
- Kumar K, Railton C, Tawfic Q. Tourniquet application during anesthesia: “What we need to know?”. J Anaesthesiol Clin Pharmacol. 2016;32(4):424–430.
- Fitzgibbons PG, Digiovanni C, Hares S, et al. Safe tourniquet use: a review of the evidence. J Am Acad Orthop Surg. 2012;20(5):310–319.
- Saied A, Ayatollahi Mousavi A, Arabnejad F, et al. Tourniquet in surgery of the limbs: a review of history, types and complications. Iran Red Crescent Med J. 2015;17(2):e9588.
- Zarrouki Y, Abouelhassan T, Samkaoui MA. Cardiac arrest after tourniquet deflation in upper limb. Trauma Case Rep. 2017;10(7):1–2.
- Sharma JP, Salhotra R. Tourniquets in orthopedic surgery. Indian J Orthop. 2012;46(4):377–383.
- Feng L, Zhang XG, Yang QG, et al. Effects of tourniquet on cardiac function in total knee arthroplasty with trans-esophageal echocardiography. Zhonghua Yi Xue Za Zhi. 2013;93(47):3755–3757.
- Huang GS, Wang CC, Hu MH, et al. Bilateral passive leg raising attenuates and delays tourniquet deflation-induced hypotension and tachycardia under spinal anaesthesia: a randomised controlled trial. Eur J Anaesthesiol. 2014;31(1):15–22. .
- Cao Q, He Z, Fan Y, et al. Effects of tourniquet application on enhanced recovery after surgery (ERAS) and ischemia-reperfusion post-total knee arthroplasty: full- versus second half-course application. J Orthop Surg. 2020;28(1):230949901989602.
- Van M, Olguner C, Koca U, et al. Ischaemic preconditioning attenuates haemodynamic response and lipid peroxidation in lower-extremity surgery with unilateral pneumatic tourniquet application: a clinical pilot study. Adv Ther. 2008;25(4):355–366.
- Dy CJ, Wilkinson JD, Tamariz L, et al. Influence of preoperative cardiovascular risk factor clusters on complications of total joint arthroplasty. Am J Orthop (Belle Mead NJ). 2011;40(11):560–565.
- Halladin NL, Zahle FV, Rosenberg J, et al. Interventions to reduce tourniquet-related ischaemic damage inorthopaedic surgery: a qualitative systematic review of randomised trials. Anaesthesia. 2014;69(9):1033–1050.
- Arnaoutoglou H, Vretzakis G, Souliotis D, et al. The effects of propofol or sevoflurane on free radical production after tourniquet induced ischaemic reperfusion injury during knee arthroplasty. Acta Anaesthesiologica Belgica. 2007;58(1):3–6.
- Bostankolu E, Ayoglu H, Yurtlu S, et al. Dexmedetomidine did not reduce the effects of tourniquet-induced ischemia-reperfusion injury during general anesthesia. Kaohsiung J Med Sci. 2013;29(2):75–81. .
- Saricaoglu F, Dal D, Salman AE, et al. Ketamine sedation during spinal anesthesia for arthroscopic knee surgery reduced the ischemia-reperfusion injury markers. Anesth Analg. 2005;101(3):904–909. .
- Turan R, Yagmurdur H, Kavutcu M, et al. Propofol and tourniquet induced ischaemia reperfusion injury in lower extremity operations. Eur J Anaesthesiol. 2007;24(2):185–189.
- Lee JY, Kim CJ, Chung MY. Effect of high-dose vitamin C on oxygen free radical production and myocardial enzyme after tourniquet ischaemia-reperfusion injury during bilateral total knee replacement. J Int Med Res. 2010;38(4):1519–1529.
- Peker K, Ökesli S, Kıyıcı A, et al. The effects of ketamine and lidocaine on free radical production after tourniquet-induced ischemia-reperfusion injury in adults. Ulus Travma Acil Cerrahi Derg. 2019;25(2):111–117.
- Karaca Ö, Pinar HU, Özgür AF, et al. The effect of pregabalin on tourniquet-induced ischemia-reperfusion injury: a prospective randomized study. Turk J Med Sci. 2019;49(6):1693–1700.
- Pinar HU, Pinar A, Mavioğlu Ö, et al. Effect of hydroxyethyl starch 130/0.4 on ischemia-reperfusion determinants in minor lower extremity surgery with tourniquet application. J Clin Anesth. 2015;27(2):105–110.
- Omer K, Nermin G, Ali A, et al. Lesão de isquemia‐reperfusão induzida por torniquete: comparação dos efeitos antioxidantes de propofol e cetamina em doses baixas [Tourniquet-induced ischaemia-reperfusion injury: the comparison of antioxidative effects of small-dose propofol and ketamine]. Rev Bras Anestesiol. 2017;673:246–250. Portuguese. .
- Leurcharusmee P, Sawaddiruk P, Punjasawadwong Y, et al. The Possible Pathophysiological Outcomes and Mechanisms of Tourniquet-Induced Ischemia-Reperfusion Injury during Total Knee Arthroplasty. Oxid Med Cell Longev. 2018;2018:1–15.
- Westman B, Weidenhielm L, Rooyackers O, et al. Knee replacement surgery as a human clinical model of the effects of ischaemia/reperfusion upon skeletal muscle. Clin Sci. 2007;113(7):313–318.
- Murphy T, Walsh PM, Doran PP, et al. Transcriptional responses in the adaptation to ischaemia-reperfusion injury: a study of the effect of ischaemic preconditioning in total knee arthroplasty patients. J Transl Med. 2010;8(1):46.
- Jason V, Leon S, Gabriella L. Reducing the potential for tourniquet-associated reperfusion injury. Eur J Emerg Med. 2013;20(6):391–396.
- Almeida M, De Sousa E, De Castro R. Escalonated Tourniquet Deflation Strategy Reduces the Incidence of Hypotension After Total Knee Replacement Surgery: a Randomized Double-Blinded Controlled Trial. ARC J Orthopedics. 2019;4(1):14–18.
- Koca K, Yurttas Y, Cayci T, et al. The role of preconditioning and N-acetylcysteine on oxidative stress resulting from tourniquet- induced ischemia-reperfusion in arthroscopic knee surgery. J Trauma. 2011;70:717–723.