ABSTRACT
Background: Shoulder pain secondary to laparoscopic interventions may cause more discomfort to the patient than the incision site pain, with a reported incidence varies from 35% to 80%.
Aim of the study:–To evaluate the effect of intrathecal dexmedetomidine on incidence and severity of laparoscopy-triggered shoulder tip pain.
Methods: Sixty patients, 1st patient recruited on the 1 July 2017, undergoing elective laparoscopic ovarian cystectomy under spinal anesthesia were randomly allocated to one of the two groups. Group C received intrathecal hyperbaric Bupivacaine 3.5 ml plus 0.5 ml normal saline. Group D received intrathecal hyperbaric Bupivacaine 3.5 ml plus 10 μg dexmedetomidine (0.5 ml). Measurements: Data on the severity of intraoperative shoulder pain were collected using a visual analogue scale.
Results: Twenty-four patients in Group C complained of intraoperative shoulder tip pain, 16 patients (53.3%) required fentanyl which was given in 25 μg increments, and total fentanyl consumption for 16 patients was 875 μg. Two patients were converted into general anaesthesia as pain was intolerable (≥ 4). In Group D, five patients (16.7%) experienced shoulder pain intraoperative with a mean VAS score 0.37 ± 0.9.
Conclusion: Intrathecal dexmedetomidine can effectively decrease the incidence and severity of shoulder tip pain during laparoscopic ovarian cystectomy under spinal anesthesia.
1. Introduction
Laparoscopy was first introduced to the medical field in the middle of the last century. It revolutionized the surgical interventions; it reduced the total medical expenditures and hastened postoperative recovery [Citation1]. General anaesthesia (GA) was considered the only anesthetic technique suitable for laparoscopic interventions. Respiratory and Cardiovascular impairment were the main aspects of concern which are thought to be better controlled by GA [Citation2].
Shoulder tip pain (STP) is a major concern during and after laparoscopy with a reported incidence varies from 35% to 80% [Citation3]. It is usually associated with significant morbidity secondary to pain severity. Laparoscopy induced shoulder tip pain is thought to be originated from retention of carbon dioxide inside the abdomen, eventually irritating the phrenic nerve and provoking referred pain in the C4 dermatome [Citation4].
Recently, application of RA in laparoscopic procedures started to gain familiarity due to its considerable advantages over GA concerning better postoperative analgesia, no airway manipulation, reduced risk of aspiration, and reduced postoperative nausea and vomiting [Citation5].
The two major concerns of laparoscopy under spinal anesthesia are Trendelenburg’s position and upper abdominal pressure. Pneumoperitoneum could initiate neck and shoulder pain within a few minutes, which is highly stressful to the patients and surgeons [Citation6].
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist which is approved by the Food and Drug Administration as an intravenous sedative and general anaesthetic adjuvant. However, intrathecal use of α2-receptor agonists was found to possess antinociceptive effects on somatic and visceral pain, which are believed to be mediated by suppression of neurotransmitters released from C-fibre and post-synaptic dorsal horn neurons hyperpolarization [Citation7]. Clinical trials revealed that dexmedetomidine decreases proinflammatory cytokines production, subsequently mitigates the systemic inflammatory response, and reduces mortality due to surgery and anaesthesia [Citation8]. Previous studies also suggested that intrathecal use of dexmedetomidine in doses varied from 0.2 to 1 µg/kg was free of any neurotoxic manifestations [Citation9]. Moreover, Its use in spinal cord injury revealed a neuroprotective effect resembles methylprednisolone [Citation9,Citation10].
Therefore, we hypothesized that dexmedetomidine by having an anti-inflammatory and immune-modulatory effects could reduce the incidence and severity of shoulder tip pain, which is the main concern in laparoscopic surgery, and facilitates laparoscopic interventions under spinal anaesthesia. To the best of our knowledge, no much literatures available regarding the use of intrathecal dexmedetomidine to reduce STP in laparoscopic ovarian cystectomy.
2. Aim of work
The objective of this study was to evaluate the effect of adding dexmedetomidine to intrathecal bupivacaine on the incidence and severity of shoulder tip pain during laparoscopic ovarian cystectomy.
3. Methods
Ethical consideration and Trial registration: This prospective, double-blind, randomized controlled study was done at Assiut University Hospital after approval of the Faculty of Medicine Institutional Review Board (IRB) registration number (No. 13,700,422). The study was registered on clinical-trials.gov (I.D NCT03172065). The procedures followed in this study were compliant with the Helsinki Declaration of 1975, revised in 2013. Informed written consent was obtained from the participants at the end of the preoperative visit after explaining the study protocol, benefits, risks, and alternative modalities of anaesthesia and postoperative analgesia. Participants were able to withdraw from participation at any time.
Inclusion criteria: Elective laparoscopic ovarian cystectomy, age 20–40 years, expected surgery time of 30 minutes, and inflation pressure ≤14 Cm H2O. Exclusion Criteria: Contraindications to neuroaxial anaesthesia, pregnancy, body mass index >30 and patients refusal.
4. Study outcome
Primary outcome: incidence and severity of shoulder tip pain between the two groups.
Secondary outcome: intraoperative hemodynamic stability, et CO2, SaO2.
Randomization: Consented participants were randomly allocated to one of the two groups using computer-generated number (random allocation software). Numbers were kept in a sealed envelope, which was opened 30 minutes before an anaesthesia induction by an anaesthesiologist, who is responsible for preparing the local anaesthetic mixture and labeling them with the patient′s number, but he was not involved in block administration or data collection. Another anesthesiologist who was responsible for the administration of spinal anesthesia remained blind to the selected group until the end of the study. Participants, data collectors, and anaesthetist involved in block administration and intraoperative data collection were kept blind to the study group.
Study Groups: Sixty patients were randomly allocated to one of the two groups. Group C (control n = 30) received intrathecal hyperbaric Bupivacaine 3.5 ml (17.5 mg) plus 0.5 ml normal saline. Group D (dexmedetomidine n = 30) received intrathecal hyperbaric Bupivacaine 3.5 ml (17.5 mg) plus 10 μg (0.5 ml) dexmedetomidine (Precedex; Fersenius Kabi).
Anaesthesia technique: All patients were premedicated with I.V ondansetron 4 mg and preloaded with 1000 ml of ringer lactate 30 minutes before surgery. At the operating theatre intraoperative monitoring with non-invasive blood pressure (NIBP), electrocardiograph (ECG), capnography, and pulse oximetry were attached to the patient and baseline data were recorded. Spinal anesthesia was given in sitting position at L3–4 interspace, using 25 gauge Quincke’s spinal needle under full aseptic precautions to achieve T6 block level. Hypotension was considered if systolic blood pressure ≤90 mmHg and was treated with incremental shots of 6 mg ephedrine and I.V fluids. Intraoperative heart rate, oxygen saturation, and etCO2, by a side stream capnography at the nostrils, were continuously monitored during the procedure. Bradycardia was considered if HR ≤ 55 beat/m and was treated with I.V atropine 0.05 mg/kg.
Surgical Technique: All operations were done by the same surgical team. The main (10 mm) trocar was introduced through an infra-umbilical incision, and two lateral trocars (5 mm) were placed at the level of the iliac crest lateral to the rectus muscle and inferior epigastric vessels and inflation pressure was kept below 14 mmHg.
Data collection: Intraoperative and postoperative data collection was done by the anaesthesiologist who did the block but was unaware of patient allocation. Intraoperative Data: Time to reach Bromage score III, intraoperative STP was assessed by visual analogue scale (VAS).
Pain was treated with 25 μg fentanyl which was repeated as needed. Conversion to general anesthesia was considered if pain was intolerable and not responding to fentanyl.
Hemodynamic parameters (blood pressure and Heart rate) were recorded every 5 minutes till the end of the procedure. Sedation was assessed by Ramsay score.
Postoperative data: STP was assessed for up to 24 hours postoperative (being a one-day surgery, patients were discharged on the second-day morning). Time of two segment regression to S1 tested by cold sensation, time to Bromage scale I (time from Bromage scale III after block to the ability of the patient to move the ankle and feet) and time of the first analgesic request: It is time from sensory block to patient′s request for analgesia. Rescue analgesia was in the form of Ketorolac 0.75 mg/kg if VAS ≤ 4 and Morphine sulfate 0.5 mg/kg if VAS > 4
Sample size calculation: The sample size was performed using G*Power version 3.1 based on a previous study [Citation11]. This sample size was estimated to be able to detect a significant difference in intraoperative pain score of 2 between the groups. This sample size was estimated to be able to detect a significant difference in postoperative STP score of two between the groups. An estimated standard deviation (SD) of 2.2 and α of 0.05 in 30 patients of each group would yield 80% power.
Statistical analysis: Data analysis was done using the IBM SPSS 20.0 software. Normality was tested by Kolmogorov–Smirnov test before further statistical analysis. Categorical variables were represented as number and percent (N, %), while continuous variables were described by mean and standard deviation (Mean, SD). Analysis of categorical data was done by Chi-square test, while continuous variables were analyzed by (Independent-samples T Test) for parametric data and (Mann–Whitney U) for nonparametric data. A two tailed p value < 0.05 was considered to be statistically significant.
5. Results
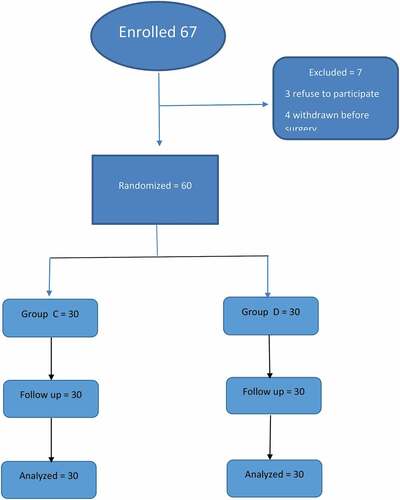
Sixty-seven patients were enrolled in the study (), seven were excluded, three refuse to participate from the start, four cases withdraw after consent and asked for general anaesthesia in the operating room before the start of surgery. Sixty patients were randomly allocated to two groups, with 30 patients in each. No statistical significant difference was detected in demographic and surgical data between both groups ().
Table 1. Demographic and surgical data
As shown in , we found a significant difference in the block characteristics between both groups. The time needed to reach T6 sensory dermatome was longer in Group C, compared to Group D. The time needed to reach Bromage 3 was shorter in Group D compared to Group C. Sensory and motor block regression was delayed in Group D. The time required reaching S1 sensory dermatome and the time to Bromage 0 were longer in Group D.
Table 2. Block characteristics
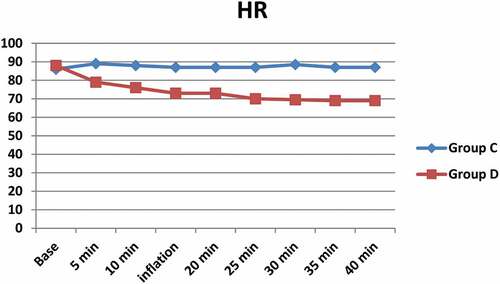
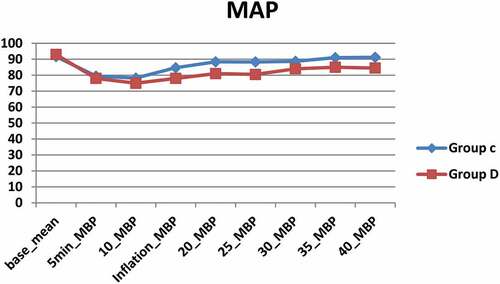
shows a significant difference between both groups with lower heart rate () and blood pressure () in Group D compared to group C (). No significant hypotension occurred in any of the study groups. Two cases in Group D represented bradycardia with a heart rate of less than 55 bpm, both of them treated with atropine 0.05 mg/kg. O2 saturation remained ≥ 96% in both groups; an insignificant decrease from the baseline has occurred in both groups, changes in both groups were not significant. A slight increase in the EtCO2 was noticed after pneumoperitoneum, but it was comparable and not significant between both groups. Patients in Group D were calmer and tranquil than Group C. only one patient was deeply sedated (score 4) and required airway support.
Table 3. Intraoperative clinical data (30 min after block)
Figure 4. Incidence of shoulder pain in the study group. Group C is control group, Group D is dexmedetomidine group
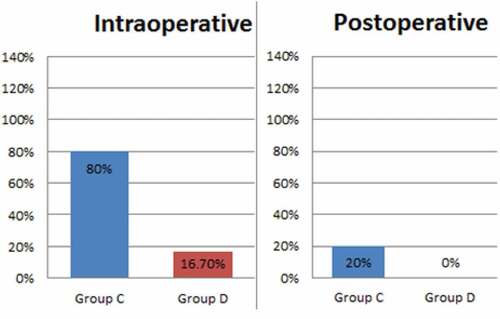
As shown in , we found a reduction in the incidence of intraoperative shoulder tip pain from 80% to 16.7%, and 79% reduction in intraoperative VAS severity, where 24 patients in Group C (80%) complained of intraoperative shoulder tip pain with a mean VAS 1.80 ± 1.21. Two patients were converted into general anaesthesia (GA). In Group D, five patients out of 30 (16.7%) experienced shoulder tip pain intraoperative with a mean VAS 0.37 ± 0.9. No patient required general anaesthesia conversion. Total intraoperative fentanyl consumption for Group C was 875 μg versus 150 μg in Group D, with 83% reduction in the amount of intraoperative analgesia required. The average amount of fentanyl consumed in all patients of each group was 29 ± 34.8 in group C versus 5 ± 15.3 in group D; P < 0.001. Six patients (20%) developed STP within 24 hours postoperative, VAS was ≤ 4 with good response to I.V ketorolac infusion, while in Group D, no patient complained of STP up to 24 hours postoperative.
Table 4. Intraoperative and postoperative shoulder pain
6. Discussion
The major finding of our study is that intrathecal administration of dexmedetomidine reduces the incidence of intraoperative (80% in Group C versus 16.7% in Group D) and postoperative (20% versus 0%) shoulder tip pain in patients undergoing Laparoscopic Ovarian Cystectomy. Also, intrathecal dexmedetomidine significantly decreased the severity of intraoperative shoulder tip pain by 79% pain (mean VAS in Group C 1.80 ± 1.2 versus 0.37 ± 0.9 Group D). Moreover, we found a reduction in intraoperative analgesic requirement (fentanyl) by 83%. We think that this action might be due to the central antinociceptive, anti-inflammatory, and immune modulatory effects of dexmedetomidine. Dexmedetomidine could inhibit somatic and visceral pain transmission, due to its ability to suppress the release of neurotransmitters from C-fiber and induce post-synaptic dorsal horn neurons hyperpolarization [Citation7]. Laparoscopy induced shoulder pain may cause more discomfort to the patient than the incision site pain [Citation4]. Different mechanisms are accused; clinical trials were conducted to estimate the mechanism of laparoscopic induced shoulder pain. Tasi et al. (2011) concluded that laparoscopy induced shoulder pain is mainly caused by retention of carbon dioxide inside the abdomen, eventually irritating the phrenic nerve and precipitating referred pain in the distribution of C4 dermatome [Citation4]. In contrast to the Tsi et al.’s finding, Wang et al. in 2001 found that direct irritation of the diaphragm by retained CO2 may not be the leading cause of shoulder pain. They conducted a study on 90 patients who underwent laparoscopic cholecystectomy and were randomly allocated into three groups (30 patients in each group): Group C; nothing was done after laparoscopic procedures, Group D; residual CO2 was absorbed in the end of the procedure. Group C; O2 was insufflated three times and then complete absorption of CO2 was done. They found that shoulder pain incidence and severity were higher in group C (O2 group) than in the other two groups. Therefore, they deduced that excess traction on the triangular ligament and diaphragmatic fibers over-stretching secondary to pneumoperitoneum might be the major causes of shoulder pain [Citation12]. Pneumoperitoneum can cause blood vessels tearing, traumatic traction of the nerves, and inflammatory mediators overflow [Citation13]. If CO2 accumulated under the diaphragm, it will irritate the phrenic nerve, and subsequently results in shoulder tip pain which in turn induces inflammatory mediators release and stimulates the nociceptors at the nerve endings [Citation14]. According to previous studies, the percentage of patients that experienced laparoscopic induced shoulder tip pain ranges from 35% to 80%. The severity could extend from mild to severe, and the duration could persist for up to 72 hours after interventions [Citation15]. Inflammatory modulation of dexmedetomidine was tested in many trials; Li et al. conducted a metaanalysis, they found that perioperative use of dexmedetomidine as a general anesthesia adjuvant resulted in a significant reduction of the postoperative serum levels of IL-6, IL-8, and TNF-α [Citation16]. Anti-inflammatory effects of dexmedetomidine are explained by its ability to module cytokine production during stress response which might be mediated by α2-adrenoceptors, suppress apoptosis, and central stimulation of the cholinergic anti-inflammatory pathway [Citation17].
Also, intrathecal dexmedetomidine significantly enhanced the onset of sensory and motor
blocks and significantly prolongs block duration.
Ghodki et al. reported that intrathecal clonidine in a dose of (30 ug) provides good sedation and adequate intraoperative and postoperative analgesia, and simultaneously abolishes laparoscopic induced shoulder tip pain [Citation18]. Zhang and colleagues performed a meta-analysis to evaluate the characteristics of clonidine and dexmedetomidine when used as local anesthetic adjuvants. They concluded that dexmedetomidine could sensory block onset and duration, and delays the time of first analgesic request [Citation19].
Bhure et al. concluded that intrathecal dexmedetomidine (10 ug and 15 ug) significantly reduced the sensory and motor blocks onset, prolonged the time of two segment regressions in a dose-related manner, increased sensory block duration and delayed the first rescue analgesia demand with an overall reduction in analgesics consumption [Citation20]. Gupta and colleagues reported the same results as previously mentioned studies regarding the block onset and duration, in addition to temperate degree sedation, which was significantly higher in patients who received 10 ug intrathecal dexmedetomidine compared to those who received 5 ug intrathecal dexmedetomidine [Citation21]. Dexmedetomidine significantly enhances the neuraxial block onset and duration, and the consequent analgesia compared to placebo in a dose dependent manner [Citation22]. Rahimzadeh et al. compared intrathecal dexmedetomidine versus fentanyl, and they reported that dexmedetomidine has longer duration of sensory and motor block, longer postoperative analgesia and lower side effects than intrathecal fentanyl [Citation23]. Sun and colleagues in their meta-analysis of nine studies confirmed that intrathecal dexmedetomidine, compared to intrathecal fentanyl, enhanced the duration of neuraxial block, improved postoperative analgesia, minimized pruritus incidence, and did not increase the incidence of hemodynamic instability [Citation24].
Our study showed a significant difference in blood pressure and heart rate between both groups after pneumoperitoneum and up to the end of operation with lower blood pressure and heart rate in dexmedetomidine group compared to control group. It is possibly due to a higher dose (10ug) of dexmedetomidine.
Sarma and colleagues found that intrathecal dexmedetomidine 10 ug induced a significant reduction in mean arterial blood pressure and heart rate without causing hypotension [Citation25].
Halder and colleagues found that intrathecal dexmedetomidine doses of 5ug and 10ug resulted in statistically and clinically significant bradycardia and a decrease in mean arterial pressure. Other side effects, such as hypotension, nausea, vomiting and shivering, although noted in both groups, were statistically insignificant [Citation26]. Gupta et al. also observed similar side effects without any significant difference between intrathecal dexmedetomidine 5ug and 10 ug [21]. Naaz and colleagues found that the mean values of MAP and HR were comparable between the intrathecal dexmedetomidine 5ug, 10ug, 15ug and 20ug groups with dose-dependent reduction in the side effects.
Therefore, we can conclude that intrathecal dexmedetomidine can effectively decrease the incidence and severity of shoulder tip pain, an action mostly mediated by the anti-inflammatory and antinociceptive effect of dexmedetomidine, in laparoscopic ovarian cystectomy under spinal anesthesia.
Study limitations: The sample size was small, so we recommend additional randomized controlled trials with larger sample sizes. Visual analogue score is a subjective indicator, which may not be so precisely described by patients.
Recommendations: Further study on this may help us to clarify the mechanism of post-operative pain after laparoscopic gynaecological surgery and develop more effective methods to reduce the intensity of pain in different types of patients receiving gynaecological laparoscopic surgery.
7. Main points
Laparoscopic induced shoulder pain may cause more discomfort to the patient than the pain at the incision site, with a reported incidence varies from 35% to 80%.
Many mechanisms are in charge; diaphragmatic irritation by CO2 insufflation which also stimulates inflammatory mediators release, excessive traction of the triangular ligament and over-stretching of the diaphragmatic fibers due to gas insufflation and rapid distension of the peritoneum may be associated with tearing of blood vessels, traumatic traction of the nerves and release of inflammatory mediators.
Dexmedetomidine is a highly selective α2 adrenoceptor agonist with central antinociceptive and immunomodulatory effects, it reduces secretion of pro-inflammatory cytokines, which in turn alleviates systemic inflammatory response.
In our study, intrathecal dexmedetomidine was proven to facilitate conduction of laparoscopic gynecological procedures under spinal anaesthesia by reducing incidence and severity of laparoscopic induced shoulder tip pain.
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper, or might be considered as potential competing interests.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
References
- Bajwa SJ, Kulshrestha A. Anaesthesia for laparoscopic surgery: general vs regional anaesthesia. J Minim Access Surg. 2016;12(1):4–9.
- Bajwa SJ, Takrouri MS. Innovations, improvisations, challenges and constraints: the untold story of anesthesia in developing nations. Anesthesia: Essays and Researches. 2014;8(1):1–2.
- Demco L. Effect of heating and humidifying gas on patients undergoing awake laparoscopy. J Am Assoc Gynecol Laparosc. 2001;8(2):247–251.
- Tsai HW, Chen YJ, Ho CM, et al. Maneuvers to decrease laparoscopy-induced shoulder and upper abdominal pain: a randomized controlled study. Arch Surg. 2011;146(12):1360–1366.
- Jun G-W, Kim M-S, Yang H-J, et al. Laparoscopic appendectomy under spinal anesthesia with dexmedetomidine infusion. Korean J Anesthesiol. 2014 Oct;67(4):246.
- Asgari Z, Rezaeinejad M, Hosseini R, et al. Spinal anesthesia and spinal anesthesia with subdiaphragmatic lidocaine in shoulder pain reduction for gynecological laparoscopic surgery: a randomized clinical trial. Pain Res Manag. 2017;2017:1–6.
- Bogra J, Kohli M, Kumar S, et al. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55(4):347.
- Lee JM, Han HJ, Choi WK, et al. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: a prospective, randomised, double-blind, doseresponse clinical study. BMC Anesthesiol. 2018;18(1):164.
- Elhadary IH, El Kenany S, Elhadary HM, et al. Evaluation of intrathecal dexmedetomidine for spine surgery. Research and Opinion in Anesthesia and Intensive Care. 2019 Apr;1(6):220.
- Celik F, Göçmez C, Kamaşak K, et al. The comparison of neuroprotective effects of intrathecal dexmedetomidine and metilprednisolone in spinal cord injury. Int J Surg. 2013 Jun;11(5):414–418.
- Jabbour-Khoury SI, Dabbous AS, Gerges FJ, et al. Intraperitoneal and intravenous routes for pain relief in laparoscopic cholecystectomy. JSLS. 2005;9(3):316–321.
- Wang Z, Cao Y, Chang Y. Shoulder pain after laparoscopic cholecystectomy. Zhonghua Wai Ke Za Zhi. 2001 Nov;39(11):858–860.
- Choi JB, Kang K, Song MK, et al. Pain characteristics after total laparoscopic hysterectomy. Int J Med Sci. 2016;13(8):562.
- Phelps P, Cakmakkaya OS, Apfel CC, et al. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Dixon JB, Reuben Y, Halket C, et al. Shoulder pain is a common problem following laparoscopic adjustable gastric band surgery. Obes Surg. 2005;15(8):1111–1117.
- Li B, Li Y, Tian S, et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5(1):12342.
- Sh KANG, YS KIM, Th HONG, et al. J. Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013 Apr;57(4):480–487.
- Ghodki PS, Sardesai SP, Thombre SK. Evaluation of the effect of intrathecal clonidine to decrease shoulder tip pain in laparoscopy under spinal anaesthesia. Indian Journal of Anaesthesia. 2010;54(3):231–234.
- Zhang C, Li C, Pirrone M, et al. Comparison of dexmedetomidine and clonidine as adjuvants to local anesthetics for intrathecal anesthesia: a meta-analysis of randomized controlled trials. J Clin Pharmacol. 2016;56(7):827–834.
- Sudheesh K, Rao RR, Kavya M, et al. Comparative study of two doses of intrathecal dexmedetomidine as adjuvant with low dose hyperbaric bupivacaine in ambulatory perianal surgeries: a prospective randomised controlled study. Indian J Anaesth. 2015;59(10):648–652.
- Gupta M. Effect of 3 Different Doses of Intrathecal Dexmedetomidine (2.5µg, 5µg, and 10 µg) on Subarachnoid Block Characteristics: a Prospective Randomized Double Blind DoseResponse Trial. Pain Physician. 2016;3;19(3;3):E411–20.
- Naaz S, Bandey J, Ozair E, et al. Optimal dose of intrathecal dexmedetomidine in lower abdominal surgeries in average Indian adult. J Clin Diagn Res. 2016;10(4):Uc09–13.
- Rahimzadeh P, Faiz SHR, Imani F, et al. Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiology. 2018;18(1):62.
- Sun S, Wang J, Bao N, et al. Comparison of dexmedetomidine and fentanyl as local anesthetic adjuvants in spinal anesthesia: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3413–3424.
- Sarma J, Narayana PS, Shivakumar MC, et al. A comparative study of intrathecal clonidine and dexmedetomidine on characteristics of bupivacaine spinal block for lower limb surgeries. Anesthesia: Essays and Researches. 2015;9(2):195–207.
- Halder S, Das A, Mandal D, et al. Effect of different doses of dexmedetomidine as adjuvant in bupivacaine -induced subarachnoid block for traumatized lower limb orthopaedic surgery: a prospective, double-blinded and randomized controlled study. J Clin Diagn Res. 2014;8(11):Gc01–6.