ABSTRACT
Background: This study aims to investigate the analgesic efficacy of ketamine and neostigmine as adjuvants to local anesthetic in ultrasound-guided Serratus anterior plane block for patients undergoing Modified Radical Mastectomy.
Methods: Ninety female patients aged 20–65 who were scheduled for a modified radical mastectomy under-combined general anesthesia and preoperative Serratus Anterior Plane Block were included. Three local anaesthetic mixtures were used, either 30 ml bupivacaine 0.25% + 1 ml ketamine (50 mg) (Group K) or 30 ml bupivacaine 0.25% + 1 ml neostigmine (500 μg) (Group N) or 30 ml bupivacaine 0.25% + 1 ml normal saline (Group S). The first 24 hours of postoperative morphine consumption was set as the primary outcome
Results: the 24 hr postoperative morphine consumption median was 3.0 (0.0–9.0) for group (S), 1.5 (0.0–4.0) for group (N) and 0.0 (0.0–4.0) mg for group (K) with statistical significant (P value 0.045). The first postoperative analgesic request was insignificant between group (K), group (N) and group (S) was (6.14 ± 5.17), (6.67 ± 3.18) and (5.89 ± 4.4 hr), respectively. The Intraoperative Fentanyl consumption showed a significant reduction in group (N) and group (K) (111.67 ± 30.64 and 110.00 ± 20.34, respectively) compared to group (S) (131.67 ± 42.51 μg). The numerical rating scale did not differ between study groups except at 8th and 16th hrs. Postoperatively.
Conclusion: The addition of 50 mg ketamine to 0.25% bupivacaine during preoperative ultrasound-guided SAPB combined with GA in female patients undergoing modified radical mastectomy decreased the 24 hr postoperative morphine consumption and the intraoperative fentanyl requirements while adding 500 µg neostigmine decreased the intraoperative fentanyl requirements.
Clinical trial registration: The study was registered at clinicaltrials.gov (NCT 04544228).
1. Introduction
Modified Radical Mastectomy is the fundamental surgical management for breast cancer. It accounts for 31% of all breast surgeries [Citation1]. Nearly 40–60% of patients experience severe acute postoperative pain. This pain might persist for 6–12 months and result in post-mastectomy pain syndrome and complex regional pain syndrome (causalgia) [Citation2,Citation3].
In the ultrasound-guided serratus anterior plane block (SAPB), the local anesthetic (LA) is injected in the compartment between the serratus anterior and latissimus dorsi muscles. SAPB anesthetizes the intercostobrachial nerve, lateral cutaneous branches of the intercostal nerves (T3–T9), long thoracic nerve, and thoracodorsal nerve. SAPB provides analgesia for breast and lateral thoracic wall surgeries [Citation4].
Several pharmaceuticals, including dexamethasone, nalbuphine, and dexmedetomidine, have been used as adjuvants to LA during SAPB. They revealed a better analgesic profile with a reduced postoperative opioid consumption [Citation5–7].
Ketamine, an intravenous anesthetic, has been used as an adjuvant to LA during combined sciatic-femoral nerve block [Citation8], axillary block [Citation9], infraclavicular brachial plexus block [Citation10], thoracic paravertebral block [Citation11], Modified Pectoral Block [Citation12], interscalene brachial plexus block [Citation13], and femoral nerve block [Citation14]. However, the ketamine results were controversial, varying from enhancing the LA’s onset and prolonged duration to not improving the onset or duration of the sensory block.
Neostigmine, a parasympathomimetic drug, proved efficacy as an adjuvant to LA during spinal anesthesia [Citation15–17]. However, its analgesic efficacy as an adjuvant to LA in peripheral nerve blocks or interstitial plane blocks is still unclear [Citation18].
This study was designed to investigate the analgesic efficacy of ketamine compared to neostigmine as adjuvants to LA in ultrasound-guided SAPB in patients undergoing Modified Radical Mastectomy. We hypothesized that adding either neostigmine or ketamine to bupivacaine in ultrasound-guided SAPB would increase the total analgesic duration and decrease the total 24 hr postoperative morphine consumption compared to SAPB with bupivacaine only. The first 24 hr of postoperative morphine consumption was set as the primary outcome. The time of the first request of analgesia and the total amount of intraoperative fentanyl consumption were set as the secondary outcomes.
2. Methods
This randomized controlled double-blinded study was conducted in Cairo University Hospitals after being approved by the Research Ethics Committee of the Faculty of Medicine, Cairo University (email: [email protected] ID: MS-101 − 2020). The study was registered on ClinicalTrials.gov identifier: (ID: NCT 04544228 on September 2020) before any patient was enrolled. Written informed consent was obtained from all patients. The Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed.
Ninety female patients aged 20 to 65 years with ASA physical status II–III, BMI 20 − 35 kg/m2, who were scheduled for modified radical mastectomy under general anesthesia were included in the study. Patients with ischemic heart disease, impaired pulmonary, liver, or kidney functions, long-standing diabetes, preexisting peripheral neuropathies, coagulopathy, and history of chronic pain or prolonged use of opioids were excluded from the study.
In the operating room, intravenous access was inserted, and Ringer acetate was started. Standard patient monitoring, including electrocardiogram (ECG), non-invasive blood pressure (NIBP), pulse oximetry, were connected. All patients were premedicated with IV midazolam 0.02 mg\kg. Patients were randomly allocated into one of the three groups using computer-generated random numbers kept in sealed envelopes. Group K: Patients received SAPB with an injection of 30 ml bupivacaine 0.25% + 1 ml ketamine (50 mg). Group N: Patients received SAPB with an injection of 30 ml bupivacaine 0.25% + 1 ml neostigmine (500 μg). Group S: Patients received SAPB with an injection of 30 ml bupivacaine 0.25% + 1 ml normal saline. The specific LA mixture solutions were prepared by a pharmacist who was not involved in the study.
2.1. Serratus Anterior Plane Block Technique (SAPB)
The patient was placed in the lateral decubitus position with the surgical side upwards and arm abducted. A linear ultrasound transducer (6–13 MHz) (Fujifilm Sonosite M-Turbo Ultrasound System) was used. The probe was placed on the transverse plane of the midaxillary line at the fifth rib level. The rib, pleural line, and overlying serratus anterior and latissimus dorsi muscles were visualized. After local skin infiltration with 3 ml of lidocaine 2%, a 38-mm 22-gauge (22-G, 50-mm Stimuplex A, BBraun, Melsung, Germany) regional block needle was advanced in-plane at an angle of approximately 45 degrees towards the fifth rib with 4 cm depth. After aspiration, a LA mixture was injected anteriorly to the rib and deep to the serratus anterior muscle.
General anesthesia was then induced intravenously using fentanyl 2 μg/kg, propofol 2 mg /kg, and rocuronium 0.5 mg/kg. Anesthesia was maintained with inhaled sevoflurane 2–3% in oxygen/air (FiO2 = 0.5) and rocuronium 0.1 mg\kg every 30–40 minutes guided by a nerve stimulator. Patients were mechanically ventilated to keep the end-tidal CO2 at 30–35 mmHg. Intraoperative fentanyl 1 μg/kg was given if MAP or HR increased >20% of the baseline values. The intraoperative MAP and HR were recorded before induction of GA (baseline reading), after induction of GA, after endotracheal intubation, before surgical incision, and at 30-min intervals until the end of surgery. Hypotension, defined as a reduction in the MAP > 20% of the baseline value, was treated by fluids and 5 mg ephedrine to be repeated to maintain MAP above 70 mmHg. Bradycardia, defined as HR < 50 beats/min, was treated by 0.4 atropine to be repeated if necessary. At the end of the surgery, the residual neuromuscular blockade was reversed using neostigmine (0.05 mg/kg) and atropine (0.02 mg/kg), and the patients were extubated after complete recovery of the airway reflexes.
The patients were transferred to the post-anesthesia care unit (PACU), where the Numeric Pain Rating Scale (NRS), MAP, and HR were recorded on arrival (T0), and then at 2, 8, 12, and 24 hr postoperatively. Analgesia in the form of IV paracetamol 500 mg \6 hours were provided. Rescue analgesia, in the form of morphine 3 mg IV bolus dose, was provided when the patient indicated NRS ≥ 3 and to be repeated every 15 minutes until NRS < 3. The maximum morphine daily dose was 0.5 mg/kg. The total morphine consumption over the first 24 hr was recorded. The block was considered failed block if the patient required more than two doses of rescue analgesia in the first hour postoperatively. Postoperative nausea and vomiting (PONV) were recorded and treated by 0.1 mg/kg of IV ondansetron. The morphine consumption in the first 24 hr postoperatively was set as the primary outcome of this study. The following were set as secondary outcomes: (i) Time of the first request of analgesia (defined as the time interval between the end of LA injection until the NRS was ≥3). (ii) The total amount of intraoperative fentanyl consumption. (iii) The intraoperative and postoperative HR and MAP. (vi) Block-related complications including LA toxicity, hematoma formation, and pneumothorax. (vii) Postoperative nausea and vomiting.
2.2. Sample size and Statistical analysis
Based on a previous study [Citation13] and the assumption that neostigmine would give the same effect as ketamine when added to bupivacaine in serratus anterior block and would decrease the total amount of morphine consumption in the first 24 hr postoperatively by (20%) when compared to control group (bupivacaine only). With α = 0.05, power of 80%, and an effect size of (0.34), a sample size of 90 patients (30 per group) was required. The G Power 3.1.9.2 program was used for the sample size calculation
Results are expressed as mean ± standard deviation, median (minimum-maximum), or number (%). Comparison between categorical data was performed using Chi-square test or Fisher exact test as appropriate. Normality of datat was tested using Kolmogorov-Smirnov test. Comparison between normally distributed variables in the three groups was performed using one-way ANOVA test followed by Tukey test as a post hoc test if significant results occurred. The comparison was performed using the Kruskal Wallis test followed by the Mann–Whitney test as a post hoc test if significant results occurred in not normally distributed variables. Statistical analysis was performed using the Science software program (SPSS), version 23 (Chicago, IL, USA). P value ≤ 0.05 was considered significant.
3. Results
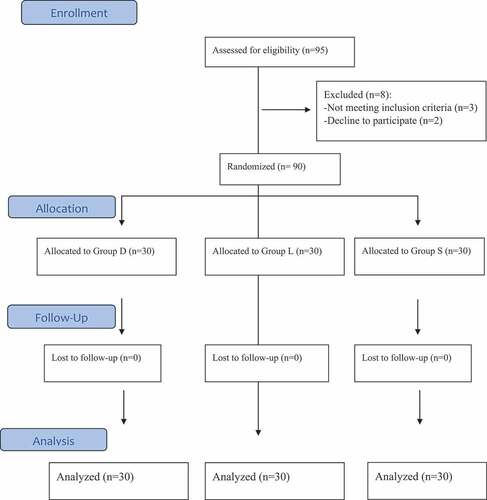
Ninety-five patients were screened for eligibility. Three patients were excluded for not meeting the inclusion criteria, and two patients refused to participate. Ninety patients (30 per group) were included and completed the study. () The demographic data and duration of the surgery were comparable in the three study groups. ().
Table 1. Demographic data and characteristics of patient
The total morphine consumption over the first 24 hours postoperatively revealed a significant difference among the three studied groups (P-value 0.045). The recorded median value for the group (S) was 3.0 (0.0–9.0) mg, versus 1.5 (0.0–4.0) mg for the group (N) and 0.0 (0.0–4.0) mg for the group (K). There was a significant reduction in group K compared to group S (P-value 0.013). However, there was no significant difference between group S and N (P-value 0.07) nor between groups K and N (P value 0.56). The time of the first analgesic request and the number of patients who required rescue analgesia were both comparable between the three groups. ()
Table 2. Intraoperative fentanyl consumption, postoperative morphine consumption, number of patients required morphine/fentanyl, and time to first postoperative analgesia (hours)
NRS did not show significant differences between the three study groups during the first 8 hours postoperatively. While at the eighth hr postoperatively, the NRS in group K [1.0 (1.0–2.0)] was significantly lower than that of both N and S groups [1.0 (1.0–3.0) and 1.0 (1.0–4.0), respectively] with P-value of 0.009 and 0.02, respectively. At the 16th hr postoperatively, the NRS was significantly lower in group K compared to group N [1.0 (0.0–2.0) versus 1.0 (0.1–3.0) P-value 0.011] and was significantly lower in group N compared to group S [1.0 (1.0–4.0) P-value 0.011]. ()
Table 3. Postoperative numerical rating scale
The intraoperative fentanyl consumption was comparable between (K&N) groups. However, it revealed a significant reduction in the groups K&N (111.67 ± 30.64 μg and 110.00 ± 20.34 μg, respectively) compared to Group S (131.67 ± 42.51 μg) with a P-value of 0.019. The number of patients who required additional intraoperative fentanyl doses was comparable among the three groups.
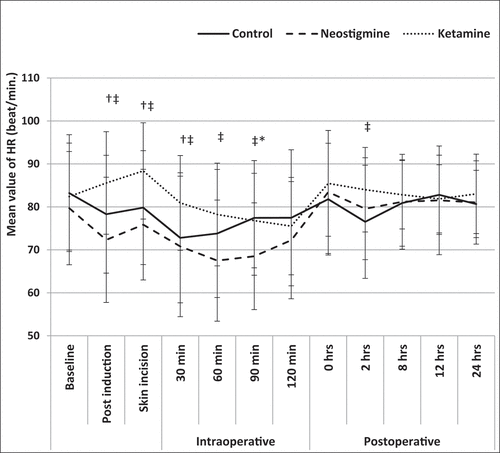
The intraoperative hemodynamics revealed a significant increase in the HR in group K compared to groups N & S after induction of general anesthesia (P-value >0.001, 0.04, respectively), at skin incision (P-value >0.001, 0.01, respectively) and at 30th min intraoperative (P-value >0.001, 0.03, respectively). Group N revealed a decrease in the HR to reach the maximum at 90 min of the operative time compared to groups K &S with P-value 0.02 & 0.009, respectively. However, there was no statistically significant difference for each of the three studied groups compared to the baseline preoperative values. ()
Figure 2. Pre, Intra, and postoperative change in mean HR
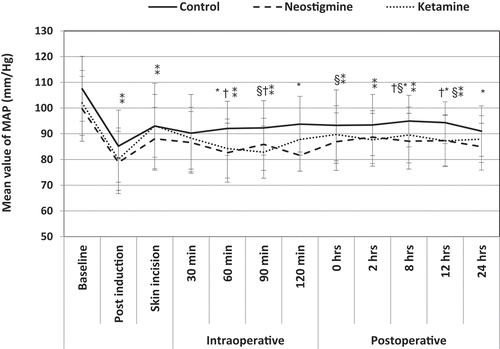
The intraoperative MAP revealed a statistically significant decrease in groups K & N compared to group S in 60 (P-value 0.005, 0.001, respectively) and 90 min (P-value 0.001, 0.03, respectively). Also, group N showed a significant decrease compared to group S in the 120th min (P-value 0.013). Group K showed a significant decrease in the postinduction, 60th and 90th minutes compared to baseline preoperative values (P-value 0.001, 0.005, 0.01, respectively). While no significant changes in groups S & N compared to baseline values. ()
Figure 3. Changes in MAP pre, intra, and postoperative
The postoperative HR revealed only a significant increase in group K during the 2nd hr compared to group S (P-value 0.02). (). The postoperative MAP showed a significant decrease in group k and N compared to group S in the 8th hr (P-value 0.03 & 0.002, respectively) and 12th hr (P-value 0.002 & 0.002, respectively) . Group N showed a significant decrease compared to group S in the 24th hr (P-value 0.01). ()
Compared to the baseline preoperative values, there was a statistically significant decrease in postoperative MAP in group S and K in immediate postoperative hour (P-value 0.01& 0.028, respectively), 2nd hr (P-value 0.03 & 0.008, respectively), 8th hr (P-value 0.03 & 0.005, respectively) and 12th hr (P-value 0.01 & 0.029 respectively). While in group N, there were no statistically significant differences relative to baseline readings within the same group throughout the study. ()
The three groups were comparable regarding the PONV, Patients’ complaint of postoperative nausea was 7 (23.3%) in the group (N), and 10 (33.3%) in the group (K) compared to 7 (23.3%) in the group (S). Regarding postoperative vomiting, the number of patients for the group (N) was 4 (13.3%), and 3 (10.0%) for the group (K) compared to 4 (13.3%) in the group (S).
For group (N), two patients showed intraoperative bradycardia, and three patients complained of preoperative abdominal colic. For group (K), two patients complained of preoperative hallucinations and nystagmus.
4. Discussion
The main findings of this study are that the addition of ketamine to bupivacaine during preoperative ultrasound-guided SAPB combined with GA in female patients undergoing modified radical mastectomy decreased the 24 hr postoperative morphine consumption and the intraoperative fentanyl requirements compared to the control group. At the same time, adding neostigmine as an adjuvant decreased the intraoperative fentanyl requirements compared to the control group with no difference regarding the 24 hr postoperative morphine consumption.
Ketamine exerts its antinociceptive effect through the blocking of N-methyl-d-aspartate receptors, increases the sensitization of the opioid system, and activation of aminergic (serotonergic and noradrenergic) with inhibition of its reuptake. Moreover, ketamine has a direct inhibitory effect on nitric oxide synthase, which probably contributes to its analgesic effects [Citation5,Citation19].
The use of ketamine as an adjuvant to LA has been investigated in epidural analgesia with favorable outcomes, but its analgesic effect in peripheral nerve blocks showed variable results [Citation20]. It was previously demonstrated that ketamine has a local anesthetic-like action through interaction with the rat myocyte’s sodium channel. Ketamine also blocks the N-methyl-D-aspartate receptors incorporated in the pain pathway [Citation8–14]. The aforementioned effects could explain the trial of ketamine as an adjuvant during fascial plane blocks.
The analgesic efficacy of ketamine as an adjuvant to bupivacaine was previously studied by Omar et al., 2012 [Citation11] during the thoracic paravertebral block for breast surgery using a dose of (0.5 mg/kg). The authors found no difference in the 24 hr opioid consumption and analgesic duration. Authors attributed their results to the rapid absorption of the hydrophilic ketamine into the systemic circulation. This systemic absorption could explain the resultant psychomimetic events in two ketamine group patients’ and the intraoperative and postoperative increase in heart rate observed in our study compared to the control group.
In agreement with our results, a study by Othman et al., 2016 [Citation12] compared the analgesic efficacy of 1 mg/kg ketamine added to 30 mL of 0.25% Bupivacaine in the ultrasound-guided modified pectoral block in patients undergoing modified radical mastectomy demonstrating a prolonged time to first request of analgesia, reduced total morphine consumption compared to control group. Another study by El Mourad et al., 2018 [Citation21] compared the effects of adding 4 mg dexamethasone or 50 mg ketamine to 0.5% bupivacaine in the thoracic paravertebral block; authors revealed that ketamine significantly prolonged the time to first analgesic demand and lowered the pain scores when compared to the dexamethasone and control groups.
In contrary to our results, Ketamine as an adjuvant to LA did not show superiority to the control group when added to patient control analgesia during interscalene brachial plexus block [Citation13], femoral nerve block [Citation14], or even in wound infiltration for cesarean section [Citation22].
In our study, the nonsignificant change in the morphine consumption and first analgesic request in the neostigmine group compared to the control group can be explained by increased systemic absorption of neostigmine due to its hydrophilic nature, and this can be evidenced by abdominal colics of three of neostigmine group and bradycardia in two patients immediately after block application. The exact mechanism of action of neostigmine in peripheral nerve blocks is still unclear. However, its peripheral analgesic effect could be attributed to the inhibition of the presynaptic glutamatergic afferents, hens increasing the endogenous acetylcholine. These cholinergic neurons terminate in nearby primary afferents, which represent muscarinic receptors [Citation18]. The spinal neostigmine exerts its action by inhibiting spinal cholinesterase and increases the endogenous acetylcholine, which is released from intrinsic cholinergic neurons within the dorsal horn of the spinal cord [Citation19]. Naguib M et al.,1997 [Citation20] suggested that the spinal neostigmine analgesic effect is mediated by spinal M1 and/or M3 receptor subtypes.
In a study by McCartney et al., 2003 [Citation23], authors demonstrated that adding 1 mg neostigmine to 0.5% lidocaine during intravenous regional anesthesia has no anesthetic or analgesic effect. Another study by Bouaziz et al., 1999 [Citation24] revealed that 500 mcg of neostigmine did not affect sensory and motor block in both axillary block and subcutaneous wound infiltration. On the other hand, Bouderka et al., 2003 [Citation25] demonstrated a lower pain score and decreased postoperative analgesic requirement when 500 mcg neostigmine was added to the axillary plexus block.
It seems that the neuraxial effect of both ketamine and neostigmine is different from the peripheral nerve block effect. In agreement with our results, Kamali et al., 2016 [Citation26] studied the analgesic effect of both neostigmine and ketamine as adjuvants to 0.25% bupivacaine in epidural anesthesia. The authors revealed a longer analgesic duration and less analgesic consumption with ketamine compared to neostigmine. However, it is not clear if the extended postoperative analgesic effect of ketamine as an adjuvant to LA is due to its local action at the injection site or its systemic absorption or both.
There are some limitations of the current study: first, the long-term effect of the studied drugs on chronic pain was not considered. Second, the effect of the studied drugs on the onset of the sensory block was not recorded as the patients were sedated. Third, the plasma level of ketamine was not measured, so we recommend further investigation using different doses of ketamine with measuring the plasma level to assess their influence on the duration of the postoperative analgesia.
5. Conclusion
The addition of 50 mg ketamine to 0.25% bupivacaine during preoperative ultrasound-guided SAPB combined with GA in female patients undergoing modified radical mastectomy decreased the 24 hr postoperative morphine consumption and the intraoperative fentanyl requirements while adding 500 µg neostigmine decreased the intraoperative fentanyl requirements.
Consent for publication
The authors accept the responsibility for releasing this material. This transfer of publication rights covers the non-exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online) or any other reproductions of similar nature.
Ethics approval and consent to participate
Approval was obtained from the Research Ethics Committee of Kasr Alainy Faculty of Medicine, Cairo University (email: [email protected]; ID MS-101-2020). Written informed consents were obtained from all participants before inclusion.
Data availability statement
Data are available from the authors upon reasonable request after Cairo University’s permission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Garg R, Bhan S, Vig S. Newer regional analgesia interventions (fascial plane blocks) for breast surgeries: review of literature. Indian J Anaesth. 2018 Apr;62(4):254.
- De AC, Bonvicini D, Correale C, et al. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019 Mar;85(3):308–319.
- Fecho K, Miller NR, Merritt SA, et al. Acute and persistent postoperative pain after breast surgery. Pain Med. 2009 May 1;10(4):708–715.
- Razek AA, AbouAllo MM, El Hamid SA, et al. Ultrasound-guided pectoral nerve blocks versus serratus intercostal plane block in breast surgeries. Res Opin Anesthesia Intensive Care. 2018 Jul 1;5(3):162.
- Kumar V, Sirohiya P, Gupta N, et al. Effect of adding dexamethasone to ropivacaine for ultrasound-guided serratus anterior plane block in patients undergoing modified radical mastectomy: a preliminary trial. Indian J Anaesth. 2020 Dec;64(12):1032–1037.
- Rashwan DAEK, Mohammed AR, Kasem Rashwan SA, et al. Efficacy of serratus anterior plane block using bupivacaine/ magnesium sulfate versus bupivacaine/ nalbuphine for mastectomy: a randomized, double-blinded comparative study. Anesth Pain Med. 2020 June;10(3):e103141.
- Abdelzaam EM, Abd Alazeem ES. Efficacy of dexmedetomidine as an adjuvant to bupivacaine in the ultrasound-guided serratus anterior plane block for postmastectomy analgesia. Egypt J Anaesth. 2020;36(1):319–323.
- Zekry J, Sayed N, Hasanin A, et al. Ketamine versus dexamethasone as an adjuvant to local anesthetics in combined sciatic-femoral nerve block for below knee surgeries. MJMR. 2015;26(1):32–38.
- Zaman B, Hojjati Ashrafi S, Seyed Siamdoust S, et al. The effect of ketamine and dexamethasone in combination with lidocaine on the onset and duration of axillary block in hand and forearm soft tissue surgery. Anesth Pain Med. 2017;7(5):e15570.
- Bakheet A, Hassan A, Darweesh E, et al. Comparative study between ketamine and dexamethasone added to bupivacaine in ultrasound guided infraclavicular brachial plexus block for upper limb surgeries. Sohag Med J. 2017;21(2):19–30.
- Omar AM, Mansour MA, Abdelwahab HH, et al. Role of ketamine and tramadol as adjuncts to bupivacaine 0.5% in paravertebral block for breast surgery: a randomized double-blind study. Egypt J Anaesth. 2011;27(2):101–105.
- Othman AH, El-Rahman AM, El Sherif F. Efficacy and safety of ketamine added to local anesthetic in modified pectoral block for management of postoperative pain in patients undergoing modified radical mastectomy. Pain Physician. 2016 Sep 1;19(7):485–494.
- Lee IO, Kim WK, Kong MH, et al. No enhancement of sensory and motor blockade by ketamine added to ropivacaine interscalene brachial plexus blockade. Acta Anaesthesiol Scand. 2002;46:821–826.
- Rahimzadeh P, Faiz SH, Ziyaeifard M, et al. Effectiveness of adding ketamine to ropivacaine infusion via femoral nerve catheter after knee anterior cruciate ligament repair. J Res Med Sci. 2013;18:632–636.
- Pandey V, Mohindra BK, Sodhi GS. Comparative evaluation of different doses of intrathecal neostigmine as an adjuvant to bupivacaine for postoperative analgesia. Anesth Essays Res. 2016 Sep;10(3):538.
- Yoganarasimha N, Raghavendra TR, Amitha S, et al. A comparative study between intrathecal clonidine and neostigmine with intrathecal bupivacaine for lower abdominal surgeries. Indian J Anaesth. 2014 Jan;58(1):43.
- Joshi-Khadke S, Khadke VV, Patel SJ, et al. Efficacy of spinal additives neostigmine and magnesium sulfate on characteristics of subarachnoid block, hemodynamic stability and postoperative pain relief: a randomized clinical trial. Anesth Essays Res. 2015 Jan;9(1):63.
- Elbahrawy K, El-Deeb A. The effects of adding neostigmine to supraclavicular brachial plexus block for postoperative analgesia in chronic renal failure patients: a prospective randomized double-blinded study. Res Opin Anesthesia Intensive Care. 2016 Jan 1;3(1):36.
- Chen S-R, Pan H-L. Activation of muscarinic receptors inhibits spinal dorsal horn projection neurons: role of GABAB receptors. Neuroscience. 2004;125(1):141–148.
- Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg. 1997;85(4):847–853.
- El Mourad MB, Amer AF. Effects of adding dexamethasone or ketamine to bupivacaine for ultrasound-guided thoracic paravertebral block in patients undergoing modified radical mastectomy: a prospective randomized controlled study. Indian J Anaesth. 2018 Apr;62(4):285.
- Zohar E, Luban I, Zunser I, et al. Patient-controlled bupivacaine wound instillation following cesarean section: the lack of efficacy of adjuvant ketamine. J Clin Anesth. 2002;14(7):505–511.
- McCartney CJL, Brill S, Rawson R, et al. No anesthetic or analgesic benefit of neostigmine 1 mg added to intravenous regional anesthesia with lidocaine 0.5% for hand surgery. Reg Anesth Pain Med. 2003;28(5):414–417.
- Bouaziz H, Paqueron X, Bur ML, et al. No enhancement of sensory and motor blockade by neostigmine added to mepivacaine axillary plexus block. Anesthesiol J Am Soc Anesthesiol. 1999;91(1):78–83.
- Bouderka MA, Al-Harrar R, Bouaggad A, et al. Neostigmine added to bupivacaine in axillary plexus block: which benefit? Ann Fr Anesth Reanim. 2003 Jun;22(6):510–513.
- Kamali A, Zareei A, Moshiri E, et al. Comparing the effect of adding ketamine and neostigmine to bupivacaine 0.25% for epidural analgesia among patients candidated for elective femoral fracture surgery. Int J Med Res Heal Sci. 2016;5(11):63–67.