ABSTRACT
Objective: To study the role for preoperative CT chest scans in suspected COVID-19 patients requiring emergent surgery.
Design: Retrospective – observational.
Participants: A total of 98 patients admitted for emergency surgery with COVID-19 infection and underwent preoperative CT chest scanning.
Main outcome measurements: Incidence of clinical symptoms of COVID-19 infection upon presentation, imaging characteristics in chest CT and semi-quantitative CT severity score.
Results: The median age of the study cohorts was 50 years (interquartile range (IQR): 40–60 years) and 52/98 (53.1%) were males. The most common symptoms were fever (80.6%) and cough (65.3%). 50/98 had positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR test prior to CT scan, while 48/98 had positive reverse transcriptase-polymerase chain reaction (RT-PCR) result returned after imaging. The imaging characteristics were bilateral infiltrates on CT of 90/98, with 70/98 of infiltrates located peripherally and 28/98 located peripherally and centrally. The most common disease pattern was ground-glass opacities, observed in 95/98. The median total COVID-19 CT severity score was 7 (IQR: 4–14), corresponding to 5–25% global lung involvement.
Conclusion: Patients with mild symptomatic COVID-19 in this study displayed CT evidence of SARS-CoV-2 infection. Preoperative CT imaging should be considered for identifying suspected active SARS-CoV-2 cases in resource limited environments with high community spread, to aid in resource allocation and personal protective equipment (PPE) rationing.
1. Introduction
The ongoing outbreak of coronavirus disease-2019 (COVID-19), caused by the SARS-CoV-2, has led to an unprecedented strain on healthcare resources [Citation1]. Although COVID-19 has led to a change in clinical practice and postponement of many elective surgical procedures, emergent surgery, in patients with severe or life-threatening pathologies, has continued despite the ongoing pandemic and the limited medical supplies [Citation2]. In an ideal setting, proper PPE, including N95 respirators, would be used in all patients requiring emergent surgery during the outbreak, regardless of their individual SARS-CoV-2 status. Nonetheless, global shortages of PPE, in particular N95s, have challenged medical and surgical practice and are likely to continue well into 2021, placing physicians, nurses, and other healthcare professionals at potentially high risk of contagion [Citation2]. As such, in order to properly allocate and ration limited PPE supplies and ensure the health and safety of both medical staff and patients, identification of patients with active SARS-CoV-2, in particular those who pose higher risk of SARS-CoV-2 transmission, is essential before emergent surgery.
To-date, nucleic acid amplification tests (NAATs) remain the gold standard for diagnosing SARS-CoV-2 infection [Citation3]. However, for emergency procedures, the turnaround time (TAT) of many routinely available NAATs, requiring between 3–6 hours for a final test result, may be unacceptable. Waiting for NAAT results could delay the surgery, which may increase patient’s risk of severe morbidity and mortality. Moreover, many worldwide laboratories are still plagued by inadequate resources as well as by a shortage of diagnostic kits, or personnel to perform testing [Citation4]. A recent survey from the American Association of Clinical Chemistry (AACC) has revealed that over 60% of worldwide laboratories are currently reporting to face serious challenges in obtaining sufficient amount of reagents and test kits needed for routine SARS-CoV-2 diagnostics [Citation4]. While newer rapid antigen assays with TAT as short of 15–30 min are now commercially available, they are still not routinely used and have variable sensitivity for detecting SARS-CoV-2 [Citation5]. As such, preoperative CT imaging has been explored as a potential tool for screening patients for SARS-CoV-2 infection [Citation6]. Overall, CT imaging has seemed to perform well at identifying a typical disease pattern of COVID-19 [Citation7], leading to recommendation by some as a clinical screening tool in regions with high viral circulation [Citation6]. However, the limited studies on preoperative CT reported conflicting findings, and preoperative CT screening remains controversial [Citation6]. Thus, in this cross-sectional study, we aimed to further explore the role of preoperative imaging in patients requiring emergent surgery.
2. Patients and methods
This study was a retrospective, multi-center, cross-sectional study performed between March and July 2020. This study was registered on ClinicalTrials.gov (NCT04560530). This study was approved by the Institutional Review Board (IRB) and received a waiver of informed consent due to no greater than minimal risk to participants. All examinations were performed at the Nile Radiology Center at Aswan city, Egypt (Center 1) and Hurghada Governmental Hospital, Egypt (center 2).
Patients were eligible for inclusion in the study if (1) they required an emergent surgical procedure, (2) received a standard-of-care chest CT, (3) had confirmed SARS-CoV-2 infection by RT-PCR on nasopharyngeal swabs collected before CT examination, and (4) had symptoms suggestive for COVID-19 with mild severity (ambulatory COVID-19 not requiring hospitalization for therapy) at time of diagnostic imaging. The exclusion criteria were age <18 years, pregnancy, past medical history pulmonary diseases or connective tissue diseases, severe or critical COVID-19 at time of imaging, and poor quality of CT images. The study was performed under a waiver of informed consent and in compliance with the Healthcare Information Portability and Accountability Act. All procedures and practices were in accordance with the Declaration of Helsinki.
All examinations were performed at 2 radiology centers (Center 1) and (center 2). At Center 1, CT scans were performed a 64 × 2-slices CT scanner (Aquilion CXL, Toshiba/Canon Medical Systems, Ōtawara, Japan). At Center 2, scans were performed on a 16-slices CT scanner (LightSpeed RT 16, General Electric Healthcare, Chalfont St. Giles, UK). No contrast agents were employed. Two radiologists, each with over 10-year experience, independently evaluated the images. Both radiologists were blinded to the aim of the study and its protocol.
3. Measurements
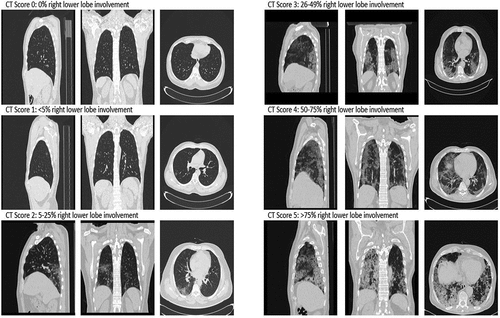
COVID-19 pulmonary involvement was assessed using semi-quantitative CT severity score proposed by Pan et al. [Citation8], which has shown to be predictive of outcome, and was found to be positively correlated with age, inflammatory biomarkers, severity of clinical categories, and disease phases [Citation9]. In short, each of the five lung lobes was assessed on a scale of 0 to 5, with 0 indicating no involvement; 1, less than 5% involvement; 2, 5–25% involvement; 3, 26–49% involvement; 4, 50–75% involvement; and 5, more than 75% involvement (). The individual lobar scores were then summed to calculate the total CT score, which ranged from 0 (no involvement) to 25 (maximum involvement) [Citation8]. Statistical analysis was performed using Prism 8.4.3 (GraphPad Software, San Diego, CA, USA).
4. Results
A total of 120 patients were enrolled, 22 of whom were excluded for presence of emphysema, pulmonary tuberculosis, pre-existing interstitial lung disease, or pregnancy, leaving a final sample of 98 SARS-CoV-2 positive patients. The median age was 50 years (interquartile range (IQR): 40–60 years) and 52/98 (53.1%) were males. The most common symptoms were fever (80.6%), cough (65.3%), dyspnea (35.7%), headache (25.5%), and anosmia (13.3%). The type of emergency surgery was abdominal in 44/98 (44.9%), orthopedic in 26/98 (26.5%), and abscess drainage in 28/98 (28.6%). Abdominal surgeries were exploratory laparotomy in 15/44 (34%), appendicectomy in 20/44 (45%) and intestinal obstruction in 5/40 (13%). Orthopedic surgeries were 20/26 (77%) closed reduction of Colles’ fractures and 6/26 (23%) open reduction and internal fixation of femur fractures. Among the cohort, 50/98 (51.0%) had positive SARS-CoV-2 RT-PCR test prior to CT scan, while 48/98 (49.0%) had positive RT-PCR result returned after imaging. All patients displayed radiologic evidence of SARS-CoV-2 involvement, thus equating to a 100% positive percent agreement between the CT scan and RT-PCR. The imaging characteristics of the cohort are summarized in . All but 8/98 (8.2%) patients displayed bilateral infiltrated on CT (90/98, 91.8%), with 70/98 (71.4%) of infiltrates located peripherally and 28/98 (28.6%) located peripherally and centrally. The most common disease pattern was ground-glass opacities, observed in 95/98 (96.9%) (). The median total COVID-19 CT severity score was 7 (IQR: 4–14), corresponding to 5–25% global lung involvement.
Figure 2. Disease pattern of COVID-19 lesions in on CT axial images: ground glass opacities (GGO) (A); crazy-paving pattern (GGO with superimposed inter and intra-lobular septal thickening) (B); consolidation (C)
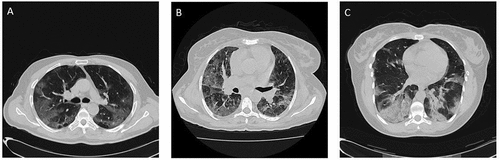
Table 1. CT Imaging characteristics of the cohort (n = 98)
5. Discussion
In this study, we found that all SARS-CoV-2 infected patients with mild COVID-19 and requiring emergent surgery demonstrated clear radiologic evidence of SARS-CoV-2 infection. Most patients had bilateral lung infiltrates with ground-glass opacities. These findings suggest that CT imaging may be useful in patients with mild COVID-19 and no preexisting pulmonary disease, who require emergency surgery and cannot wait to receive the results of a NAAT.
Agrawal et al. [Citation6] recently reported in a mini-review of 6 studies that CT imaging has variable sensitivity, and positive predictive value that decreases in parallel as disease prevalence declines. We agree that screening patients with asymptomatic disease, in which only half may have CT findings [Citation10], may have limited utility. However, in one study with emphasis on emergency surgery, CT was identified as the most accurate diagnostic test [Citation11]. Moreover, as observed in our study, if imaging is restricted to mildly symptomatic cases (regardless of NAAT result at time of investigation), CT imaging identified pulmonary infiltrates in 100% of cases.
A couple of points should be considered for interpreting these results. First, imaging has been reported to correlate with disease severity [Citation9], while disease severity seems to correlate with viral load [Citation12], and in consequence, the ability to effectively transmit the virus [Citation13]. As such, patients with asymptomatic or pre-symptomatic disease, while capable of transmitting the virus [Citation14], appear at significantly less risk to do so [Citation15]. As these patients are also likely to have more limited CT involvement, patients without active symptoms are probably poor candidates for CT screening. Nonetheless, while imaging may exclude those without clear signs of significant disease burden, it cannot rule out all cases of COVID-19 [Citation10]. As CT negative patients with undiagnosed SARS-CoV-2 infection are still potentially capable (though at much lower risk) of transmitting the virus, we would suggest that in patients with negative CT, anesthesiologists performing airway procedures (i.e. intubation) should always use optimal PPE, in a negative pressure room, with only the absolute necessary support staff. Notably, although PPE (including N95) should hence be used whenever possible in regions of high community transmission, in resource limited environments, where N95s are of restricted supply, CT screening of potentially symptomatic patients may help in effective resource allocation and PPE rationing. We also recommend diagnostic imaging only be used as a screening method in patients with mild symptoms and in whom a NAAT cannot be rapidly obtained, or such that waiting for a NAAT would delay an emergent procedure, thus placing the patients at unacceptable risk of morbidity or death.
A few negatives and safety hazards of CT screening should also be considered. First, CT screening may expose radiologic staff to high-risk contagion. In the event of suspect findings, shutdown of CT until properly decontamination would be highly advisable, in such way that the safety of subsequent patients is guaranteed [Citation6]. In addition, CT may further stretch hospital resources (especially radiology departments), exposes patients to potentially unnecessary radiation, could delay surgery, and increase the overall health care costs [Citation6]. As such, CT screening of potential SARS-CoV-2 infected patients only seems reasonable in regions of high transmission and low PPE availability.
6. Conclusion
All patients with mild symptomatic COVID-19 in this study displayed CT evidence of SARS-CoV-2 infection. Preoperative CT imaging should be considered for identifying suspected active SARS-CoV-2 cases in resource limited environments with high community spread, to aid in resource allocation and PPE rationing. More sensitive and rapid diagnostics tests would be urgently welcomed to enable rapid identification and safe triage of patients with suspected COVID-19 [Citation5].
Declerations
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lippi G, Sanchis-Gomar F, Henry BM. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8(7):497.
- Jessop ZM, Dobbs TD, Ali SR, et al. Personal Protective Equipment (PPE) for surgeons during COVID-19 pandemic: a systematic review of availability, usage, and rationing. Br J Surg. Published online. 2020 May 12;107(10):1262–1280.
- Lippi G, Henry BM, Sanchis-Gomar F, et al. Updates on laboratory investigations in coronavirus disease 2019 (COVID-19). Acta Bio Medica Atenei Parmensis. 2020;91(3): ahead of print-ahead of print. doi:10.23750/abm.v91i3.10187
- American Association of Clinical Chemistry. coronavirus testing survey. Accessed November 1, 2020. https://www.aacc.org/science-and-research/covid-19-resources/aacc-covid-19-testing-survey
- Mattiuzzi C, Henry B, Lippi G. Making sense of rapid antigen testing in SARS-CoV-2 diagnostics. Diagnosis (Berl). 2020; Published online. DOI:10.1515/dx-2020-0131
- Agrawal V, Yadav SK, Sharma D. Pre-operative CT Chest as a screening tool for COVID-19: an appraisal of current evidence. BJS (British Journal of Surgery). 2020;107(12):e596–e597.
- Prokop M, Van Everdingen W, Van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296(2):E97–E104.
- Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel Coronavirus (COVID-19) pneumonia. Radiology. Published online. 2020 February 13;295(3):715–721.
- Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020 July 4;1–10. Published online. DOI: 10.1007/s00330-020-07033-y
- Ikehara H, Gotoda T, Kusano C. Chest computed tomography for severe acute respiratory syndrome coronavirus 2 infection screening for COVID-19 before emergency and elective upper endoscopy: pilot study. Digestive Endosc. 2020;32(7):1112.
- Hernigou J, Valcarenghi J, Safar A, et al. Post-COVID-19 return to elective orthopaedic surgery—is rescheduling just a reboot process? which timing for tests? is chest CT scan still useful? safety of the first hundred elective cases? how to explain the “new normality health organization” to patients? International Orthopaedics (SICOT). 2020;44(10):1905–1913.
- Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38(5):661–671.e2.
- Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A. 2020;117(28):16587–16595.
- Lippi G, Plebani M. Asymptomatic COVID-19 transmission: the importance of avoiding official miscommunication. Diagnosis (Berl). 2020;7(4):347–348.
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17(9):e1003346.