ABSTRACT
Background: Secondary analyses of randomized controlled trials found that driving pressure (DP) ≤ 14 cm H2O may be associated with improved clinical outcomes in acute respiratory distress syndrome (ARDS) patients. Therefore, the aim of this study was to evaluate the effect of DP-guided ventilation compared to conventional protective lung ventilation (PLV) on clinical outcomes in ARDS patients.
Methods: In this prospective, controlled trial, 110 patients with ARDS were randomly assigned according to ventilatory strategy into conventional PLV group and DP-guided ventilation group (by maintaining DP value ≤ 14 cm H2O). Clinical outcomes were incidence of mortality at 28th day (primary outcome), PaO2/FiO2, static compliance (Cstat), organ(s) dysfunction, mechanical ventilation (MV) duration, and length of ICU stay.
Results: Incidence of mortality at 28th day was reduced in DP-guided ventilation group compared to PLV group (20% vs. 5.45%); the hazard ratio was 0.26)95% CI: 0.09 to 0.73). The PaO2/FiO2, C stat and MV-free days were higher in in DP-guided ventilation group compared to PLV group. Organ dysfunction, duration of MV and length of ICU stay were significantly lower DP-guided ventilation group compared to PLV group.
Conclusions: In patients with ARDS, DP-guided ventilation showed improved survival, Cstat and oxygenation and lower incidence of organ dysfunction, duration of MV and length of ICU stay compared to PLV.
1. Introduction
Protective lung strategy using tidal volume (4–6 ml/kg of predicted body weight (PBW)), FiO2 guided PEEP, and plateau pressure ≤ 30 cm H2O in patients with ARDS improves survival [Citation1]. The optimum value of PEEP reflects the balance between lung recruitment, hyperinflation, and/or hemodynamic dysfunction [Citation2]. The use of conventional protective lung ventilation (PLV) is associated with improved oxygenation and survival in acute respiratory distress syndrome (ARDS) patients [Citation3,Citation4].
To date, mortality is still high despite the use of PLV strategy, which may reflect the imbalance between tidal volume, manipulation of PEEP, lung recruitment, and hyperinflation, as shown in clinical trials. Also, there are conflicting responses for manipulating PEEP and tidal volume during PLV strategy [Citation5–7].
Calculating tidal volume to PBW does not consider the heterogeneous pathology of the lung in ARDS with different respiratory system compliance [Citation8]. Driving pressure (DP) is the ratio of tidal volume to C stat. It can be calculated simply at the bedside as plateau pressure minus PEEP [Citation9]. Amato et al. have shown in their secondary analyses that DP was the primary variable that should be optimized during mechanical ventilation (MV) in ARDS patients and associated improved survival [Citation10].
Our trial suggested that keeping DP ≤ 14 cm H2O in patients with ARDS may improve the clinical outcomes of the patients due to decrease ventilator induced lung injury. Therefore, the aim of this study was to evaluate the impact of DP-guided ventilation strategy compared to conventional PLV on clinical outcomes in ARDS patients. The primary outcome was the incidence of mortality at the 28th day. Secondary outcomes included oxygenation, lung compliance, organ(s) dysfunction, MV duration, and length of ICU-stay.
2. Methods
This prospective randomized controlled parallel study was conducted at Surgical Intensive Care Unit (SICU) of Tanta University Hospital, Egypt, after it had been approved by the institutional ethical committee (approval code 32,422/06/18) and registered at Pan African Clinical Trials Registry (ID number; PACTR201807132391075). Informed written consent was obtained from the relatives of the patients. All collected data were used for this research only. The study was open labelled as blindness of the study wasn’t accessible. The study was done from August 2018 to January 2020.
Criteria of study inclusion were as follows: Patients aged ≥ 18 years old of both sexes, under MV and fulfilling Berlin definition of ARDS [Citation11] as indicated by acute onset within one week, P/F ratio ≤ 300 mmHg with PEEP of ≥5 cm H2O, Bilateral lung opacities shown by chest X-ray or lung sonography consistent with pulmonary edema and exclusion of fluid overload and cardiac failure in the absence of a definite cause of ARDS.
Pregnant females and patients with unstable hemodynamic parameter (MAP less than 65 mmHg or any dose of vasopressor and/or inotropic support), pneumothorax, or organ(s) dysfunction (other than lung dysfunction) by sequential organ failure assessment (SOFA) score at the time of allocation were excluded.
Closed envelopes were used for randomization of patients based on choice of PEEP and ventilation strategy into conventional PLV group (control group) and DP-guided ventilation group. Randomization and enrollment were done by a doctor who didn’t participate in the study.
2.1. Initiation of mechanical ventilation
All patients were kept in a semi-recumbent position and were managed by protective lung strategy using volume-controlled (VC) mode. Tidal volume was set at 6 mL/kg, based on PBW. Plateau pressure (Pplat) was kept ≤ 30 cmH2O throughout the study by TV reduction in 1 ml/kg steps to levels down to 4 ml/kg. Accepted oxygenation levels (SpO2 88–95% or PaO2 60–80 mmHg) maintained by setting of FiO2 initially at 0.4 and then titrated if target oxygenation was not met. PEEP was set initially at 5 cmH2O, while the ventilator rate was set to keep adequate minute ventilation (7 to 9 L/min) and arterial pH >7.25–7.44. The inspiratory to expiratory ratio (I/E ratio) was set initially at 1:2. Sedation was given to all patients by continuous infusion of midazolam (0.1 mg/kg/h), and muscle relaxation with a bolus injection of 3 mg cis-atracurium on demand during titration of PEEP that was adjusted during the morning shift according to the allocated group once daily. The choice of PEEP was guided by the ARDS network [Citation3] as in in group I while the choice of PEEP was set to keep DP ≤ 14 cm H2O in group II through manipulating PEEP value that was increased by 2 cm H2O increments to achieve the target value of DP provided that maintaining stable hemodynamics of patients. If the target DP was not met, tidal volume was decreased by 1 ml/kg steps as low as 4 ml/kg PBW.
Table 1. FiO2 (%) and PEEP (cmH2O) combinations according to ARDS network [Citation3]
All patients were managed according to ventilation strategy in each group until fulfilling weaning criteria and by the same protocol of weaning, as per unit protocol.
2.2. Collection of data and measurements
For each patient, the following data were collected: 28th day mortality (the primary outcome), P/F ratio, C stat, organ/s dysfunction by SOFA score, hemodynamics (mean arterial pressure and heart rate), barotrauma, duration of MV, weaning categories (simple, difficult or prolonged weaning), MV free days (without assisted breathing after successful extubation) at 28th day, organ(s) dysfunction free days at 28th day, and length of ICU stay. Patients were followed up for 28 days.
2.3. Statistical methods
2.3.1. Sample size
The calculation of sample size using the Epi-Info software statistical package (version 2002) created by World Health Organization (WHO) and Center for Disease Control (CDC) and Prevention, Atlanta, Georgia, USA. The number of patients in the sample size was calculated at N = 55 in each group as a result of 95% confidence interval (CI), power of the study 80%, group: group ratio is (1: 1) and the primary outcome in the control group (incidence of mortality at 28th day) is 32%, while in the study group is 10%.
2.3.2. Statistical analysis
Statistical analysis was done using SPSS v25 (IBM Inc. Chicago, USA). Shapiro-Wilks test was used for checking of normality of data. Quantitative data with normal distribution were expressed as mean and standard deviation (mean ± SD) and were compared by unpaired t-test. Quantitative data with abnormal distribution were expressed as median and interquartile ranges (IQR) and were compared using the Mann-Whitney test between both groups. Qualitative data were expressed as frequency and percentage (%) and compared using Chi-square or Fisher Exact test. Kaplan-Meier curve with log-rank test was used to compare survival of patients at 28th day in the two studied groups. A two-tailed P value ˂ 0.05 represented statistical significance.
3. Results
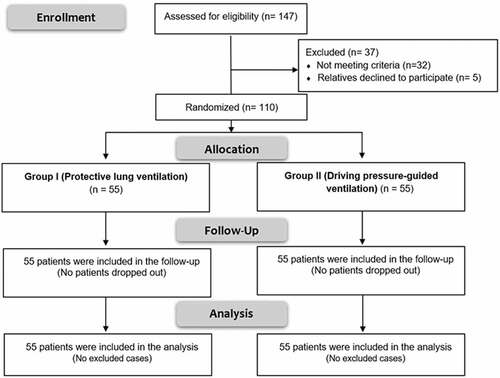
In this research, 147 patients were assessed for eligibility; 32 patients did not fulfill the criteria of inclusion, while 5 relatives refused to participate in the study. The remaining 110 patients were allocated randomly into two groups (55 patients in each of them) (). Regarding patients, characteristics (age, sex, and weight), causes, and severity of ARDS, there were no significant differences between both groups (P values = 0.396, 0.872, 0.095, 0.925, and 0.608 respectively) ().
Table 2. Patients’ characteristics, causes and severity of ARDS
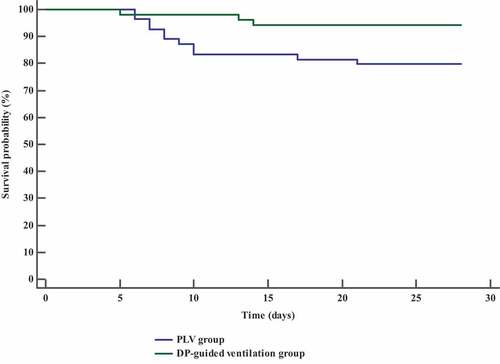
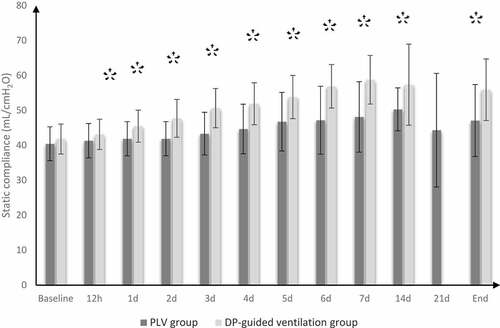
Incidence of mortality at 28th day was reduced in DP-guided ventilation group compared to control group (20% vs. 5.45%); the hazard ratio was lower in DP-guided ventilation group (0.26 times; 95% CI: 0.09 to 0.73). Length of ICU stay, duration of MV, number of patients who developed organ dysfunction, and the number of patients who showed hemodynamic instability (MAP ˂ 65 mmHg), in DP-guided ventilation group were significantly lower as compared to control group (P value = 0.004, 0.023, 0.010, and 0.041, respectively) (). PaO2/FiO2 and C stat were significantly better in DP-guided ventilation group compared to control group ().
Table 3. Clinical outcomes in the two studied groups
Figure 2. PaO2/FiO2 in both groups
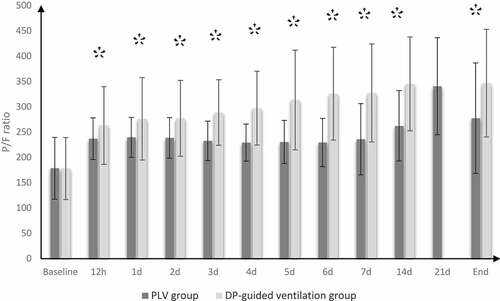
Figure 3. Static compliance in both groups
MV free days at 28th day, free days of organ/s dysfunction at 28th day, and patients with successful weaning in DP-guided ventilation group were significantly higher in comparison to control group (P value <0.001, 0.045 and 0.004, respectively), (). The rate and duration of survival were significantly higher in DP-guided ventilation group compared with contol group ().
4. Discussion
In the present study, the incidence of mortality at 28th day and organ/s dysfunction were reduced in DP-guided ventilation strategy. Also, DP-guided ventilation improved oxygenation, lung compliance, and weaning outcomes. Furthermore, it reduced the length of ICU stay and MV duration. DP 14 cm H2O was the variable associated with improved hospital survival in ARDS patients, as reported by Amato et al. [Citation10] and Laffey et al. [Citation12]. Amato et al., in a retrospective analysis of data from several RCTs, concluded that DP was a better mortality predictor than Cstat or tidal volume. They explained the benefit of DP ventilator variable to the optimization of MV in ARDS patients by adopting ventilation to the aerated lung units only. Laffey and his colleagues, in the Lung Safe study, found that DP ≤ 14 cmH2O was associated with better survival outcomes in patients with moderate to severe ARDS.
Moreover, the results of this study came in agreement with those of other researches [Citation13,Citation14]. Kassis et al. [Citation15] reported improved 28th day mortality, improved oxygenation, and respiratory system compliance with ventilator strategy leading to decreased DP. Guerin et al. [Citation16] said that DP was a risk parameter of mortality, along with Pplat and C stat. They noticed that patients with lower DP values have better survival outcomes (decreased 90th day mortality) with a significant decreased in SOFA score among survivors compared to those with higher values of DP.
The beneficial effects of DP-guided ventilation compared to PLV in patients with ARDS can be explained by the heterogeneous distribution of lung pathology in ARDS [Citation17]. The non-aerated units of the lung are responsible for the reduction in C stat [Citation18], which reflects the end-expiratory lung volume. In turn, the tidal volume/end-expiratory lung volume ratio represents lung strain. Therefore, this ratio, also called DP, can be considered a substitute for lung strain. Use of tidal volume according to PBW in PLV strategy exposes the lungs to the forces of cyclic stretch and inflation that release inflammatory mediators to the systemic circulation, which in turn have a negative impact on organ/s function. On the other hand, in DP-guided ventilation, manipulation of tidal volume tailored to the size of the aerated lung, which prevents cyclic or dynamic strain of the lung [Citation19].
Serpa Neto et al. [Citation20] performed a meta-analysis study, which included nine studies of patients with refractory hypoxemia receiving ECMO. They found that a significant decrease of tidal volume and DP was accompanied by ECMO initiation, which resulted in improved oxygenation. They concluded that DP was the only independent ventilator parameter that was associated with mortality during ECMO. Also, they found that lower values of DP were associated with better survival outcomes. Aoyama et al. [Citation21], in another meta-analysis, concluded that higher DP value was associated with lower survival outcomes during MV of ARDS patients.
Considering P/F and C stat, DP-guided ventilation improved both of them compared to PLV. These findings are in the same context as Estenssoro et al. [Citation22], De Jong et al. [Citation23], and Kacmark et al. [Citation24].
In disagreement with this study, Villar et al. [Citation25] stated that Pplat was better than DP in predicting hospital mortality. They found that in a secondary analysis of observational studies, including patients with moderate to severe ARDS managed with PLV strategy, comparing the effect of Pplat versus DP on the prediction of mortality. They found that there were insignificant differences between both groups regarding oxygenation, C stat, and organ dysfunction. This disagreement can be explained by the higher cut-off value of DP (19 cmH2O) in Villar et al. study. Cavalcanti et al. [Citation26] studied the effect of PLV strategy versus lung recruitment with titrated PEEP. They concluded that the use of PLV with tidal volume 4–6 ml/kg improved survival with reduction of the duration of MV and ICU stay compared with lung recruitment using titrated PEEP, despite the low value of DP at the 7th day in the lung recruitment group. This disagreement may be explained by potential alveolar distention in the lung recruitment group.
Limitations: a) It was not a multi-center trial b) The study was non-blind c) The majority of ARDS cases were mild to moderate.
5. Conclusions
In patients with ARDS, DP-guided ventilation showed improved survival, Cstat and oxygenation and lower incidence of organ dysfunction, duration of MV and length of ICU stay compared to PLV.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Author contributions
Khaled M. Hamama: Conduct the study, data collection, analysis and preparation of the manuscript.
Sameh M. Fathy: Analysis of the data and manuscript revision.
Reda S. Abd Arahman: Supervising the conduct of the study and revision of the manuscript.
Salah El-din I. Alsherif: The idea of the research and the final approval of the manuscript.
Sameh Abdelkhalik Ahmed: Supervising the conduct of the study and revision of the manuscript.
Abbreviations
C stat: static lung complianceDP: driving pressureICU: intensive care unit MAP: mean arterial blood pressure MV: mechanical ventilation PaO2/FiO2: partial pressure of arterial oxygen tension/fraction of inspired oxygen PEEP: positive end expiratory pressure PLV: protective lung ventilation
Additional information
Funding
References
- Holman RR, Bethel, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239.
- Gattinoni L, Carlesso E, Brazzi L, et al. Positive end-expiratory pressure. Curr Opin Crit Care. 2010;16:39–44.
- The Acute Respiratory Distress Syndrome Network. Ventilation with low tidal volumes as compared with traditional tidal volumes for acute respiratory distress syndrome. N Eng J Med. 2000;243(18):1301–1308.
- Valentini R, Aquino-Esperanza J, Bonelli I, et al. Gas exchange and lung mechanics in patients with acute respiratory distress syndrome: comparison of three different strategies of positive end expiratory pressure selection. J Crit Care. 2015;30(2):334–340.
- Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245.
- Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120.
- Tonelli AR, Zein J, Adams J, et al. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med. 2014;40(6):769–787.
- Staffieri F, Stripoli T, De Monte V, et al. Physiological effects of an open lung ventilator strategy titrated on elastance-derived end-inspiratory trans-pulmonary pressure: study in a pig model. Crit Care Med. 2012;40(7):2124–2131.
- Loring SH, Malhotra A. Driving pressure and respiratory mechanics in ARDS. N Engl J Med. 2015;372(8):776–777.
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755.
- Ranieri VM, Rubenfeld GD, Thompson BT, ARDS definition Task Force. Acute respiratory distress syndromr: the Berlin definition. JAMA. 2012;307(23):2526–2533.
- Laffey J, Bellani G, Pham T, et al. Potential modifiable factors contributing to outcome from acute respiratory distress syndrome: theLUNG SAFE study. Intensive Care Med. 2016;42(12):1865–1876.
- Borges JB, Hedenstierna G, Larsson A, et al. Altering the mechanical scenario to decrease the driving pressure. Crit Care. 2015;19:342.
- Grieco DL, Chen L, Dres M, et al. Should we use driving pressure to set tidal volume? Curr Opin Crit Care. 2017;23(1):38–44.
- Kassis EB, Loring SH, Tamlor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressure in ARDS. Intensive Care Med. 2016;42:1206–1213.
- Guerin C, Papazian I, Reignier J, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care. 2016;20:384.
- Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784.
- Girard TD, Bernard GR. Mechanical ventilation in ARDS: a state-of-the-art review. Chest. 2007;131:921–929.
- Arnal J-M, Saoli M, Garnero A. “Airway and transpulmonary driving pressures and mechanical powers selected by INTELLiVENT-ASV in passive, mechanically ventilated ICU patients. Heart Lung. 2020;49(4):427–434.
- Serpa Neto A, Schmidt M, Azevedo LC, et al. Association between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: mechanical ventilation during ECMO. Intensive Care Med. 2016;42:1672–1684.
- Aoyama H, Pettenuzzo T, Aoyama K, et al. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: a Systematic review and Meta-analysis. Crit Care Med. 2018;46(2):300–306.
- Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456.
- De Jong A, Cossic J, Verzilli D, et al. Impact of the driving pressure on mortality in obese and non-obese ARDS patients: a retrospective study of 362 case. Intensive Care Med. 2018;44(7):1106–1114.
- Kacmarck RM, Villar J, Sulemanji D, et al. Open lung approach for the acure respiratory distress syndrome: a pilot, randomizex controlled trial. Crit Care Care Med. 2016;44(1):32–42.
- Villar J, Martin-Rodriguez C, Domingues-Berrot AM, et al. Analysis of plateau and driving pressures: effects on mortality in patients with acute respiratory distress syndrome receiving lung-protective ventilation. Crit Care Med. 2017;17(5):843–850.
- Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure vs. Low PEEP o mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345.