ABSTRACT
Background: This study aimed to evaluate safety and efficacy of adding two different doses of neostigmine to the local anesthetic lidocaine in peribulbar block anesthesia for cataract surgery.
Methods: This randomized, double blind, parallel group, controlled trial (Trial registration number: IRCT20210106049952N1) included 60 patients (30–70 years-old), ASA physical status I, II, or III who were scheduled for cataract surgery. Patients received standard peribulbar block (Group C) or standard block plus 0.25 mg neostigmine (Group N25) or 0.5 mg neostigmine (Group N50). All patients were assessed for primary outcomes including hemodynamics and onsets of motor and sensory blocks. Secondary outcomes included duration of block, patient’s and surgeon’s satisfaction, postoperative pain, and time to first analgesic dose.
Results: Mean onset of motor block was significantly shorter in N25 and N50 groups (72.25 ± 20.3 and 44.3 ± 10.4 sec, respectively) than in control group (110.25 ± 35.6 sec). Onset of sensory block was significantly shorter in N50 group compared to control group as well as N25 group (P < 0.001). A significantly longer mean duration of block was observed in N50 group (173.55 ± 31.09 min) in comparison to control group (79.75 ± 12.51 min) and N25 group (81.25 ± 6.25 min). Mean time till first analgesic dose showed significant differences between the three groups C, N25, and N50 (2.85 ± 0.47, 3.45 ± 1.28, and 7.1 ± 1.25 h, respectively, P < 0.001). Only two patients belonging to N50 group developed postoperative nausea and vomiting.
Conclusion: Neostigmine in 0.5 mg was superior to 0.25 mg dose when added to the local anesthetic lidocaine in peribulbar anesthesia for cataract surgery regarding both sensory and motor block as well as postoperative analgesia.
1. Introduction
General anesthesia has many complications especially in elderly patients with concurrent comorbidities. Alternatively, regional anesthesia is safer and can provide the required analgesia and akinesia for the majority of ophthalmic surgical procedures in adults [Citation1].
Eye block using local anesthetics has been done for a long time through retrobulbar injection. Further, peribulbar technique has been developed because it has lesser risk of globe perforation and optic nerve injury [Citation2].
Peribulbar block is commonly used for cataract surgery but using only local anesthetics for peribulbar anesthesia has some drawbacks. These include delayed onset of globe akinesia and corneal anesthesia as well as short duration of analgesia. So, the block frequently needs extra doses of rescue analgesics [Citation3].
Various adjuvants to regional anesthesia (e.g., hyaluronidase, adrenaline, fentanyl, and magnesium sulphate) have been investigated to provide the optimal surgical conditions for the surgeon as well as the patients. They may enhance and prolong analgesia of local anesthetics and may also produce analgesia themselves. As well, they may lower the dose requirements of local anesthetics, and thus reduce their dose-dependent side effects, for example, motor block and nausea, with possible increase in the degree of satisfaction [Citation4,Citation5].
This study aimed to evaluate the efficacy and safety of adding two different doses of neostigmine to the local anesthetic lidocaine during peribulbar anesthesia in cataract surgeries.
2. Methods
2.1. Ethical considerations
The study protocol was approved by the Ethics Committee of the Research Institute of Ophthalmology (1–12–2020). The trial was registered at the Iranian Registry of Clinical Trials (IRCT Id: IRCT20210106049952N1, Registration date: 10–01–2021). An informed, written consent was obtained from each patient prior to commencement of the study.
We intend to share the individual de-identified participants’ data. Data will be accessible through direct contact with the corresponding author, beginning 6 months and ending 24 months following article publication.
2.2. Study design, setting, and date
This was a randomized, double blind, parallel group, controlled trial with 1:1:1 allocation ratio. It was conducted at the Research Institute of Ophthalmology, Giza, Egypt during January 15 to 26 February 2021.
2.3. Eligibility criteria
We included adult (30–70 years-old), male or female patients, indicated for cataract surgery who were ASA I, II, or III. We excluded patients with any of the following conditions: coagulopathy or had been on anticoagulant drugs, infection at the site of the surgery, posterior staphyloma, allergy to any of the used drugs, bronchial asthma, or bradyarrhythmia.
2.4. Outcomes
The primary outcome variables included the onset of motor and sensory blocks as well as the patients’ hemodynamics (heart rate, blood pressure, oxygen saturation). The secondary outcomes included the duration of the block, patient’s and surgeon’s satisfaction, postoperative pain, and time to first analgesic dose. Safety was assessed looking for complications or drug-related adverse effects.
2.5. Intervention
The trial recruited 60 patients who were randomized into three groups (20 participants each). Each patient in the intervention groups received 9 ml of lidocaine 2% (containing 1 ml = 10 units of hyaluronidase) plus 0.5 mg or 0.25 mg of neostigmine (Group N1 or Group N2, respectively). Each patient in the control group (Group C) was treated the same way but received 1 ml of saline instead of the neostigmine dose. The peribulbar block was done with two injections using a 25-G, one-inch-long needle. The first injection was inferotemporal, where the needle was inserted through the fornix below the lateral limbus. In the second injection, the needle was advanced between the caruncle and the medial canthus in a medially backward direction, away from the globe.
2.6. Randomization and blinding
We used the sealed, opaque, sequentially numbered envelopes method for randomization and allocation concealment of patients included in this trial. We used 60 identical, opaque, letter-sized envelopes into which sheets of the household aluminum cooking foil were inserted to assure its opacity. We prepared 60 envelope-sized sheets of white paper and 60 envelope-sized sheets of single sided carbon paper. We wrote “Treatment A” on 20 paper sheets, “Treatment B” on another 20 sheets, and “Treatment C” on the last 20 sheets. To prepare 20 “Treatment A” envelops, we selected one envelope-sized sheet of Treatment A and placed one sheet of carbon paper on top of the Treatment A allocation paper with the carbon side facing the paper, then we put both papers inside a foil wrapper. Then, the completed insert was placed into a blank envelope with the carbon paper closest to the front of the envelope. Finally, the envelop was sealed and we signed across the seal. We completed all the 20 “Treatment A” envelops the same way. We prepared 20 “Treatment B” and 20 “Treatment C” envelops the same way as “Treatment A” envelops. The three sets of envelops were combined and we shuffled them thoroughly. Then, using a pen we marked a number on the front of each envelope sequentially from 1 to 60. The carbon paper inside the envelope transferred this number to the allocation paper inside. Finally, we placed these envelopes into a plastic container, in numerical order, ready for use.
Participants, health professionals carrying out the peribulbar block, and those assessing the participants’ outcomes were blinded to the type of intervention.
2.7. Monitoring
All patients were fasted for 6 h preoperatively. In the operating room, 22 G cannula was inserted and the patient was attached to a multichannel monitor to record the baseline ECG, heart rate, systolic and diastolic blood pressures, and oxygen saturation. The patient was lying supine with a nasal cannula that delivered oxygen at a rate of 3 L/min. Then, the patient received 0.03 mg of fentanyl, 2 mg of dormicum, and 20 mg of propofol for sedation before carrying out the block. The local anesthetic solution was prepared by health professionals not included in the study in syringes of equal volume for the purpose of the blinding. After cannulation, the eye was sterilized using bovidone iodine, and benoxinate eye drops was applied. Then, the peribulbar anesthesia was accomplished and the outcome parameters and adverse effects were monitored.
2.8. Statistical analysis
All data were analyzed using SPSS software, version 22 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Continuous data were tested for normality by Shapiro Wilk test. Normally distributed continuous data were expressed as means and standard deviations. One-Way ANOVA test was used to compare the studied groups. When its results were significant, post-hoc Tukey test was applied to determine pairwise comparison between the studied groups. The skewed data were expressed as median and interquartile range (25th-75th percentiles) and were compared using Kruskal–Wallis test followed by post-hoc Bonferroni test. Categorical data were expressed as numbers and percentages and were compared using Chi-Square test. P < 0.05 was considered statistically significant.
3. Results
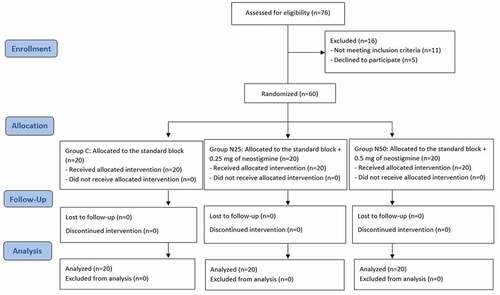
In the present study, 76 patients were assessed for eligibility, out of whom 16 patients were excluded. This study included 60 patients who were randomly assigned into 3 groups with 1:1:1 allocation ratio. The final analysis involved 20 subjects in each group, and each of them was analyzed in the group to which he was originally assigned to ().
shows homogenous distribution of age and gender among the studied groups with no significant differences (P > 0.05).
Table 1. Demographic data of the studied groups
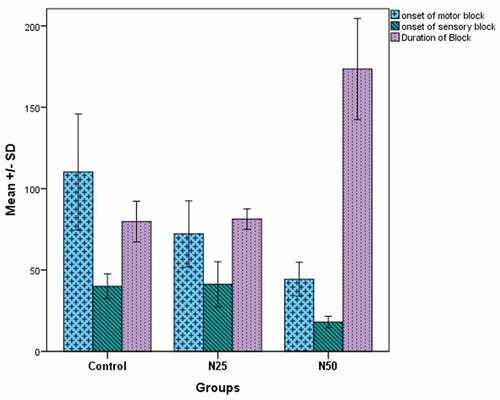
The mean onset time of motor block was significantly shorter in groups N25 and N50 (72.25 ± 20.3 s and 44.3 ± 10.4 s, respectively) than in the control group (110.25 ± 35.6 s). As well, the onset of motor block was significantly shorter in group N 50 compared to group N25. Additionally, the onset of sensory block was significantly shorter in group N50 compared to the control group as well as the N25 group (P < 0.001), while there was no significant difference between group N25 and the control group (P = 0.933). Also, a significantly longer mean duration of the block was observed in group N50 (173.55 ± 31.09 min) in comparison to the control group (79.75 ± 12.51 min) and group N25 (81.25 ± 6.25 min), with no significant difference between group N25 and the control group (P = 0.881) ().
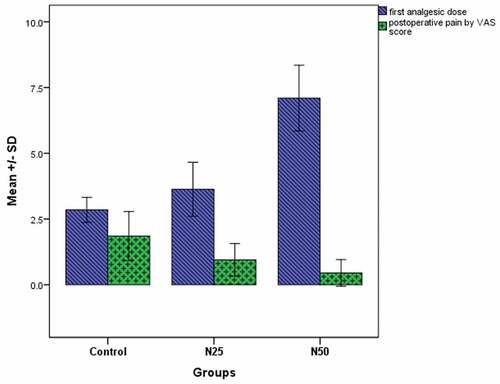
shows that the mean time till the first analgesic dose was significantly different among the three groups C, N25, and N50 (2.85 ± 0.47 h, 3.45 ± 1.28 h, and 7.1 ± 1.25 h, respectively, P < 0.001). Also, the median visual analogue score (VAS) was significantly lower in the groups N25 and N50 compared to the control group (P = 0.005 and P < 0.001, respectively), while there was no significant difference between groups N25 and N50 (P = 0.165).
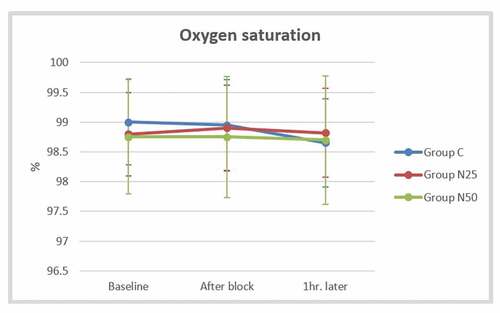
The mean arterial pressure did not display a significant difference between the studied groups after block and one hour later (P > 0.05) (). The mean heart rate after the block was significantly lower in group N25 (71.8 ± 8.16 beats/min) than in the control group (80.3 ± 7.51 beats/min) as well as the group N50 (78.85 ± 6.96 beats/min), but there was no significant difference between group N50 and the control group (P = 0.803). The mean heart rate recorded one hour later showed similar findings to that observed after the block (). The mean oxygen saturation showed non-significant differences among the three groups at all studied time points (P > 0.05) ().
Figure 4. Comparison of the mean arterial pressure between the studied groups at the baseline, after the block, and one hour later
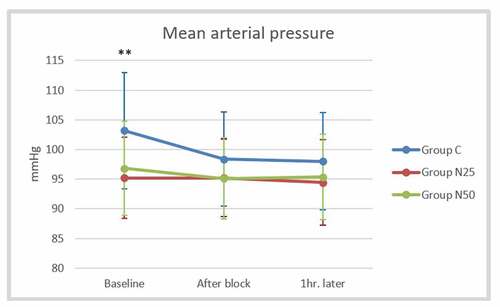
Figure 5. Comparison of the mean heart rate between the studied groups at the baseline, after the block, and one hour later
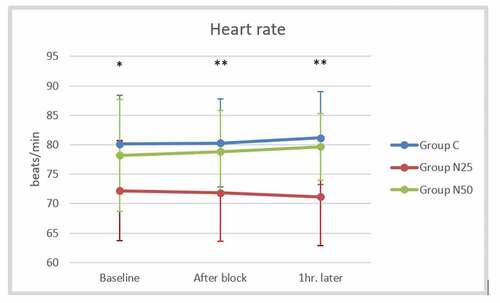
Figure 6. Comparison of the mean oxygen saturation between the studied groups at the baseline, after the block, and one hour later
illustrates the patient and surgeon satisfaction and the incidence of adverse effects in the studied groups. Furthermore, the percentages of the patients’ as well as the surgeons’ satisfaction in groups N25 and N50 (100% each) were comparable to that of the control group (90%), with no significant difference. Only two patients belonging to group N50 developed postoperative nausea and vomiting, with no significant differences in comparison to the other groups.
Table 2. Assessment of patient and surgeon satisfaction and the incidence of adverse effects in the studied groups
4. Discussion
Compared to general anesthesia, regional anesthesia is safer and can offer adequate analgesia and akinesia for most ophthalmic surgeries in adults [Citation1]. Despite being a common anesthetic procedure for cataract surgery, peribulbar block is associated with a short duration of analgesia [Citation3]. A number of adjuvants to regional anesthesia have been investigated with the aim of enhancing analgesia and reducing the dose of the local anesthetics and their accompanying adverse effects [Citation4,Citation5]. The current study aimed to evaluate safety and efficacy of adding neostigmine (in two different doses) to the local anesthetic lidocaine during peribulbar anesthesia in cataract surgeries.
The present study demonstrated that addition of 0.25 mg neostigmine to lidocaine 2% gave rise to an earlier onset of motor block, but it did not significantly change the onset of sensory block. Additionally, the use of neostigmine at 0.5 mg shortened both motor and sensory block onset times. Moreover, neostigmine at a dose of 0.5 mg caused a more rapid onset of motor and sensory block than 0.25 mg dose. The duration of the block was significantly prolonged only with the addition of 0.5 mg neostigmine. The adjuvant uses of neostigmine at 0.25 and 0.5 mg enhanced the postoperative analgesia with significantly lower VAS scores than in the control group. Each of the 0.25 and 0.5 mg neostigmine doses significantly prolonged the time to first postoperative rescue analgesic; however, the time was significantly more prolonged with the 0.5 mg dose. Adding neostigmine at 0.5 mg dose to the local anesthetic lidocaine 2% was more effective than 0.25 mg dose as it produced a better quality of the peribulbar block. Neostigmine at 0.5 mg dose resulted in faster onset and more prolonged duration of the sensory and motor globe anesthesia, and it reduced the postoperative pain and the need for analgesia.
Concerning the safety of the investigated neostigmine regimens, intraoperative and postoperative bradycardia was more evident with 0.25 mg neostigmine. Furthermore, only two patients out of 20 patients who received 0.5 mg neostigmine developed postoperative nausea and vomiting. All patients and surgeons were satisfied with the two regimens of neostigmine as they provided the sedation that enables full cooperation.
A comparable study assessed the effect of adding neostigmine 0.5 mg to local anesthetic mixture in peribulbar anesthesia for patients scheduled for trabeculectomy. Neostigmine improved the quality of surgical conditions where it accelerated the onset and prolonged the duration of sensory and motor blocks, delayed the time to first analgesic request, and increased satisfaction of the patients without any adverse effects [Citation6]. As well, Kayalha et al. [Citation7] evaluated the analgesic efficacy and the safety of adding 25 µg neostigmine to 20 mg bupivacaine during spinal anesthesia in a randomized, controlled study involving patients scheduled for lower limb orthopedic surgery. The authors concluded that neostigmine prolonged the time to the first analgesic request, and its use was not associated with any side effects. Another study proved that addition of 1 µg of neostigmine to intrathecal fentanyl increased the duration of analgesia and decreased the analgesic consumption in patients undergoing total knee replacement [Citation8].
A systematic review regarding the use of neostigmine as an adjuvant in neuraxial anesthesia reported postoperative analgesia following cesarean section. The risk of nausea was found with the intrathecal but not with the epidural neostigmine administration [Citation9].
Furthermore, intrathecal 50 µg neostigmine did not enhance the onset of sensory block, but prolonged the sensory and motor blockade of bupivacaine, together with effective postoperative analgesia in patients admitted for lower abdominal and lower limb surgeries. Furthermore, slower heart rates were detected in the neostigmine group compared to the placebo group at 5, 10, 60, 120, 240, 300, and 420 min after the surgery [Citation10].
Evaluation of additive effects of 500 µg of neostigmine to supraclavicular brachial plexus block in chronic renal failure patients revealed no effects on the duration of block. However, it resulted in rapid onset of sensory and motor blockade and enhancement of postoperative analgesia, with no significant side effects [Citation11].
In contrast to our findings, earlier studies that investigated the adjuvant effects of neostigmine reported that intrathecal neostigmine produced shorter duration of analgesia and the time to first request for analgesia compared to morphine in patients listed for elective total knee replacement under spinal anesthesia [Citation12]. Further, intrathecal neostigmine at a dose of 50 μg was inadequate for analgesia with increased incidence of vomiting, whereas neostigmine at a dose of 150 μg enhanced the postoperative analgesia up to 10 h. However, the later dose was associated with high incidence of postoperative nausea and vomiting, sweating, and salivation [Citation13]. Moreover, Gupta [Citation14] reported increased occurrence of bradycardia, hypotension, and postoperative nausea and vomiting with intrathecal 75 μg of neostigmine.
A recent study assessed the effect of nalbuphine in comparison to neostigmine as an adjuvant to intrathecal hyperbaric bupivacaine in patients who underwent hemorrhoidectomy under spinal anesthesia. The onset of motor block was significantly faster with neostigmine additive than nalbuphine. However, compared to neostigmine, nalbuphine showed greater prolongation of the duration of motor block and postoperative analgesia with decreased postoperative analgesic requirement [Citation15].
The anesthetic effect of lidocaine has been linked to inhibition of both substances P binding and substance P-evoked increase in intracellular calcium [Citation16]. Alternatively, the analgesic properties of neostigmine have been attributed to the release of nitric oxide and the eversible inhibition of cholinesterase enzyme, which results in an increased concentration of acetylcholine with consequent binding to both muscarinic (M1, M3, M2, and M4) and nicotinic receptors [Citation17].
The present study showed that two patients in the N50 group developed postoperative nausea and vomiting. Bradycardia was significantly prominent in the N25 group. An earlier phase I safety assessment of intrathecal neostigmine methyl sulfate in humans showed a dose-dependent incidence of the adverse effects; in particular, nausea and vomiting [Citation18]. Further, randomized clinical trials proved the same dose-dependent effects of neostigmine [Citation19,Citation20]. A more recent study investigated intrathecal neostigmine at doses of 50 and 150 μg as adjuvant to bupivacaine for postoperative analgesia under spinal anesthesia, and the incidence of nausea and vomiting was more in the 150 μg-neostigmine group [Citation21].
5. Conclusions
This study indicates that adding 0.5 mg of neostigmine to the local anesthetic lidocaine 2% in peribulbar anesthesia for cataract surgery enhanced the onset of sensory and motor blocks of the globe and prolonged the duration of the block. Thus, its use produced more suitable surgical conditions. Moreover, it showed effective postoperative analgesia, with prolonged time to the first analgesic dose without any side effects. Neostigmine at 0.5 mg was safe and exhibited a non-significant increase in the incidence of postoperative nausea and vomiting.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Weller CL, Tabor MH, Ryan LE, et al. Orbital Surgery: approaches and Techniques. Int Ophthalmol Clin. 2018;58:61–84.
- Jaichandran V. Ophthalmic regional anaesthesia: a review and update. Indian J Anaesth. 2013;57:7–13.
- Fayed SM, Mahdy MM, Ahmed AM, et al. Comparative study between effects of addition of fentanyl versus dexmedetomidine to local anesthetic mixture for peribulbar block for cataract surgery. Egypt J Hosp Med. 2018;72:4984–4989.
- Swain A, Nag DS, Sahu S, et al. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5:307–323.
- Schäfer M, Mousa SA, Shaqura M, et al. [Background and current use of adjuvants for regional anesthesia: from research to evidence-based patient treatment]. Anaesthesist. 2019;68:3–14.
- Mohamed A, Genidy M. Evaluation of the effect of neostigmine as adjuvant to local anesthetic mixture in peribulbar anesthesia. MJMR. 2014;25:211–219.
- Kayalha H, Mousavi Z, Sadat Barikani A, et al. The effects of intrathecal neostigmine added to bupivacaine on postoperative analgesic requirement in patients undergoing lower limb orthopedic surgery. Middle East J Anaesthesiol. 2015;23:199–204.
- Jain A, Jain K, Bhardawaj N. Analgesic efficacy of low-dose intrathecal neostigmine in combination with fentanyl and bupivacaine for total knee replacement surgery. J Anaesthesiol Clin Pharmacol. 2012;28:486–490.
- Cossu AP, De Giudici LM, Piras D, et al. A systematic review of the effects of adding neostigmine to local anesthetics for neuraxial administration in obstetric anesthesia and analgesia. Int J Obstet Anesth. 2015;24:237–246.
- Kumari Vasantha NS, Madhusudhana R. Intrathecal bupivacaine with neostigmine and bupivacaine with normal saline for postoperative analgesia: a cost-effective additive. Anesth Essays Res. 2018;12:328–332.
- Elbahrawy K, El-Deeb A. The effects of adding neostigmine to supraclavicular brachial plexus block for postoperative analgesia in chronic renal failure patients: a prospective randomized double-blinded study. Res Opin Anesth Intensive Care. 2016;3:36–41.
- Tan PH, Chia YY, Lo Y, et al. Intrathecal bupivacaine with morphine or neostigmine for postoperative analgesia after total knee replacement surgery. Can J Anaesth. 2001;48:551–556.
- Saini S, Sethi S, Malhotra N. Evaluation of intrathecal neostigmine for postoperative analgesia. J Anaesth Clin Pharmacol. 2005;21:419.
- Gupta S. Postoperative analgesia with intrathecal neostigmine; two different doses of 75 µgms and 50 µgms with heavy bupivacaine. Internet J Anesthesiol. 2010;25:1–7.
- Ashrey E, Bosat B. Nalbuphine versus neostigmine as an adjuvant to intrathecal hyperbaric bupivacaine-induced postoperative analgesia. Sci J Al-Azhar Med Fac Girls. 2020;4:153–158.
- Li YM, Wingrove DE, Too HP, et al. Local anesthetics inhibit substance P binding and evoked increases in intracellular Ca2+. Anesthesiology. 1995;82:166–173.
- Lauretti GR. The evolution of spinal/epidural neostigmine in clinical application: thoughts after two decades. Saudi J Anaesth. 2015;9:71–81.
- Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–343.
- Lauretti GR, Mattos AL, Gomes JM, et al. Postoperative analgesia and antiemetic efficacy after intrathecal neostigmine in patients undergoing abdominal hysterectomy during spinal anesthesia. Reg Anesth. 1997;22:527–533.
- Lauretti GR, Reis MP. Postoperative analgesia and antiemetic efficacy after subarachnoid neostigmine in orthopedic surgery. Reg Anesth. 1997;22:337–342.
- Pandey V, Mohindra BK, Sodhi GS. Comparative evaluation of different doses of intrathecal neostigmine as an adjuvant to bupivacaine for postoperative analgesia. Anesth Essays Res. 2016;10:538–545.